
GEMSTONE-302: “If you keep doing the same thing you get the same result”
Lung cancer is the leading cause of cancer-related mortality globally (1,2). Over the last decade, the treatment of advanced non-small cell lung cancer (NSCLC) has changed dramatically with the emergence of immune checkpoint inhibitors (ICIs). A growing number of phase III clinical trials have demonstrated that the first-line combination of ICIs with standard chemotherapy improves survival outcomes in patients with metastatic NSCLC, regardless of programmed death-ligand 1 (PD-L1) expression (3-7).
Sugemalimab is a fully human, full-length anti-PD-L1 immunoglobulin G4 (IgG4) monoclonal antibody that is developed for the treatment of various advanced solid tumors and lymphoma (8). The GEMSTONE-302 trial did what has been done in numerous trials over the past 5 years. It was a randomized, double-blind phase III clinical trial, demonstrating that the addition of sugemalimab to chemotherapy significantly improved survival outcomes among patients with treatment-naïve metastatic NSCLC without actionable genomic tumor alterations including epidermal growth factor receptor (EGFR) mutations and anaplastic lymphoma kinase (ALK) rearrangements. Based on the encouraging results of GEMSTONE-302, sugemalimab plus chemotherapy has been approved for the first-line treatment of metastatic squamous and non-squamous NSCLC without EGFR and ALK mutations in China (9). Did we really expect a different result? Herein, we will compare the efficacy and safety data of GEMSTONE-302 to other clinical trials that led to several ICIs being approved for the first-line treatment of metastatic NSCLC in the US (Table 1) (3-7).
Table 1
| Study | GEMSTONE-302 | KEYNOTE-189 | KEYNOTE-407 | IMpower130 | IMpower150 | CheckMate 9LA |
|---|---|---|---|---|---|---|
| Treatment regimen | Suge-CP/pemCT vs. CP/pemCT | Pembro-pemCT vs. pemCT | Pembro-C(n)P vs. C(n)P | Atez-CnP vs. CnP | Atez-Bev-CP vs. Bev-CP | Niv-Ipi-CP/pemCT vs. CP/pemCT |
| Sample size, ITT (experimental vs. control) | 320 vs. 159 | 410 vs. 206 | 278 vs. 281 | 451 vs. 228† | 356 vs. 336† | 361 vs. 358 |
| Histology | Non-squamous 60%; squamous 40% | Non-squamous | Squamous | Non-squamous | Non-squamous | Non-squamous 69%; squamous 31% |
| Biomarkers | PD-L1 unselected; EGFR−/ALK− | PD-L1 unselected; EGFR−/ALK− | PD-L1 unselected; EGFR−/ALK− | PD-L1 unselected; including EGFR+/ALK+ | PD-L1 unselected; including EGFR+/ALK+ | PD-L1 unselected; EGFR−/ALK− |
| On-study crossover permitted | Yes | Yes | Yes | Yes‡ | No | No |
| Crossover rate in the control arm | ||||||
| On-study ICI, % | 28.3 | 40.8 | 40.6 | 40.8 | NA | NA |
| Any ICI, % | 43.4 | 55.8 | 50.5 | 59.2 | ||
| PD-L1 status, % | ||||||
| TPS <1% | 39.2 | 30.8 | 34.7 | 52.4 | 48.8 | 39.3 |
| TPS ≥1% | 60.8 | 69.2 | 65.3 | 47.6 | 51.2 | 60.7 |
