The way to precision medicine of our team
Introduction
Breast cancer (BC) is the most common cancer in women around the world, which is becoming an increasingly urgent health problem in China. However, comparing with the other cancers, BC has a better outcome attributing to the development of diagnosis and multiple treatments. During the past 20 years, our center has been consistently strengthening our efforts on the way to individual medicine and striving for more clinical benefits (CBs) for our patients. With all the achievements these years, we are very pleased to see that our center keeps an advanced level in China and even in the world, either individual treatment and the concept of full course management we brought up or the more sensitive and less invasive liquid biopsies we researched on. In this paper, we retrospect our way to precision medicine in chronological order, hoping to draw on the experience and promote the future development.
From entirety to classification: the more individual treatment
One-size-fits-all approach is the first treatment method for BC, but the disappointing outcome hints that the individual characteristic influences the efficacy. Fortunately, the finding of estrogen receptor (ER) has brought us to classification medicine. Now along with the development of genomics and biomedicine, the individual treatment is coming.
Chemotherapy: benefits all patients? And the more the better?
Chemotherapy was established for early BC in the 1980s and its efficacy continues to improve; however, side effects remain a concern, particularly since chemotherapy does not benefit most patients. In the past 20 years, the chemotherapy has developed from the entirety treatment to classified treatment (1), which has avoided some patients from excessive chemotherapy, and made the treatment more precise.
NSABP B-15 compared the efficacy and safety between CMF and AC chemotherapy, and established the cornerstone status of anthracycline, for there’s no difference between the two groups in disease-free survival (DFS), DDFS and overall survival (OS). Paclitaxel was first used in BC patients in 1994. Our center evaluated the efficacy and the side effects of paclitaxel in Chinese BC patients (2), showing that paclitaxel is effective in the treatment of BC (63.6%) and the side effects are tolerable. Since then, anthracycline and paclitaxel has been the first two essential chemotherapy agents in BC treatment. Even though there are several chemotherapy regimens appears for adjuvant chemotherapy, we think the AC, TC, AC-T regimens are enough considering the efficacy and side effects up to several large-scale clinical trials like B-15, US9735, E1199 (Table 1).
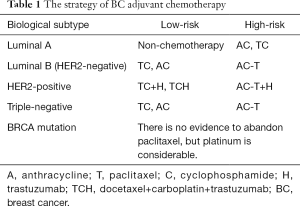
Full table
Even though anthracycline and taxanes have a considerable effective rate, the resistance still exists. Platinum is one of the applicable second line regimens, particularly for HER2-negative MBC, but to combine which agent is unclear. We compared the efficacy and safety of the regimens: gemcitabine plus cisplatin and vinorelbine plus cisplatin (3). Totally 186 HER2 negative MBC patients pretreated with anthracycline and taxanes were enrolled, and the result shows there is no statistical significance difference between the two groups. So for these patients, they can benefit from cisplatin-based regimens with acceptable toxicity, no matter combining with gemcitabine or vinorelbine.
In recent years, oncologists were placing much greater emphasis on patient quality of life, especially on the agents and cycles of chemotherapy. In most MBC cases, combination chemotherapy may be recommended for patients with good performance status and rapidly progressing disease in order to obtain control of the disease, although the optimal duration of treatment remains to be determined. In addition, although combination chemotherapy has demonstrated greater efficacy than monotherapy, it carries a higher toxicity burden. Oncologists face the challenge of selecting the optimal therapeutic regimen for these patients, maintenance therapy may be a good choice. We performed an analysis of the efficacy of capecitabine monotherapy as maintenance treatment for MBC after response to capecitabine-based chemotherapy [capecitabine plus docetaxel (XT) or vinorelbine (XN)] as a first-line or a second-line treatment (4). Sixty-four Chinese patients with histologically confirmed MBC received capecitabine maintenance therapy after disease stabilization or maximal response to capecitabine based combination chemotherapy. This work is the first study of the use of single-agent maintenance chemotherapy in Chinese patients with MBC. Our results indicate that capecitabine monotherapy is an effective maintenance treatment in MBC with a favorable safety profile. Capecitabine may offer the oncologist an alternative treatment approach when effective combination chemotherapy is discontinued before disease progression.
In conclusion, chemotherapy has been one of the most effective systemic therapies for decades of years. At first it was used for all patients who can tolerate it, but now we can choose the suitable population and the individual chemotherapy depend on the pathology and immunohistochemical information. In another word, the chemotherapy has become more precise, and become less essential especially for patients with low risk.
Endocrine therapy: beyond the standard, what can we do?
