
A patient-centered, multidisciplinary approach to treating borderline resectable pancreatic adenocarcinoma
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is the fourth leading cause of cancer-related deaths in the United States but accounts for only 3% of new cancer diagnoses (1). Although the cancer-related death rate for many other solid organ malignancies has declined, the cancer-related death rate for PDAC has not shown similar improvement over the past decade, and PDAC is expected to be the second leading cause of cancer-related deaths by 2030 (2). This is partly because most patients with PDAC present with metastatic (40%) or locally advanced (40%) disease (3). A minority of patients (20%) present with resectable or borderline resectable (BR) PDAC and are considered potential candidates for pancreatectomy, the only curative treatment available. Here, we outline the evolution of the pancreatic cancer staging system as it pertains to surgical resectability, describe the influence this staging system has had on treatment, and review the evidence that guides a multidisciplinary approach to workup, staging, and treatment of patients with BR PDAC.
Defining BR PDAC: an evolving staging system
Anatomic origin
Historically, PDAC was considered resectable if it appeared radiographically localized without involvement of adjacent mesenteric vessels—the celiac axis, common hepatic artery, superior mesenteric artery and vein, and portal vein. Involvement of these vessels represented a high risk for positive margins at surgery and poor oncologic outcomes following it. Some surgeons recognized that complete resection could be achieved with vascular resection and reconstruction in highly selected patients (4,5). Enhanced selection through the use of first-line chemotherapy subsequently broadened the potential for complete resection and was hypothesized to improve longevity (4,6-8). As such, “borderline resectable” became a term to signify tumors that are technically resectable, with or without vascular resection and reconstruction, but that are at high risk of harboring occult metastases at the time of diagnosis or positive margins if pancreatectomy is performed de novo (9,10).
At its inception, “marginally resectable” (as it was reported in literature published in 2001) represented an entirely anatomic designation, and this concept became the foundation for the first clinical classification of BR PDAC outlined by the National Comprehensive Cancer Network in 2006 (9-12). BR PDAC staging now differentiates pancreatic head/uncinate process tumors from pancreatic body/tail tumors. Staging has also evolved to omit confusing nomenclature (e.g., “abutment”, “encasement”, “occlusion”, and “impingement”) used in prior classification systems to describe the tumor-vessel interface, or the relationship between the tumor and the adjacent blood vessels, in favor of a detailed descriptor of the degree of tumor-vessel interface between the tumor and each vessel (<180° or ≥180°) to help standardize radiologists’ reporting and trial enrollment inclusion.
Over the past two decades, several pancreatic oncology societies and cancer centers have outlined their preferred anatomic definitions, including those proposed by the Americas Hepato-Pancreato-Biliary Association, the Society of Surgical Oncology, the Society for Surgery of the Alimentary Tract (13,14), the Alliance for Clinical Trials in Oncology (15), The University of Texas MD Anderson Cancer Center (9,14), and Medical College of Wisconsin (16). Each group of definitions is designed to outline objective radiographic criteria to inform treatment decisions. Table 1 provides a review of the anatomic differences among the more commonly used criteria to define resectable, BR, and locally advanced PDAC.
Table 1
| Vessel | Group definition | AHPBA/SSAT/SSO (13,14) | Alliance (15) | MDACC (9,14) | MCW (16) |
|---|---|---|---|---|---|
| CA | Resectable | Clear fat planes, no involvement | No extension | No extension | No evidence of TVI |
| BR | TVI <180° without stenosis or deformity | TVI <180° | TVI <180° without stenosis or deformity; periarterial stranding forming a convexity | TVI <180° | |
| Locally advanced | TVI ≥180° | TVI ≥180° | TVI ≥180° and no technical option for reconstruction | Type A: TVI ≥180° but does not extend to aorta and amenable to reconstruction. Type B: TVI ≥180° with extension beyond bifurcation of PHA | |
| SMA | Resectable | Clear fat planes, no involvement | No extension | No extension; normal fat plane between the tumor and the artery | No evidence of TVI |
| BR | TVI <180° without stenosis or deformity | TVI <180° | TVI <180° without stenosis or deformity; periarterial stranding forming a convexity against the vessel | TVI <180° | |
| Locally advanced | TVI ≥180° | TVI ≥180° | TVI ≥180° | Type A: TVI ≥180° but <270°. Type B: ≥270° | |
| CHA | Resectable | Clear fat planes, no involvement | No extension | No extension | No evidence of arterial abutment |
| BR | Short segment TVI <180° without tumor contact with the PHA; GDA TVI ≥180° with short segment TVI ≥180° at CHA without CA involvement | Short segment TVI (of any degree) amenable to resection and reconstruction | Short segment TVI of any degree that is amenable to reconstruction, typically at the GDA | TVI <180° or short segment TVI ≥180° without extension to CA or PHA | |
| Locally advanced | Involvement not amenable to reconstruction | Non-reconstructible involvement | Encased and no technical option for reconstruction | Type A: TVI ≥180° with extension to CA and amenable to reconstruction. Type B: TVI ≥180° with extension beyond the PHA | |
| SMV/PV | Resectable | No evidence of TVI, distortion, tumor thrombus, or venous encasement | TVI <180°, without occlusion | Patent | Tumor-induced narrowing of ≤50% |
| BR | TVI of any degree with or without occlusion and amenable to reconstruction | TVI ≥180° and/or short-segment occlusion of the SMV-PV amenable to reconstruction | TVI ≥180° with or without occlusion; amenable to reconstruction | Tumor-induced narrowing of >50% amenable to reconstruction | |
| Locally advanced | Any non-reconstructible involvement or major venous thrombosis extending several centimeters | Any non-reconstructible involvement | Occluded and no technical option for reconstruction | Occlusion without obvious option for reconstruction |
TVI, tumor-vessel interface; BR, borderline resectable; PDAC, pancreatic ductal adenocarcinoma; AHPBA, Americas Heapto-Pancreato-Biliary Association; SSAT, Society for Surgery of the Alimentary Tract; SSO, Society of Surgical Oncology; MDACC, MD Anderson Cancer Center; MCW, Medical College of Wisconsin; CA, celiac axis; PHA, proper hepatic artery; SMA, superior mesenteric artery; CHA, common hepatic artery; GDA, gastroduodenal artery; SMV, superior mesenteric vein; PV, portal vein.
Beyond anatomy: BR PDAC dimensional staging
The University of Texas MD Anderson Cancer Center proposed that borderline resectability should not be limited to a solely anatomic designation but should also reflect the biologic thumbprint of an individual cancer and the baseline physiologic capacity of each patient. This model is a much more patient-centric approach to staging compared with the original tumor-centric approach. This comprehensive definition was first introduced in 2008 and was included in the guidelines for potentially curable pancreatic cancer by the American Society of Clinical Oncology (ASCO). This definition was also the foundation for the international consensus criteria for BR PDAC in 2017 (15,17).