| Ethnicity, % | ||||||
| Asian | 100 | 1.6 | 19.0 | 2.2 | 9.5 | 8.0 |
| Non-Asian | 0 | 98.4 | 81.0 | 97.8 | 90.5 | 92.0 |
| Male, % | 80.0 | 58.9 | 81.4 | 59.0 | 61.4 | 70.0 |
| Median age, year (range) | 62 [56–67] | 65 [34–84] | 65 [29–87] | 64 [18–86] | 63 [31–89] | 65 [59–70] |
| Smoking status, % | 26.7 | 11.9 | 7.3 | 9.6 | 15.6 | 13.6 |
| Never smoker | 73.3 | 88.1 | 92.7 | 90.4 | 84.4 | 86.4 |
| Current or former smoker | ||||||
| ECOG, % | 17.5 | 43.0 | 29.2 | 41.3 | 40.6 | 31.0 |
| 0 | 82.5 | 56.2 | 70.8 | 58.5 | 59.4 | 68.0 |
| 1 | ||||||
| Median PFS, month, all HR (95% CI) | 9.0 vs. 4.9, 0.48 (0.39–0.60) | 8.8 vs. 4.9, 0.52 (0.43–0.64) | 6.4 vs. 4.8, 0.56 (0.45–0.70) | 7.0 vs. 5.5, 0.64 (0.54–0.77) | 8.3 vs. 6.8, 0.62 (0.52–0.74) | 6.8 vs. 5.0, 0.70 (0.57–0.86) |
| Median PFS, month, subgroups HR (95% CI) | ||||||
| PD-L1 negative | 7.4 vs. 4.9, 0.56 (0.40–0.77) | 6.2 vs. 5.1, 0.67 (0.49–0.93) | 6.3 vs. 5.9, 0.67 (0.49–0.91) | 6.2 vs. 4.7, 0.72 (0.56–0.91) | 7.1 vs. 6.9, 0.77 (0.61–0.99) | 5.8 vs. 4.9, 0.68 (0.51–0.89) |
| PD-L1 positive | 10.9 vs. 4.9, 0.46 (0.35–0.62) | 10.9 vs. 4.9, 0.42 (0.33–0.53) | 8.2 vs. 4.6, 0.50 (0.39–0.63) | 0.61 (0.43–0.85)§, 0.51 (0.34–0.77)¶ | 11.0 vs. 6.8, 0.50 (0.39–0.64) | 7.0 vs. 5.0, 0.67 (0.53–0.84) |
| Squamous | 8.3 vs. 4.8, 0.34 (0.24–0.48) | NA | NA | NA | NA | 5.6 vs. 4.3, 0.60 (0.44–0.81) |
| Non-squamous | 9.6 vs. 5.8, 0.59 (0.45–0.79) | NA | NA | NA | NA | 7.0 vs. 6.0, 0.72 (0.59–0.88) |
| Median OS, month, all HR (95% CI) | NYR vs. 17.7, 0.67 (0.50–0.90); updated analysis: 25.4 vs. 16.9, 0.65 (0.50–0.84) | NYR vs. 11.3, 0.49 (0.38–0.64); updated analysis: 22.0 vs. 10.6, 0.56 (0.46–0.69) | 15.9 vs. 11.3, 0.64 (0.49–0.85); updated analysis: 17.1 vs. 11.6, 0.71 (0.58–0.88) | 18.6 vs. 13.9, 0.79 (0.64–0.98) | 19.2 vs. 14.7, 0.78 (0.64–0.96); updated analysis: 19.5 vs. 14.7, 0.80 (0.67–0.95) | 15.6 vs. 10.9, 0.66 (0.55–0.80); updated analysis: 15.8 vs. 11.0, 0.72 (0.61–0.86) |
| Median OS, month, subgroups HR (95% CI) | ||||||
| PD-L1 negative | 19.4 vs. 14.8, 0.66 (0.45–0.97) | 17.2 vs. 10.2, 0.51 (0.36–0.71) | 15.0 vs. 11.0, 0.79 (0.56–1.11) | 15.2 vs. 12.0, 0.81 (0.61–1.08) | 16.9 vs. 14.1, 0.9 (0.71–1.14) | 17.7 vs. 9.8, 0.67 (0.51–0.88) |
| PD-L1 positive | 27.0 vs. 19.0, 0.64 (0.46–0.91) | 23.0 vs. 11.3, 0.63 (0.48–0.81) | 18.9 vs. 12.8, 0.67 (0.51–0.87) | 0.70 (0.45–1.08)§, 0.84 (0.51–1.39)¶ | 22.5 vs. 16.0, 0.73 (0.57–0.94) | 15.8 vs. 10.9, 0.70 (0.56–0.89) |
| Squamous | 23.3 vs. 12.2, 0.56 (0.38–0.82) | NA | NA | NA | NA | 14.5 vs. 9.1, 0.63 (0.47–0.85) |
| Non-squamous | 26.9 vs. 19.8, 0.72 (0.51–1.01) | NA | NA | NA | NA | 17.8 vs. 12.0, 0.78 (0.63–0.96) |
| Objective response rate, % | 63.4 vs. 40.3 | 48.3 vs. 19.9 | 62.6 vs. 38.4 | 49.2 vs. 31.9 | 63.5 vs. 48.0 | 38.0 vs. 25.4 |