Undoubtedly, there will always be standard treatment in each era and eventually will be broken by new evidence. When retrospect to the endocrine therapy in BC in the last 30 years in China, it is no exaggeration to say that you may find the footprint of our team as the leader in exploring a better way of endocrine therapy. That is what a precursor will do beyond the standard.
It is now a common sense that the individual treatment depends on the BC molecular classification. Hormone receptor (HR) positive BC occupies 70% of the general patients. Endocrine therapy targeting the HR or reduce the level of estrogen could be the earliest ancestor of precision medicine.
As early as in 1983, our center has paid great attention to the aspect of different pathological types in BC, especially the ER as a very essential prognosis factor among BC molecular markers. The published paper by our team focused on ER-positive BC clinical features, pathological characteristics and its prognosis significance (5), which laid a solid foundation for the subsequent endocrine treatment and research.
On the other hand, our center insisted on the standard endocrine therapy, meanwhile, with the ambitions to break the standard with the research results by our team as well as the updated information in the worldwide. Speaking of the standard treatment for early BC, we have been making efforts on not only the standard drug but also the standard duration of medication. Fifteen years ago, we published a paper of the prospective clinical research on the adjuvant endocrine therapy for HR positive BC. It proved that the endocrine therapy is equivalent or even better than chemotherapy for some of the HR positive patient, which consolidated the concept of classification treatment (6). Although with the well-known results of aTTom and ATLAS, the duration of adjuvant endocrine therapy is prolonged to 10 years in some patients, the fact of poor persistence needs more attention. We attended the publication of the paper on this problem and found that patients’ persistence to adjuvant aromatase inhibitor (AI) medication for postmenopausal women with early stage BC is relatively high in the first year (over 95%) and is not significantly increased by adding a patient support program to standard treatment (7). The attention on the adherence and persistence of medication should be continuously paid in the future.
Since the birth and the utilization of the AI in BC in postmenopausal women, with the result of the several multicenter prospective clinical trials including ATAC, BIG1-98 and TEAM, AI has played a more and more important role in endocrine therapy and has replaced of Tamoxifen as the standard treatment in both the early and metastasis BC in postmenopausal. Several clinical researches have helped us to find more appropriate treatment choices in the post AI era. We firstly reported the high dose medroxyprogesterone (HD-MPA) in China in 1995. Sixty-two cases of metastasis BC receiving oral HD-MPA 500 mg, 2–3 times per day, 30 days as one cycle were observed in our study. Objective effect was CR 1.6% (1/62), PR 53.2% (3/62), SD 17.7% (11/62), PD 27.4% (17/62) and patients with bone metastasis, lung metastasis, soft tissue metastasis of the chest wall have a relatively better outcome (8). Then we carried out and attended several clinical trials of fulvestrant, a new ER regulator in the past few years, aiming to evaluate the efficacy of fulvestrant for metastatic BC following progression on AI. 56 metastatic BC patients who were resistant to AI and underwent fulvestrant therapy were analyzed. Ten patients (17.9%) experienced CB, overall response rate was 3.6%, median progression-free survival (PFS) was 3.0 months (range, 1–17 months). Cox regression showed that the PFS of fulvestrant had associated with DFS and treatment lines (P=0.017; P=0.021). This data proved that fulvestrant was the important choice of treatment for the patients resistant to AI in metastasis BC (9). And the recent prospective multicenter trial China CONFIRM lead by our center showed a better result in terms of HR than the global study in the post-AI subgroup (HR 0.65 vs. HR 0.85), proving a promising utilization of fulvestrant in post AI patients (10).
Plenty of exploration has also been made in the endocrine therapy in premenopausal BC. Date back to 13 years ago, our team firstly reported combined Goserelin (GnRHa) with anastrozole (AI) in premenopausal women with MBC (11) and then a larger amount of patients were observed in 2004 and 2012 respectively, with a more convincing result of over 59% CB rate in the combination endocrine therapy (12,13). As mentioned before, with a much more utilization of AI with ovarian function suppression (OFS) in premenopausal either in adjuvant therapy or advanced therapy, a tough question is bringing up that what the choice will be after AI with OFS in premenopausal women. In 2013, we reported the exploring research on combining goserelin and fulvestrant in premenopausal women with metastasis BC, all of the patients having been treated with AI (14). With the result of 53% patients achieving SD, it proposed a promising option for this kind of patients and an ongoing prospective multicenter clinical trial named PROOF lead by our team may help provide new data and solve this issue.