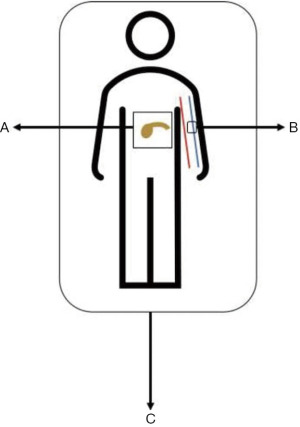
Three distinct dimensions of disease are captured within this system: BR-anatomic (BR-A), BR-biology (BR-B), and BR-condition (BR-C). Anatomic factors include the historic criteria for tumor-vessel interface as outlined in Table 1. Biologic factors include potentially resectable disease based on anatomic criteria but with clinical findings suspicious for, but without radiographic confirmation of, distant metastases or regional lymph node metastases diagnosed by biopsy or positron emission tomography. Biologic factors also include elevated (>500 units/mL) serum carbohydrate antigen 19-9 (CA 19-9), the most widely used serologic tumor marker in PDAC. Conditional factors include potentially resectable disease based on anatomic and biologic criteria but Eastern Cooperative Oncology Group (ECOG) performance status of two or more, making resection a riskier treatment option.
The definition of BR PDAC can be unidimensional or multidimensional (e.g., A, B, C, AB, AC, BC, or ABC). By incorporating anatomy, biology, and condition into a staging system, it is possible to stratify a patient’s unique clinical phenotype into a nomenclature that is understandable among all oncologists. This approach to staging has resulted in a shift in the way oncologists view pancreatic cancer: it is readily accepted that occult disease is present in most patients and, therefore, pancreatectomy is considered more cautiously, the unique role that each dimension has on resectability is critically assessed, and there is greater reliance on input from all medical disciplines as to the best strategy to optimize treatment success. Figure 1 illustrates the multidimensional staging of BR PDAC.
The multidisciplinary team: identifying and engaging key members
When starting a clinical journey with a patient who is diagnosed with BR PDAC, it is essential to recognize that the path should be founded on the individual. The best treatment plan is ultimately defined by the patient: their symptoms; their unique clinical, serologic, and radiographic staging; their family history; and with their goals of care in mind.
The most important conversation is the first one. This conversation should collect valuable information about each patient’s clinical symptoms, baseline function and lifestyle, familial support, and goals of care. The conversation should also provide an opportunity to outline the treatment options and introduce the multidisciplinary team members integral to the proposed treatment.
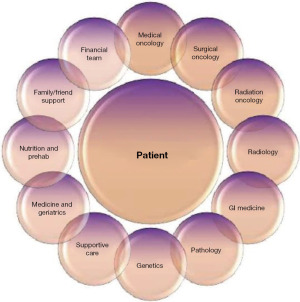
Figure 2 displays an approach to patient-centered care and depicts some of the key members necessary to deliver the highest level of multidisciplinary care. Data suggests that there is an objective value in engaging a multidisciplinary team. A multidisciplinary strategy for patients with pancreatic cancer allows more accurate diagnosis and staging, a higher receipt of guideline-concordant treatment, and higher accrual to clinical trials (18-21). Johns Hopkins reported that a multidisciplinary clinic resulted in change of management in 24% of patients and enrolled 78% of patients in the National Familial Pancreas Tumor Registry (19). Gardner et al. also demonstrated that patients who were treated in a multidisciplinary setting had a significantly shorter duration to first treatment and shorter total number of clinic consultations prior to initiating therapy (18).
Establishing a diagnosis and developing a treatment plan
In the simplest terms, the steps that should be taken when faced with a new pancreatic mass are as follows: (I) name it, (II) stage it, and (III) treat it.
Name it: pathologic confirmation and parallel treatment preparation
Diagnostic confirmation is dependent on tissue biopsy. Esophagogastroduodenoscopy with endoscopic ultrasound (EUS) is most often used to establish the diagnosis (22). EUS uses a high-frequency transducer at the tip of the endoscope that facilitates the generation of high-resolution images of the pancreas through the stomach or duodenum (23).
EUS is regarded as the most sensitive imaging modality for the detection of pancreatic lesions, with a pooled sensitivity rate of 94% (24-42). This modality is specifically useful for the detection and confirmation of small (<3 mm) pancreatic lesions and is superior to the detection offered by computed tomography or magnetic resonance imaging (93% for EUS, 67% for computed tomography, and 53% for magnetic resonance imaging) (26). On EUS, most solid pancreatic lesions are depicted as heterogeneous, hypoechoic masses that can be biopsied with fine-needle aspiration or core needle biopsy to provide pathologic confirmation (23).
Furthermore, endoscopy allows simultaneous endoscopic retrograde cholangiopancreatography-guided biliary decompression in patients who present with obstructive jaundice (43). Preoperative endoscopic retrograde cholangiopancreatography-guided biliary stenting is also a prophylactic tool and is the preferred approach for patients with non-obstructed pancreatic head masses who plan to undergo neoadjuvant chemotherapy and thus will experience delay surgical bypass (44). This procedure is often performed with sphincterotomy followed by trans-papillary placement of a metal or plastic stent over a guidewire. Metal stents are preferred over plastic stents owing to a lower risk of complications such as stent dysfunction and cholangitis (45,46). Although plastic stents are cheaper, technically more compliant, and easier to deploy, they result in more frequent replacements with a patency on the order of 3 months compared with 6–9 months or longer for metal stents (47).
Stage it: a multidimensional approach to local and systemic staging
Anatomy
A pancreatic protocolled computed tomography study of the abdomen and pelvis is the best modality to identify the anatomic relationship of a tumor to the surrounding vasculature. Intravenous iodinated contrast at a volume of 150 mL should be rapidly infused at a rate of 5 mL/second, slices should be constructed at <3 mm with overlap, and at least two postcontrast acquisitions should be included: a late arterial and venous phase. In the late arterial phase, the tumor is discernable as a hypodense mass in a background of pancreatic parenchyma. The venous phase is generally the best for determining the relationship of the tumor with surrounding vasculature and assessing the liver for hepatic metastases. Coronal and sagittal views are reformatted and aid in determining arterial and venous vascular involvement (48).