| Median DoR, month | 9.9 vs. 4.4 | 12.5 vs. 7.1 | 8.8 vs. 4.9 | 8.4 vs. 6.1 | 9.0 vs. 5.7 | 13.0 vs. 5.6 |
| Treatment-related grade 3–4 AEs, % | 53.0 vs. 56.0 | 50.3 vs. 40.6 | 52.2 vs. 53.9 | 73 vs. 60|| | 55.7 vs. 47.7|| | 47.0 vs. 38.0 |
| Treatment-related serious AEs, % | 23.0 vs. 20.0 | NA | NA | 23.7 vs. 12.9|| | 25.4 vs. 19.3|| | 30.0 vs. 18.0 |
| Treatment-related death, % | 3.0 vs. 1.0 | 2.0 vs. 1.0 | 4.3 vs. 1.8 | 2.0 vs. <1.0|| | 2.8 vs. 2.3|| | 2.0 vs. 2.0 |
†, wild type, excluding patients with EGFR or ALK genomic alterations; ‡, crossover to receive atezolizumab at disease progression was permitted only for patients enrolled before June 15, 2016; §, PD-L1 expression in ≥1% and <50% of tumor cells or ≥1% and <10% of tumor-infiltrating immune cells; ¶, PD-L1 expression in ≥50% of tumor cells or ≥10% of tumor-infiltrating immune cells; ||, including patients with EGFR or ALK genomic alterations. NSCLC, non-small cell lung cancer; US, United States; ITT, intention to treat; ICI, immune checkpoint inhibitor; PD-L1, programmed death-ligand 1; TPS, tumor proportion score; ECOG, Eastern Cooperative Oncology Group; PFS, progression-free survival; HR, hazard ratio; CI, confidence interval; OS, overall survival; DoR, duration of response; AE, adverse event; Suge, sugemalimab; CP, carboplatin and paclitaxel; pemCT, pemetrexed and platinum chemotherapy; Pembro, pembrolizumab; C(n)P, carboplatin and (nab) paclitaxel; Atez, atezolizumab; CnP, carboplatin and (nab) paclitaxel; Bev, bevacizumab; Nivo, nivolumab; Ipi, ipilimumab; EGFR, epidermal growth factor receptor; ALK, anaplastic lymphoma kinase; NYR, not yet reached.
The GEMSTONE-302 trial included patients from 35 Chinese centers, with squamous histology consisting of 40% of the study population in both the sugemalimab and placebo arms. The primary endpoint was progression-free survival (PFS), and the secondary endpoints included overall survival (OS) and objective response rate (ORR) (10). Four hundred and seventy-nine patients with histologically confirmed metastatic squamous and non-squamous NSCLC, regardless of PD-L1 expression level, were randomly assigned 2:1 to the sugemalimab arm (n=320) and the placebo arm (n=159). Patients in the sugemalimab arm received sugemalimab plus platinum-based chemotherapy (carboplatin and paclitaxel for squamous, or carboplatin and pemetrexed for non-squamous NSCLC) for four cycles, followed by either sugemalimab (squamous) or sugemalimab plus pemetrexed (non-squamous) as the maintenance therapy. Patients in the placebo arm received placebo plus the same platinum-based chemotherapy regimens for four cycles, followed by maintenance therapy with either placebo alone (squamous) or placebo plus pemetrexed (non-squamous). Among the patients in the placebo arm, crossover to the sugemalimab arm at the time of progression was permitted.