Biology
CA 19-9 is measured in every patient who presents with PDAC. Although serum CA 19-9 has little use in the 10% of patients who are nonproducers and is difficult to comprehend in patients with biliary obstruction, CA 19-9 is the only US Food and Drug Administration–approved biomarker in PDAC (49,50). Normal values range from 0 to 37 U/dL. Although most patients with PDAC present with elevated values of CA 19-9, a value ≥500 U/mL is generally considered the threshold for defining borderline resectability at MD Anderson. This value is founded on clinical observation but is statistically arbitrary.
Owing to the limitations of CA 19-9 as a reliable biomarker in nonproducers, there is growing enthusiasm for the use of circulating tumor DNA (ctDNA) and circulating tumor cells (CTCs) as universal serologic biomarkers in PDAC. ctDNA and CTCs can be collected from the peripheral and portal venous blood to potentially quantify systemic disease burden (51-56). Given the added benefit of assessing the tumor mutational profile, it is also realistic to anticipate the forthcoming ability to predict response to therapy by the presence and volume of a tumor’s ctDNA or CTCs (57,58).
Condition
ECOG performance status is used to assess each patient at the time of presentation with PDAC. Any patient who has an ECOG performance status of ≥2 is considered to have BR PDAC according to conditional criteria (15).
Age is not captured within the ECOG performance score but is an important component of condition among patients with PDAC. Because PDAC affects a predominately elderly population, with a median age at diagnosis of 70 years, it is important to engage a geriatrician, when appropriate, who can provide useful information such as the predicted non-cancer survival at 5 and 10 years based on comorbidity, functional, and mental status. This information can be helpful in making treatment decisions (59,60).
Treat it: neoadjuvant therapy and dynamic metrics to follow response
All patients with BR PDAC, when defined using the criteria outlined above, should be considered for neoadjuvant therapy according to ASCO guidelines for potentially curable pancreatic cancer (17). Although this is founded on low-quality evidence, it is considered a strong recommendation (17).
Two trials, the PREOPANC I and a Korean trial by Jang et al., have compared neoadjuvant therapy with surgery de novo in this setting. These two trials used chemoradiation as the neoadjuvant treatment arm, and no subsequent trials have compared contemporary systemic regimens with surgery de novo (61,62). Contemporary regimens include 5-fluorouracil (5-FU)/leucovorin/oxaliplatin/irinotecan (FOLFIRINOX) or gemcitabine with abraxane (nanoparticle albumin-bound paclitaxel; GemAb) and are the current first-line regimens used to treat PDAC, with proven efficacy in more advanced disease. Two recent phase II studies, the SWOG S1505 and the Alliance A021501, included these regimens in their neoadjuvant trial design but did not include a primary resection arm (63,64).
Extrapolating data from best-available trials has resulted in expert consensus that neoadjuvant chemotherapy is advantageous in patients with BR PDAC (17,65). Prioritizing a systemic or combined systemic and local therapy–first approach in the management of BR PDAC has the potential to optimize each dimension of treatment of BR PDAC, as outlined below.
Anatomy
Some prospective evidence supporting the potential of neoadjuvant therapy to optimize anatomic dimensions of resectability compared with surgery de novo can be found in the PREOPANC I and Jang trials (61,62). The PREOPANC I trial randomized patients with resectable and BR PDAC to surgery de novo or neoadjuvant gemcitabine-based chemoradiotherapy and reported a higher R0 resection rate in the neoadjuvant treatment cohort (63% compared with 31%, P<0.01) (61). In a smaller study by Jang et al., the findings were similar: the R0 resection rate was significantly higher in the neoadjuvant chemoradiation group than in the surgery de novo group (52% compared with 26%, P<0.01) (62).
The recently published Alliance A021501 trial suggested an R0 resection advantage with the use of a neoadjuvant systemic therapy-only approach, reporting a higher R0 resection rate with neoadjuvant modified FOLFIRINOX (mFOLFIRINOX) than with neoadjuvant mFOLFIRINOX followed by hypofractionated radiotherapy (mFOLFIRINOX + RT) (42% compared with 25%, P<0.01) (64). Additionally, a single-institution trial from MD Anderson showed that a tumor-parenchymal interface response to neoadjuvant mFOLFIRINOX and chemoradiation was a reliable anatomic biomarker and could be used to predict R0 resection (66). The details of these studies will be discussed below.
The available evidence suggests that neoadjuvant therapy has the potential to show a radiographically meaningful response on restaging imaging and improve the rate of R0 resection (61,62,66,67).
Biology
There is a high rate of distant failure following surgery de novo among patients with BR PDAC, validating the hypothesis that micrometastases are present even in a seemingly localized stage of disease (9,10). A retrospective study from MD Anderson showed that among CA 19-9 producers, the overwhelming majority (>90%) of patients who had a pathologic major response (pMR) had normalization of their elevated baseline CA 19-9 following neoadjuvant therapy, demonstrating the value of CA 19-9 as a biologic readout during treatment (68).
The University of Pittsburgh Medical Center reported that a pre-treatment to post-treatment CA 19-9 reduction of >50% was highly predictive of R0 resection. Additionally, they reported that 29% of patients who had a pre-treatment to post-treatment CA 19-9 reduction experienced a complete pathologic response (69).
In a recent study, more than 95% of patients with resectable PDAC had CTCs in their peripheral blood. The dynamic changes of CTCs following first-line chemotherapy were strongly associated with disease progression or response. Thus, CTCs and ctDNA offer tools to study the systemic burden of disease. Further work is needed to determine how to incorporate such tests into neoadjuvant management algorithms (70).
Condition
A neoadjuvant approach provides the opportunity for all patients to optimize the management of medical comorbidities or functional limitations that may portend poorer outcomes following pancreatectomy. All potential surgical candidates should consult with a general practitioner to optimize the management of comorbidities and a dietician to improve nutrition, as well as enroll in a prehabilitation program during their neoadjuvant therapy (71). The chemotherapy period allows an opportunity to declare improvement or decompensation from baseline while receiving systemic therapy. Just as radiographic and serologic response to therapy have important prognostic readouts, the functional dynamics that are observed during prehabilitation may help predict which patients will experience significant complications following surgery (71-73).
These data illustrate the value of neoadjuvant systemic therapy in not only managing anatomic barriers to surgical clearance, treating occult metastatic disease, and conditioning a patient for proposed pancreatectomy, but also guiding the multidisciplinary team in its understanding of each patient’s unique phenotypic profile. Furthermore, a neoadjuvant treatment strategy improves multimodal treatment completion; about 25% to 50% of patients who undergo surgery de novo never begin adjuvant therapy because of postoperative complications, failure to regain performance status sufficient to permit therapy, or rapid development of progressive disease (74-76).
Evidence-based treatment: borderline staging as an impetus for a shift in trial design
The initial multimodal clinical trials in PDAC established the use of adjuvant therapy following pancreatectomy for patients with resectable PDAC. After BR PDAC was introduced, a neoadjuvant approach was incorporated into trial designs, reflecting how BR PDAC staging conceptually influenced the approach to treating PDAC. Figure 3 outlines the evolution of clinical trial design before and after the emergence of BR PDAC staging.
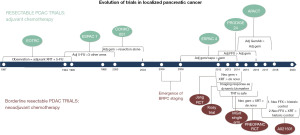
Resectable PDAC: adjuvant therapy trials
Early trials studied the efficacy of adjuvant therapies in patients with resectable PDAC and cumulatively established the multimodal strategy of resection and chemotherapy with or without radiation to be superior to resection alone in curative-intent treatment.
Between 1987 and 1995, the European Organization for Research and Treatment of Cancer (EORTC) trial enrolled 218 patients and randomized them to surgery de novo followed by observation or surgery with adjuvant 5-FU and radiotherapy. The study showed no significant difference in the primary endpoint of overall survival (OS), with a median OS of 24 months in the treatment arm and 19 months in the observation arm (P=0.21) (77).
In 2004, the European Study Group for Pancreatic Cancer (ESPAC)-1 trial compared four arms: adjuvant 5-FU-based chemoradiotherapy alone, adjuvant 5-FU-based chemoradiotherapy followed by 5-FU, adjuvant 5-FU alone, and observation (78). This trial was not powered for direct comparisons between the four cohorts but did show that patients who received systemic chemotherapy survived longer than patients who did not (median OS 20 months compared with 16 months, P=0.009). Additionally, patients who received adjuvant chemoradiotherapy experienced inferior survival (median OS 16 months compared with 18 months for 5-FU alone, P=0.05) (78).
In 2013, the Charite Onkologie 001 (CONKO-001) trial compared adjuvant gemcitabine with observation and reported a significantly improved 5-year OS rate of 21% for patients treated with adjuvant gemcitabine compared with 10% for patients who received observation only (P=0.01) (79).
In 2017, the ESPAC-4 trial compared adjuvant gemcitabine with gemcitabine plus capecitabine (80). The median OS was 26 months for patients treated with gemcitabine alone and 28 months for patients treated with gemcitabine plus capecitabine (P 0.032).
In 2018, the Partenariat de Recherche en Oncologie Digestive (PRODIGE) 24 trial compared adjuvant gemcitabine with mFOLFIRINOX and showed a robust survival advantage with the implementation of adjuvant mFOLFIRINOX (81). The median OS of patients treated with mFOLFIRINOX was 54 months compared with 35 months for patients treated with gemcitabine (P<0.01). As such, mFOLFIRINOX has become the gold standard adjuvant chemotherapy choice for patients who undergo surgery de novo.
In 2019, the results from the phase III Adjuvant Pancreatic Adenocarcinoma Clinical Trial (APACT) trial comparing adjuvant GemAb with gemcitabine alone were reported at the annual ASCO meeting: a survival advantage was observed in the GemAb arm compared with the gemcitabine arm (median OS 41.8 months compared with 37.7 months, P=0.01) (82).
BR PDAC: neoadjuvant therapy trials
The wide adoption of borderline resectability as a unique stage of PDAC introduced new ways of approaching treatment and inspired clinical trial design that investigated the potential role of neoadjuvant approaches in the management of localized pancreatic cancer.
One of the first neoadjuvant trials published was a 2012 Korean study by Jang et al. This trial randomized patients with BR PDAC to receive neoadjuvant gemcitabine with radiotherapy or surgery de novo. The primary endpoint was OS at 2-year follow-up, and the neoadjuvant arm had a longer median OS of 21 months compared with 12 months in the surgery de novo arm (P=0.03) (62).
From 2012 to 2015, a trial from MD Anderson enrolled 33 patients with BR PDAC and measured radiographic response to neoadjuvant mFOLFIRINOX and 50 Gy chemoradiation with concurrent gemcitabine using the anatomic tumor-parenchymal interface (66). The authors proposed a new radiographic biomarker in which tumors at the time of presentation were categorized using computed tomography by the Hounsfield unit difference between the visualized tumor and normal pancreatic parenchyma (66). Low-delta tumors were those that did not exhibit a change in Hounsfield units between the tumor and the parenchyma, and high-delta tumors were those that did exhibit an abrupt change in Hounsfield units between the tumor and parenchyma. Following neoadjuvant therapy, a type I interface response was described as one that remained stable or became more defined, and a type II interface response was described as one that became less defined. In total, 17 patients exhibited a type I interface response and 16 patients exhibited a type II interface response. Patients with high-delta PDAC were more likely to experience a type II interface response than were those with low-delta PDAC (P=0.026) (66). Patients with a type II interface response were more likely to have an R1 resection margin than were those who exhibited a type I response (P<0.001). Patients with low-delta tumors had significantly better OS than those with high-delta tumors (median OS not reached compared with 17 months, P<0.01), and patients with a type I response also had significantly better OS than those with a type II response (median OS 30 months compared with 14 months, P<0.01) (66). These data suggest that a novel imaging-based biomarker may exist, and that the tumor-parenchymal interface response of PDAC may be used to gauge anatomic response to neoadjuvant therapy and better select patients for pancreatectomy.
Concurrently, a single-center, single-arm trial from Massachusetts General Hospital investigated the primary outcome of R0 resection rate in patients who received 8 cycles of neoadjuvant chemotherapy with multi-agent FOLFIRINOX followed by short-course radiotherapy; a 97% R0 resection rate was observed. The initial design of this trial was to give 4 cycles of therapy preoperatively and 4 cycles postoperatively, but the study was amended to allow patients without progression on the restaging computed tomography scan to receive an additional 4 cycles of FOLFOIRINOX prior to radiotherapy (for a total of 8 cycles of neoadjuvant FOLFIRINOX) and no adjuvant chemotherapy. The authors termed this approach a “total neoadjuvant” approach, and the results showed that such an approach is safe, with favorable oncologic outcomes, including a median OS of 38 months (67).
The Dutch group’s PREOPANC phase III trial randomized patients to receive either 2 cycles of neoadjuvant gemcitabine concurrently with 15 Gy chemoradiation followed by resection and 4 cycles of adjuvant gemcitabine, or surgery de novo followed by 6 cycles of gemcitabine. The rate of resection was 61% in the neoadjuvant chemotherapy group and 72% in the surgery de novo group, although this was not statistically significant (P=0.06). In an intention-to-treat analysis, the median OS was similar between those who received neoadjuvant chemoradiation and those who received primary resection (16 months compared with 14 months, P=0.10). However, more patients in the neoadjuvant therapy cohort underwent an R0 resection (72% compared with 40%, P<0.001). In the subset of patients who underwent resection and started adjuvant therapy, there was improved OS in the neoadjuvant chemoradiation cohort compared with the surgery de novo cohort (median OS 35 months compared with 20 months, P=0.03). Although the trial was a negative study and did not show a benefit of neoadjuvant therapy using the defined endpoints, subsequent long-term analysis did show a statistically longer median OS among patients treated with neoadjuvant chemoradiation (hazard ratio 0.73, P=0.03) (61,83).