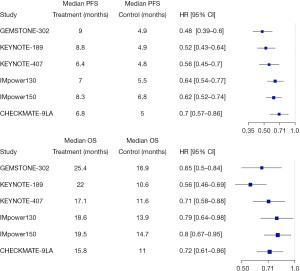
At the primary analysis (data cutoff: March 15, 2021) with a median follow-up of 17.8 months, the results of GEMSTONE-302 showed that sugemalimab plus platinum-based chemotherapy significantly improved PFS (9.0 months in the sugemalimab arm vs. 4.9 months in the placebo arm, HR 0.48 with 95% CI: 0.39–0.60) irrespective of PD-L1 expression levels and histologies (10). The OS data was not mature enough for formal analysis when the results were first published in The Lancet Oncology. Still, the preliminary analysis showed the addition of sugemalimab to chemotherapy reduced the risk of death by 33% compared with the placebo arm (median OS not yet reached vs. 17.7 months, HR 0.67 with 95% CI: 0.50–0.90) (10). With a median follow-up of 25.4 months, the updated OS result (data cutoff: November 22, 2021) showed the OS benefit remained consistently in favor of the sugemalimab arm (25.4 vs. 16.9 months, HR 0.65 with 95% CI: 0.50–0.84), despite a high crossover rate from placebo to either sugemalimab (28.3%) or other non-study programmed cell death protein 1 (PD-1)/PD-L1 containing therapies (18.2%) (11). This OS benefit was observed across all subgroups, including both squamous and non-squamous histologies and all PD-L1 categories. Compared with a 28% death risk reduction in the non-squamous subgroup (26.9 vs. 19.8 months, HR 0.72 with 95% CI: 0.51–1.01), the improvement of OS was more significant in the squamous subgroup, with the risk of death reduced by 44% in the sugemalimab arm relative to the placebo arm (23.2 vs. 12.2 months, HR 0.56 with 95% CI: 0.38–0.82) (11). In addition, a better ORR was noted in the sugemalimab arm (63.4% vs. 40.3%) (3). The results of the safety analysis demonstrated comparable incidences of treatment-related grade 3–4 adverse events (AEs) (53% vs. 56%) and fatal AEs (3% vs. 1%) between the two arms (10).
So, how do these results compare to what we have already established as the standard of care in the United States (US)? Pembrolizumab is an anti-PD-1 monoclonal antibody that has been studied in both metastatic squamous and non-squamous NSCLC. In KEYNOTE-189, the addition of pembrolizumab to platinum/pemetrexed was associated with improved PFS (8.8 vs. 4.9 months, HR 0.52 with 95% CI: 0.43–0.64), OS (22.0 vs. 10.6 months, HR 0.56 with 95% CI: 0.46–0.69) and ORR (48.3% vs. 19.9%) when compared to chemotherapy alone in patients with metastatic non-squamous NSCLC (4,12). Comparable results were also observed in KEYNOTE-407, in which pembrolizumab plus chemotherapy showed significant improvement in PFS (6.4 vs. 4.8 months, HR 0.56 with 95% CI: 0.45–0.70) and OS (17.1 vs. 11.6 months, HR 0.71 with 95% CI: 0.58–0.88) and ORR (62.6% vs. 38.4%) in metastatic squamous NSCLC patients (3,13). In both KEYNOTE-189 and KEYNOTE-407, the survival benefits of pembrolizumab plus chemotherapy were observed across all PD-L1 categories. Similar to GEMSTONE-302, a high crossover rate occurred in both KEYNOTE-189 (55.8%) and KEYNOTE-407 (50.5%), and the survival benefit remained significant (12,13).
IMpower130 is a phase III clinical trial evaluating the anti-PD-L1 antibody atezolizumab in combination with chemotherapy for the first-line treatment of metastatic non-squamous NSCLC. Improvements in PFS (7.0 vs. 5.5 months, HR 0.64 with 95% CI: 0.54–0.77), OS (18.6 vs. 13.9 months, HR 0.79 with 95% CI: 0.64–0.98) and ORR (49.2% vs. 31.9%) were observed in the atezolizumab arm (5). In IMpower 150, atezolizumab in combination with bevacizumab plus carboplatin and paclitaxel as the first-line therapy for metastatic non-squamous NSCLC was compared with bevacizumab plus the same chemotherapy regimen. In the intention-to-treat (ITT) wild type population, compared to bevacizumab and chemotherapy, the addition of atezolizumab to bevacizumab and chemotherapy improved PFS (8.3 vs. 6.8 months, HR 0.62 with 95% CI: 0.52–0.74), OS (19.5 vs. 14.7 months, HR 0.80 with 95% CI: 0.67–0.95) and ORR (63.5% vs. 48.0%) (6,14). In the final OS analysis of IMpower 150, OS benefit was noted in PD-L1 high group (30.0 vs. 15.0 months, HR 0.70 with 95% CI: 0.46–1.08) and PD-L1 positive group (22.5 vs. 16.0 months, HR 0.73 with 95% CI: 0.57–0.94), while limited benefit was found in the PD-L1 negative group (16.9 vs. 14.1 months, HR 0.90 with 95% CI: 0.71–1.14) (14).
CheckMate 9LA evaluated the use of dual ICIs nivolumab and ipilimumab in combination with chemotherapy for two cycles in the first-line treatment of metastatic NSCLC with any histology. Again, improved PFS (6.8 vs. 5.0 months, HR 0.70 with 95% CI: 0.57–0.86), OS (15.8 vs.11.0 months, HR 0.72 with 95% CI: 0.61–0.86) and ORR (38.0% vs. 25.4%) were observed in the experimental arm versus the control arm, regardless of PD-L1 expression (7,15). Similar to GEMSTONE-302, in subgroup analysis, the survival benefit of combining nivolumab plus ipilimumab to chemotherapy was more noticeable in the squamous subgroup, with a 37% death risk reduction compared to the control arm (14.5 vs. 9.1 months, HR 0.63 with 95% CI: 0.47–0.85), while the risk of death was reduced by 22% in the non-squamous subgroup (17.8 vs. 12.0 months, HR 0.78 with 95% CI: 0.63–0.96).
In a perfect world, multi-regional clinical trials (MRCT) remain the optimal approach. How should we interpret and apply a trial like GEMSTONE-302 done exclusively in China to populations elsewhere? Data from clinical trials should not be overlooked solely due to the geographic location, however, potential intrinsic and extrinsic factors are important factors to consider (16). In the GEMSTONE-302 trial, there was a higher proportion of nonsmokers compared to other similar trials (26.7% vs. 7.3–15.6%), which reflected the higher prevalence of NSCLC in nonsmokers compared to former or current smokers in the Asian population (17). It has been reported that EGFR mutations occur more frequently in the East Asian population (30–60%) than in the western population (7–10%) (17-20), but patients with EGFR mutations were excluded from the GEMSTONE 302 study. Aside from ethnicity, ECOG and smoking status, the study population of GEMSTONE-302 was generally consistent with chemoimmunotherapy studies in first-line treatment of metastatic NSCLC conducted in the US (Table 1). The GEMSTONE-302 applied similar pathology guidelines, staging system, inclusion/exclusion criteria, adverse events grading system and response evaluation criteria compared to other similar trials conducted in the US. It should be noted that the placebo arm in GEMSTONE-302 was platinum-based chemotherapy without concurrent ICIs, which is not the current standard of care for metastatic NSCLC in the US. However, platinum-based chemotherapy was the standard of care for the first-line treatment of metastatic NSCLC in China when GEMSTONE-302 was first initiated in 2018. Overall, the efficacy results of GEMSTONE-302 are in line with those reported in other phase III clinical trials evaluating chemoimmunotherapy compared to platinum-based chemotherapy alone as the first-line treatment of metastatic NSCLC (Table 1 and Figure 1). Several subgroup analyses have shown durable long-term survival benefits and comparable safety profile of first-line combined immunotherapy-chemotherapy in the Asia subpopulation with metastatic NSCLC without any driver mutations, consistent with survival benefits observed in the global studies (21-24). The safety profile of sugemalimab with combined chemotherapy is also comparable to other global studies evaluating PD-1/PD-L1 inhibitors in combination with chemotherapy. In summary, the GEMSTONE-302 study provides convincing results in the Chinese population that, we believe, are applicable to the Western population.