The Alliance A021501 trial randomized patients to receive either 8 cycles of neoadjuvant mFOLFIRINOX or 7 cycles of mFOLFIRINOX + RT (64). Each cohort was then eligible to receive pancreatectomy followed by 4 cycles of adjuvant mFOLFIRINOX. The primary endpoint was 18-month OS rate, and each arm was compared with a historical control of 50%. The mFOLFIRINOX cohort had an 18-month OS rate of 67%, and the mFOLFIRINOX + RT cohort had an 18-month OS rate of 47%, which did not meet the predefined historic threshold. The study also showed that the rate of R0 resection was 43% for mFOLFIRINOX and 25% for mFOLFIRINOX + RT. The mFOLFIRINOX + RT arm closed at interim analysis owing to the low R0 rate, but the mFOLFIRINOX arm was accrued to full enrollment with a median OS of 30 months. This trial established an 8-cycle mFOLFIRINOX regimen as the contemporary reference neoadjuvant regimen for BR PDAC (64).
Inclusion of neoadjuvant therapy in resectable PDAC trial design
Published in 2020, the SWOG S1505 trial compared two multi-agent neoadjuvant regimens in patients with resectable PDAC: FOLFIRINOX and GemAb. The primary outcome was 2-year OS rate, with similar rates of 41.6% for FOLFIRINOX and 48.8% for GemAb and a median OS of 22 months for FOLFIRINOX and 24 months for GemAb (P=0.40) (63). This trial established these two neoadjuvant regimens to be equally effective (63).
The global view: a guide to dynamic response interpretation and integration of response into treatment decisions
Neoadjuvant chemotherapy administration allows time to better understand the phenotypic profile and longitudinal behavior of an individual PDAC. Although an initial clinical stage gives a single snapshot of the appearance and predicted natural history of a tumor, the integration of longitudinal treatment response provides a panoramic view of a tumor’s behavior over time and a more reliable metric of long-term outcomes, thus guiding subsequent treatment choice.
Response can be divided into three unique domains: serologic, radiographic, and pathologic response to neoadjuvant therapy. Figure 4 is a flow diagram showing an example of the integration of dynamic changes of response into treatment decisions.

Data suggest that these measures of response are reliable prognosticators. In a retrospective study from MD Anderson that included 485 patients treated with either induction FOLFIRINOX or GemAb, patients who experienced a pMR had a significantly higher median reduction in tumor volume radiographically in the restaging period following neoadjuvant therapy compared with patients who did not experience a pMR (68% compared with 34% tumor volume reduction, P<0.001) (84). Additionally, decrease and normalization of CA 19-9 levels were associated with significantly higher rates of pMR: 71% of patients who experienced a pMR had normalization of their elevated baseline CA 19-9, 25% of patients had a low baseline CA 19-9, and only 4% had a mildly elevated CA 19-9 following neoadjuvant chemotherapy (84). These are worthwhile data because they predict long-term survival. Patients who experienced a pMR had a median OS that was not reached compared with 40 months for patients without a pMR (P<0.01) (84).
Deciding to whom and when to offer pancreatectomy is a diagnostic challenge and should be a collective decision among the multidisciplinary team. Including longitudinal treatment response data enhances treatment precision among practitioners.
Authors’ reflections on type of neoadjuvant regimen and approach to BR PDAC
The data presented here have shown that patients can tolerate and often benefit from neoadjuvant chemotherapy and subsequently undergo successful pancreatectomy without prohibitive perioperative complications. Current guidelines recommend the administration of neoadjuvant multi-agent chemotherapy regimens, either first-line FOLFIRINOX or GemAb, for a total of 4–6 months with consideration of subsequent radiotherapy for most patients with resectable PDAC or BR PDAC (17). This is followed by pancreatectomy and adjuvant chemotherapy.
A dynamic approach to the treatment of BR PDAC allows for an enhanced feedback system to optimize outcomes. Serologic, radiographic, physiologic, and pathologic measures of response can provide real-time readouts of the effectiveness of treatment and act to predict subsequent treatment success or failure. Integration of these putative markers into the treatment strategy allows a flexible, individualized framework. Data suggest that a total neoadjuvant therapy approach without adjuvant therapy is a safe strategy, but it is important to recognize that such an approach restricts the integration of pathologic response into adjuvant regimen decisions and limits the opportunity for dynamic response data to guide therapy (67).
Concluding remarks
The introduction of the multidimensional BR PDAC staging system has transformed the approach to management of BR PDAC. The inception of this staging system resulted in multiple neoadjuvant trials that sought to understand the role that neoadjuvant therapy may play in improving outcomes for this complex diagnosis, and evidence suggests that neoadjuvant therapy has the potential to improve each of the three dimensions of borderline resectability. Understanding the individual phenotype of a patient with BR PDAC is critical. By definition, these patients are at high risk of treatment failure following surgery de novo, so it is critical to understand the unidimensional or multidimensional barriers that are likely to hinder success, use the available evidence for neoadjuvant treatment in an effort to target and optimize all dimensions of borderline resectability, and become fluent in the dynamic metrics of response that can help select patients who would benefit from pancreatectomy.
All decisions in the management of BR PDAC should be done in the setting of a multidisciplinary conversation and only after consensus is obtained. Treatment recommendations should be founded on the multidimensional staging of BR PDAC and integrate longitudinal, dynamic data into decisions. Each individual patient and their goals should act as the foundation for all recommendations, and the proposed plan should be carried out within a broad and diverse multidisciplinary team.