In oncology, the discussion of unmet medical needs is usually centered on the lack of available effective treatment options. We believe the immense cost is another unmet need as it can be a barrier preventing patients from accessing cancer treatments that clearly offer significant clinical benefit. The inability to secure life-prolonging cancer treatment due to financial toxicities creates disparities in the delivery of cancer care. By introducing more treatment options that are both effective and safe, we believe there would be more pressure on pricing leading to a more affordable price. This could potentially bridge the gap and fulfill the unmet needs of many cancer patients making cancer care more accessible to patients with metastatic NSCLC (25).
ICIs have revolutionized NSCLC management in China and globally (Table 1). The combination of ICIs and chemotherapy has become the first-line therapy for metastatic NSCLC since 2018 in the US, with multiple immunotherapy options available. Sugemalimab significantly improves the PFS and OS in metastatic NSCLC patients irrespective of tumor histology and PD-L1 expression levels. GEMSTONE-302 shows results similar to what has been demonstrated by the currently approved agents in the US supporting the addition of sugemalimab to chemotherapy as a novel first-line treatment option for both metastatic squamous and non-squamous NSCLC. We believe sugemalimab combined with chemotherapy could further broaden treatment options for patients with metastatic NSCLC, not solely due to its similar efficacy and safety profile compared to currently available first-line treatments in the US, but also owing to its proposed relatively affordable cost, which could fit the unmet need and ease the financial hardship among many cancer patients (25). These issues should outweigh the lack of a multi-regional clinical trial, given the consistent results of GEMSTONE-302 with other currently approved agents, which could be done as a post-marketing commitment.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Chinese Clinical Oncology. The article did not undergo external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cco.amegroups.com/article/view/10.21037/cco-22-103/coif). The authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Schabath MB, Cote ML. Cancer Progress and Priorities: Lung Cancer. Cancer Epidemiol Biomarkers Prev 2019;28:1563-79. [Crossref] [PubMed]
- Thandra KC, Barsouk A, Saginala K, et al. Epidemiology of lung cancer. Contemp Oncol (Pozn) 2021;25:45-52. [Crossref] [PubMed]
- Paz-Ares L, Luft A, Vicente D, et al. Pembrolizumab plus Chemotherapy for Squamous Non-Small-Cell Lung Cancer. N Engl J Med 2018;379:2040-51. [Crossref] [PubMed]
- Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:2078-92. [Crossref] [PubMed]
- West H, McCleod M, Hussein M, et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 2019;20:924-37. [Crossref] [PubMed]
- Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N Engl J Med 2018;378:2288-301. [Crossref] [PubMed]
- Paz-Ares L, Ciuleanu TE, Cobo M, et al. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): an international, randomised, open-label, phase 3 trial. Lancet Oncol 2021;22:198-211. [Crossref] [PubMed]
- Dhillon S, Duggan S. Sugemalimab: First Approval. Drugs 2022;82:593-9. [Crossref] [PubMed]
- Ligand Pharmaceuticals. Ligand’s partner CStone Pharmaceuticals receives approval in China for sugemalimab (Cejemly®) for the first-line treatment of advanced non-small cell lung cancer in combination with chemotherapy [media release]. 2021. Available online: https://investor.ligand.com/press-releases/detail/457/ligands-partner-cstone-pharmaceuticals-receives-approval