Acknowledgments
We thank Erica Goodoff, Senior Scientific Editor in the Research Medical Library at The University of Texas MD Anderson Cancer Center, for editing this article. This work was supported by the Lockton distinguished chair for pancreatic cancer research.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Yoji Kishi) for the series “Pre- and Post-Operative Treatment for Pancreatic Cancer” published in Chinese Clinical Oncology. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://cco.amegroups.com/article/view/10.21037/cco-22-86/coif). The series “Pre- and Post-Operative Treatment for Pancreatic Cancer” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022. CA Cancer J Clin 2022;72:7-33. [Crossref] [PubMed]
- Rahib L, Smith BD, Aizenberg R, et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 2014;74:2913-21. [Crossref] [PubMed]
- Janssen QP, O'Reilly EM, van Eijck CHJ, et al. Neoadjuvant Treatment in Patients With Resectable and Borderline Resectable Pancreatic Cancer. Front Oncol 2020;10:41. [Crossref] [PubMed]
- Fuhrman GM, Leach SD, Staley CA, et al. Rationale for en bloc vein resection in the treatment of pancreatic adenocarcinoma adherent to the superior mesenteric-portal vein confluence. Pancreatic Tumor Study Group. Ann Surg 1996;223:154-62. [Crossref] [PubMed]
- Evans DB, Lee JE, Leach SD, et al. Vascular resection and intraoperative radiation therapy during pancreaticoduodenectomy: rationale and technique. Adv Surg 1996;29:235-62. [PubMed]
- Fortner JG. Regional pancreatectomy for cancer of the pancreas, ampulla, and other related sites. Tumor staging and results. Ann Surg 1984;199:418-25. [Crossref] [PubMed]
- Ishikawa O, Ohigashi H, Imaoka S, et al. Preoperative indications for extended pancreatectomy for locally advanced pancreas cancer involving the portal vein. Ann Surg 1992;215:231-6. [Crossref] [PubMed]
- Nakao A, Nonami T, Harada A, et al. Portal vein resection with a new antithrombogenic catheter. Surgery 1990;108:913-8. [PubMed]
- Katz MH, Pisters PW, Evans DB, et al. Borderline resectable pancreatic cancer: the importance of this emerging stage of disease. J Am Coll Surg 2008;206:833-46; discussion 846-8. [Crossref] [PubMed]
- Varadhachary GR, Tamm EP, Abbruzzese JL, et al. Borderline resectable pancreatic cancer: definitions, management, and role of preoperative therapy. Ann Surg Oncol 2006;13:1035-46. [Crossref] [PubMed]
- National Comprehensive Cancer Network. National Comprehensive Cancer Network (NCCN) practice guidelines for pancreatic cancer. Available online: https://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf
- Varadhachary GR, Tamm EP, Crane C, et al. Borderline resectable pancreatic cancer. Curr Treat Options Gastroenterol 2005;8:377-84. [Crossref] [PubMed]
- Lopez NE, Prendergast C, Lowy AM. Borderline resectable pancreatic cancer: definitions and management. World J Gastroenterol 2014;20:10740-51. [Crossref] [PubMed]
- Nappo G, Donisi G, Zerbi A. Borderline resectable pancreatic cancer: Certainties and controversies. World J Gastrointest Surg 2021;13:516-28. [Crossref] [PubMed]
- Isaji S, Mizuno S, Windsor JA, et al. International consensus on definition and criteria of borderline resectable pancreatic ductal adenocarcinoma 2017. Pancreatology 2018;18:2-11. [Crossref] [PubMed]
- Evans DB. What Makes a Pancreatic Cancer Resectable? Am Soc Clin Oncol Educ Book 2018;38:300-5. [Crossref] [PubMed]
- Khorana AA, Mangu PB, Berlin J, et al. Potentially Curable Pancreatic Cancer: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2016;34:2541-56. [Crossref] [PubMed]
- Gardner TB, Barth RJ, Zaki BI, et al. Effect of initiating a multidisciplinary care clinic on access and time to treatment in patients with pancreatic adenocarcinoma. J Oncol Pract 2010;6:288-92. [Crossref] [PubMed]
- Pawlik TM, Laheru D, Hruban RH, et al. Evaluating the impact of a single-day multidisciplinary clinic on the management of pancreatic cancer. Ann Surg Oncol 2008;15:2081-8. [Crossref] [PubMed]
- Mobley EM, Swami U, Mott S, et al. A Retrospective Analysis of Clinical Trial Accrual of Patients Presented in a Multidisciplinary Tumor Board at a Tertiary Health Care Center and Associated Barriers. Oncol Res Treat 2020;43:196-203. [Crossref] [PubMed]
- Page AJ, Cosgrove D, Elnahal SM, et al. Organizing a multidisciplinary clinic. Chin Clin Oncol 2014;3:43. [PubMed]
- Prager M, Prager E, Sebesta C Jr, et al. Diagnostic and Therapeutic Indications for Endoscopic Ultrasound (EUS) in Patients with Pancreatic and Biliary Disease-Novel Interventional Procedures. Curr Oncol 2022;29:6211-25. [Crossref] [PubMed]
- Kitano M, Yoshida T, Itonaga M, et al. Impact of endoscopic ultrasonography on diagnosis of pancreatic cancer. J Gastroenterol 2019;54:19-32. [Crossref] [PubMed]
- Rösch T, Lorenz R, Braig C, et al. Endoscopic ultrasound in pancreatic tumor diagnosis. Gastrointest Endosc 1991;37:347-52. [Crossref] [PubMed]
- Palazzo L, Roseau G, Gayet B, et al. Endoscopic ultrasonography in the diagnosis and staging of pancreatic adenocarcinoma. Results of a prospective study with comparison to ultrasonography and CT scan. Endoscopy 1993;25:143-50. [Crossref] [PubMed]
- Müller MF, Meyenberger C, Bertschinger P, et al. Pancreatic tumors: evaluation with endoscopic US, CT, and MR imaging. Radiology 1994;190:745-51. [Crossref] [PubMed]
- Marty O, Aubertin JM, Bouillot JL, et al. Prospective comparison of ultrasound endoscopy and computed tomography in the assessment of locoregional invasiveness of malignant ampullar and pancreatic tumors verified surgically. Gastroenterol Clin Biol 1995;19:197-203. [PubMed]
- Melzer E, Avidan B, Heyman Z, et al. Preoperative assessment of blood vessel involvement in patients with pancreatic cancer. Isr J Med Sci 1996;32:1086-8. [PubMed]
- Howard TJ, Chin AC, Streib EW, et al. Value of helical computed tomography, angiography, and endoscopic ultrasound in determining resectability of periampullary carcinoma. Am J Surg 1997;174:237-41. [Crossref] [PubMed]
- Sugiyama M, Hagi H, Atomi Y, et al. Diagnosis of portal venous invasion by pancreatobiliary carcinoma: value of endoscopic ultrasonography. Abdom Imaging 1997;22:434-8. [Crossref] [PubMed]
- Legmann P, Vignaux O, Dousset B, et al. Pancreatic tumors: comparison of dual-phase helical CT and endoscopic sonography. AJR Am J Roentgenol 1998;170:1315-22. [Crossref] [PubMed]
- Gress FG, Hawes RH, Savides TJ, et al. Role of EUS in the preoperative staging of pancreatic cancer: a large single-center experience. Gastrointest Endosc 1999;50:786-91. [Crossref] [PubMed]
- Midwinter MJ, Beveridge CJ, Wilsdon JB, et al. Correlation between spiral computed tomography, endoscopic ultrasonography and findings at operation in pancreatic and ampullary tumours. Br J Surg 1999;86:189-93. [Crossref] [PubMed]
- Harrison JL, Millikan KW, Prinz RA, et al. Endoscopic ultrasound for diagnosis and staging of pancreatic tumors. Am Surg 1999;65:659-64; discussion 664-5. [Crossref] [PubMed]
- Mertz HR, Sechopoulos P, Delbeke D, et al. EUS, PET, and CT scanning for evaluation of pancreatic adenocarcinoma. Gastrointest Endosc 2000;52:367-71. [Crossref] [PubMed]
- Rivadeneira DE, Pochapin M, Grobmyer SR, et al. Comparison of linear array endoscopic ultrasound and helical computed tomography for the staging of periampullary malignancies. Ann Surg Oncol 2003;10:890-7. [Crossref] [PubMed]
- Kitano M, Kudo M, Maekawa K, et al. Dynamic imaging of pancreatic diseases by contrast enhanced coded phase inversion harmonic ultrasonography. Gut 2004;53:854-9. [Crossref] [PubMed]
- Agarwal B, Abu-Hamda E, Molke KL, et al. Endoscopic ultrasound-guided fine needle aspiration and multidetector spiral CT in the diagnosis of pancreatic cancer. Am J Gastroenterol 2004;99:844-50. [Crossref] [PubMed]
- DeWitt J, Devereaux B, Chriswell M, et al. Comparison of endoscopic ultrasonography and multidetector computed tomography for detecting and staging pancreatic cancer. Ann Intern Med 2004;141:753-63. [Crossref] [PubMed]
- Jemaa Y, Houissa F, Trabelsi S, et al. Endoscopic ultrasonography versus helical CT in diagnosis and staging of pancreatic cancer. Tunis Med 2008;86:346-9. [PubMed]
- Sakamoto H, Kitano M, Suetomi Y, et al. Utility of contrast-enhanced endoscopic ultrasonography for diagnosis of small pancreatic carcinomas. Ultrasound Med Biol 2008;34:525-32. [Crossref] [PubMed]
- Kamata K, Kitano M, Kudo M, et al. Value of EUS in early detection of pancreatic ductal adenocarcinomas in patients with intraductal papillary mucinous neoplasms. Endoscopy 2014;46:22-9. [Crossref] [PubMed]
- Yousaf MN, Ehsan H, Wahab A, et al. Endoscopic retrograde cholangiopancreatography guided interventions in the management of pancreatic cancer. World J Gastrointest Endosc 2020;12:323-40. [Crossref] [PubMed]
- Lee PJ, Podugu A, Wu D, et al. Preoperative biliary drainage in resectable pancreatic cancer: a systematic review and network meta-analysis. HPB (Oxford) 2018;20:477-86. [Crossref] [PubMed]
- Zorrón Pu L, de Moura EG, Bernardo WM, et al. Endoscopic stenting for inoperable malignant biliary obstruction: A systematic review and meta-analysis. World J Gastroenterol 2015;21:13374-85. [Crossref] [PubMed]
- Walter D, van Boeckel PG, Groenen MJ, et al. Cost Efficacy of Metal Stents for Palliation of Extrahepatic Bile Duct Obstruction in a Randomized Controlled Trial. Gastroenterology 2015;149:130-8. [Crossref] [PubMed]
- Grimm IS, Baron TH. Biliary Stents for Palliation of Obstructive Jaundice: Choosing the Superior Endoscopic Management Strategy. Gastroenterology 2015;149:20-2. [Crossref] [PubMed]
- Tamm EP, Loyer EM, Faria S, et al. Staging of pancreatic cancer with multidetector CT in the setting of preoperative chemoradiation therapy. Abdom Imaging 2006;31:568-74. [Crossref] [PubMed]
- Distler M, Pilarsky E, Kersting S, et al. Preoperative CEA and CA 19-9 are prognostic markers for survival after curative resection for ductal adenocarcinoma of the pancreas - a retrospective tumor marker prognostic study. Int J Surg 2013;11:1067-72. [Crossref] [PubMed]
- Parikh AA, Robinson J, Zaydfudim VM, et al. The effect of health insurance status on the treatment and outcomes of patients with colorectal cancer. J Surg Oncol 2014;110:227-32. [Crossref] [PubMed]
- Groot VP, Mosier S, Javed AA, et al. Circulating Tumor DNA as a Clinical Test in Resected Pancreatic Cancer. Clin Cancer Res 2019;25:4973-84. [Crossref] [PubMed]
- Maire F, Micard S, Hammel P, et al. Differential diagnosis between chronic pancreatitis and pancreatic cancer: value of the detection of KRAS2 mutations in circulating DNA. Br J Cancer 2002;87:551-4. [Crossref] [PubMed]
- Uemura T, Hibi K, Kaneko T, et al. Detection of K-ras mutations in the plasma DNA of pancreatic cancer patients. J Gastroenterol 2004;39:56-60. [Crossref] [PubMed]
- Bettegowda C, Sausen M, Leary RJ, et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci Transl Med 2014;6:224ra24. [Crossref] [PubMed]
- Singh N, Gupta S, Pandey RM, et al. High levels of cell-free circulating nucleic acids in pancreatic cancer are associated with vascular encasement, metastasis and poor survival. Cancer Invest 2015;33:78-85. [Crossref] [PubMed]
- Hadano N, Murakami Y, Uemura K, et al. Prognostic value of circulating tumour DNA in patients undergoing curative resection for pancreatic cancer. Br J Cancer 2016;115:59-65. [Crossref] [PubMed]
- Sausen M, Phallen J, Adleff V, et al. Clinical implications of genomic alterations in the tumour and circulation of pancreatic cancer patients. Nat Commun 2015;6:7686. [Crossref] [PubMed]
- Takai E, Totoki Y, Nakamura H, et al. Clinical utility of circulating tumor DNA for molecular assessment in pancreatic cancer. Sci Rep 2015;5:18425. [Crossref] [PubMed]
- Wang H, Liu J, Xia G, et al. Survival of pancreatic cancer patients is negatively correlated with age at diagnosis: a population-based retrospective study. Sci Rep 2020;10:7048. [Crossref] [PubMed]
- Higuera O, Ghanem I, Nasimi R, et al. Management of pancreatic cancer in the elderly. World J Gastroenterol 2016;22:764-75. [Crossref] [PubMed]
- Versteijne E, van Eijck CH, Punt CJ, et al. Preoperative radiochemotherapy versus immediate surgery for resectable and borderline resectable pancreatic cancer (PREOPANC trial): study protocol for a multicentre randomized controlled trial. Trials 2016;17:127. [Crossref] [PubMed]
- Jang JY, Han Y, Lee H, et al. Oncological Benefits of Neoadjuvant Chemoradiation With Gemcitabine Versus Upfront Surgery in Patients With Borderline Resectable Pancreatic Cancer: A Prospective, Randomized, Open-label, Multicenter Phase 2/3 Trial. Ann Surg 2018;268:215-22. [Crossref] [PubMed]
- Sohal D, Duong MT, Ahmad SA, et al. SWOG S1505: Results of perioperative chemotherapy (peri-op CTx) with mfolfirinox versus gemcitabine/nab-paclitaxel (Gem/nabP) for resectable pancreatic ductal adenocarcinoma (PDA). J Clin Oncol 2020;38:abstr 4504.