- Zhou C, Wang Z, Sun Y, et al. Sugemalimab versus placebo, in combination with platinum-based chemotherapy, as first-line treatment of metastatic non-small-cell lung cancer (GEMSTONE-302): interim and final analyses of a double-blind, randomised, phase 3 clinical trial. Lancet Oncol 2022;23:220-33. [Crossref] [PubMed]
- Zhou C, Wang Z, Sun M, et al. A protocol pre-specified interim overall survival (OS) analysis of GEMSTONE-302: A phase 3 study of sugemalimab (suge) versus placebo plus platinum-based chemotherapy (chemo) as first-line (1L) treatment for patients (pts) with metastatic non–small cell lung cancer (NSCLC). J Clin Oncol 2022;40: [Crossref]
- Rodríguez-Abreu D, Powell SF, Hochmair MJ, et al. Pemetrexed plus platinum with or without pembrolizumab in patients with previously untreated metastatic nonsquamous NSCLC: protocol-specified final analysis from KEYNOTE-189. Ann Oncol 2021;32:881-95. [Crossref] [PubMed]
- Paz-Ares L, Vicente D, Tafreshi A, et al. A Randomized, Placebo-Controlled Trial of Pembrolizumab Plus Chemotherapy in Patients With Metastatic Squamous NSCLC: Protocol-Specified Final Analysis of KEYNOTE-407. J Thorac Oncol 2020;15:1657-69. [Crossref] [PubMed]
- Socinski MA, Nishio M, Jotte RM, et al. IMpower150 Final Overall Survival Analyses for Atezolizumab Plus Bevacizumab and Chemotherapy in First-Line Metastatic Nonsquamous NSCLC. J Thorac Oncol 2021;16:1909-24. [Crossref] [PubMed]
- Reck M, Ciuleanu TE, Cobo M, et al. First-line nivolumab plus ipilimumab with two cycles of chemotherapy versus chemotherapy alone (four cycles) in advanced non-small-cell lung cancer: CheckMate 9LA 2-year update. ESMO Open 2021;6:100273. [Crossref] [PubMed]
- Liu SV, Nagasaka M, Stefaniak V, et al. The Applicability of the Results in the Asian Population of ORIENT-11 to a Western Population According to the ICH-E5 Framework. Front Oncol 2022;12:859892. [Crossref] [PubMed]
- Zhou W, Christiani DC. East meets West: ethnic differences in epidemiology and clinical behaviors of lung cancer between East Asians and Caucasians. Chin J Cancer 2011;30:287-92. [Crossref] [PubMed]
- Shi Y, Au JS, Thongprasert S, et al. A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER). J Thorac Oncol 2014;9:154-62. [Crossref] [PubMed]
- Shigematsu H, Lin L, Takahashi T, et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst 2005;97:339-46. [Crossref] [PubMed]
- Marchetti A, Martella C, Felicioni L, et al. EGFR mutations in non-small-cell lung cancer: analysis of a large series of cases and development of a rapid and sensitive method for diagnostic screening with potential implications on pharmacologic treatment. J Clin Oncol 2005;23:857-65. [Crossref] [PubMed]
- John T, Sakai H, Ikeda S, et al. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in advanced non-small cell lung cancer: a subanalysis of Asian patients in CheckMate 9LA. Int J Clin Oncol 2022;27:695-706. [Crossref] [PubMed]
- Horinouchi H, Nogami N, Saka H, et al. Pembrolizumab plus pemetrexed-platinum for metastatic nonsquamous non-small-cell lung cancer: KEYNOTE-189 Japan Study. Cancer Sci 2021;112:3255-65. [Crossref] [PubMed]
- Kato T, Lee S, Cheng Y, et al. Carboplatin-paclitaxel/nab-paclitaxel with or without pembrolizumab in first-line metastatic squamous NSCLC: Results from the KEYNOTE-407 east Asia subgroup. Ann Oncol 2018;29:ix151. [Crossref]
- Cheng Y, Zhang L, Hu J, et al. Keynote-407 China Extension study: Pembrolizumab (pembro) plus chemotherapy in Chinese patients with metastatic squamous NSCLC. Ann Oncol 2019;30:ix201-2. [Crossref]
- EQRx. Innovative therapies at radically lower prices-beginning in non-small cell lung cancer. 2021. Available online: https://www.eqrx.com/perspective/innovative-therapies-at-radically-lower-prices-beginning-in-non-small-cell-lung-cancer/