- Katz MHG, Shi Q, Meyers J, et al. Efficacy of Preoperative mFOLFIRINOX vs mFOLFIRINOX Plus Hypofractionated Radiotherapy for Borderline Resectable Adenocarcinoma of the Pancreas: The A021501 Phase 2 Randomized Clinical Trial. JAMA Oncol 2022;8:1263-70. [Crossref] [PubMed]
- Khorana AA, Mangu PB, Katz MHG. Potentially Curable Pancreatic Cancer: American Society of Clinical Oncology Clinical Practice Guideline Update Summary. J Oncol Pract 2017;13:388-91. [Crossref] [PubMed]
- Koay EJ, Katz MHG, Wang H, et al. Computed Tomography-Based Biomarker Outcomes in a Prospective Trial of Preoperative FOLFIRINOX and Chemoradiation for Borderline Resectable Pancreatic Cancer. JCO Precis Oncol 2019;3. ePO. [Crossref] [PubMed]
- Murphy JE, Wo JY, Ryan DP, et al. Total Neoadjuvant Therapy With FOLFIRINOX Followed by Individualized Chemoradiotherapy for Borderline Resectable Pancreatic Adenocarcinoma: A Phase 2 Clinical Trial. JAMA Oncol 2018;4:963-9. [Crossref] [PubMed]
- Perri G, Prakash L, Qiao W, et al. Response and Survival Associated With First-line FOLFIRINOX vs Gemcitabine and nab-Paclitaxel Chemotherapy for Localized Pancreatic Ductal Adenocarcinoma. JAMA Surg 2020;155:832-9. [Crossref] [PubMed]
- Liu H, Zenati MS, Rieser CJ, et al. CA19-9 Change During Neoadjuvant Therapy May Guide the Need for Additional Adjuvant Therapy Following Resected Pancreatic Cancer. Ann Surg Oncol 2020;27:3950-60. [Crossref] [PubMed]
- Gemenetzis G, Groot VP, Yu J, et al. Circulating Tumor Cells Dynamics in Pancreatic Adenocarcinoma Correlate With Disease Status: Results of the Prospective CLUSTER Study. Ann Surg 2018;268:408-20. [Crossref] [PubMed]
- Ngo-Huang A, Parker NH, Bruera E, et al. Home-Based Exercise Prehabilitation During Preoperative Treatment for Pancreatic Cancer Is Associated With Improvement in Physical Function and Quality of Life. Integr Cancer Ther 2019;18:1534735419894061. [Crossref] [PubMed]
- Nakajima H, Yokoyama Y, Inoue T, et al. Clinical Benefit of Preoperative Exercise and Nutritional Therapy for Patients Undergoing Hepato-Pancreato-Biliary Surgeries for Malignancy. Ann Surg Oncol 2019;26:264-72. [Crossref] [PubMed]
- Bundred JR, Kamarajah SK, Hammond JS, et al. Prehabilitation prior to surgery for pancreatic cancer: A systematic review. Pancreatology 2020;20:1243-50. [Crossref] [PubMed]
- Altman AM, Wirth K, Marmor S, et al. Completion of Adjuvant Chemotherapy After Upfront Surgical Resection for Pancreatic Cancer Is Uncommon Yet Associated With Improved Survival. Ann Surg Oncol 2019;26:4108-16. [Crossref] [PubMed]
- Aloia TA, Lee JE, Vauthey JN, et al. Delayed recovery after pancreaticoduodenectomy: a major factor impairing the delivery of adjuvant therapy? J Am Coll Surg 2007;204:347-55. [Crossref] [PubMed]
- Patel H, Okamura R, Fanta P, et al. Clinical correlates of blood-derived circulating tumor DNA in pancreatic cancer. J Hematol Oncol 2019;12:130. [Crossref] [PubMed]
- Klinkenbijl JH, Jeekel J, Sahmoud T, et al. Adjuvant radiotherapy and 5-fluorouracil after curative resection of cancer of the pancreas and periampullary region: phase III trial of the EORTC gastrointestinal tract cancer cooperative group. Ann Surg 1999;230:776-82; discussion 782-4. [Crossref] [PubMed]
- Neoptolemos JP, Stocken DD, Friess H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med 2004;350:1200-10. [Crossref] [PubMed]
- Oettle H, Post S, Neuhaus P, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA 2007;297:267-77. [Crossref] [PubMed]
- Neoptolemos JP, Palmer DH, Ghaneh P, et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet 2017;389:1011-24. [Crossref] [PubMed]
- Conroy T, Hammel P, Hebbar M, et al. FOLFIRINOX or Gemcitabine as Adjuvant Therapy for Pancreatic Cancer. N Engl J Med 2018;379:2395-406. [Crossref] [PubMed]
- Tempero MA, Reni M, Riess H. APACT: phase III, multicenter, international, open-label, randomized trial of adjuvant nab-paclitaxel plus gemcitabine vs gemcitabine for surgically resected pancreatic adenocarcinoma. J Clin Oncol 2019;37:abstr 4000.
- Versteijne E, van Dam JL, Suker M, et al. Neoadjuvant Chemoradiotherapy Versus Upfront Surgery for Resectable and Borderline Resectable Pancreatic Cancer: Long-Term Results of the Dutch Randomized PREOPANC Trial. J Clin Oncol 2022;40:1220-30. [Crossref] [PubMed]
- Perri G, Prakash L, Wang H, et al. Radiographic and Serologic Predictors of Pathologic Major Response to Preoperative Therapy for Pancreatic Cancer. Ann Surg 2021;273:806-13. [Crossref] [PubMed]