From new data generated by ourselves to comments on the new therapy strategy thinking on endocrine therapy for BC, our team has published several papers in the past decades (15,16). But in the last 2 years, some critical results of clinical studies and updated guidelines solved some questions, at the same time, triggering a more heat discussion. The paper recently published by us considered and discussed on ten hot issues of endocrine therapy for BC (17), hoping to figure out some complex question.
In conclusion, endocrine therapy has effectively helped us to reduce the recurrence of early BC and provided a more important role in metastasis BC. The therapy choice with the concept of precision medicine thinking may point out a better solution to the urgent questions, including finding the appropriate medication order and combination of drugs, solving the resistance to endocrine therapy by adding targeting drugs which may inhibit particular pathway (PAM pathway etc.) or adding some epigenetic drugs. Some related clinical trials are undergoing in our center and will make current endocrine therapy a more accurate way.
Targeted therapy: derives from HER2, towards to multiple targets
Since the HER2 gene was first found by Slamon in 1971, treatment of breast cancer has entered the era of targeted treatment. HER2 has no known ligand and complex interactions between different HER family members, involving dimerization, and is overexpressed on the cell membrane in about 20% of early BCs. Overexpression of HER2 favors the production of activated homo- and heterodimers, and is associated with poorer disease prognosis. Our center investigated the expression of HER2 oncoprotein in primary BC in China and analyzed its relation to the prognosis. Immunohistochemical staining for HER2 was performed on paraffin-embedded specimens of primary BC from 284 patients (18). The total positive expression rate of HER2 was 26.8% (76/284). Expression of HER2 was positively correlated with the status of lymph node metastasis (P=0.003). Univariate analysis indicated that HER2 expression is a significant prognostic factor for the DFS (P=0.024) and OS (P=0.002), while multivariate analysis demonstrated that HER2 is an independent prognostic factor for OS (P=0.023). Moreover, tumors with HER2 positive expression are more tend to metastasis to other viscera than those with HER2 negative. HER2 expression has different prognostic values for patients with different status of ER and lymph node metastasis.
The first successful targeted agent for BC was trastuzumab—a humanized monoclonal antibody against HER2. Extensive studies in the 1990s and early 2000s established trastuzumab (added to chemotherapy) as first-line treatment for metastatic cancers—and subsequently early BCs—that highly overexpress HER2. As one of the multicenter, we participated the registry investigation of trastuzumab in China, hoping to observe the clinical efficacy and adverse effects of trastuzumab for advanced Chinese BC patients (19). Thirty-one pathologically proved advanced BC women were treated by trastuzumab, the response rate was 25.8%. In factors influencing the prognosis, age and general condition were factors favoring the results, and pathological type, site of metastasis, grade of HER2 over expression and prior treatment were irrelevant to the results. So trastuzumab is an active agent for the patients with HER2 overexpressing metastatic BC.
Although trastuzumab is generally well-tolerated, heart toxicity is a problem particularly if anthracyclines are also given. We took a prospective observational study to investigate the incidence of cardiotoxicity within 5 years of trastuzumab treatment and evaluated potential risk factors in clinical practice (20). The study cohort included 415 patients diagnosed with early BC. Cardiotoxicity incidence was evaluated in patients receiving trastuzumab and those who did not. Incidence of cardiotoxicity in patients treated with trastuzumab was significantly higher than that in controls (23.7% vs. 10.8%, P<0.001). This result was adjusted for factors that might increase the risk of cardiotoxicity, such as history of coronary artery diseases (CADs) or the use of anthracyclines for more than four cycles. So when treating EBC patients with history of CAD and prior anthracycline or RT, physicians should be aware of potential cardiotoxicity risk and thus timely initiate treatment. The use of prophylactic cardio-protective agents and careful cardiac monitoring might reduce the incidence of adverse cardiac events and improve long-term survival outcomes in patients with high risk of cardiotoxicity.
What should be noteworthy is that in clinical practice the resistance of trastuzumab, either intrinsic or secondary, is general and difficult to avoid. For those trastuzumab-resistance patients, we can choose lapatinib or continue to use trastuzumab as the second line savage therapy up to the cNCCN guideline. But which treatment is more effective? We took a prospective, nonrandomized, controlled study to compare the regimen of continuously administering trastzuzumab and capercitabine with the regimen of lapatinib plus capecitabine for metastatic BC who are resistant to trastuzumab and have previously received taxane treatment (21). The median PFS of the patients in the HX and LX groups was 4.5 vs. 6.0 months, respectively (P=0.006). The proportion of patients having a PFS ≥6 months in the HX and LX groups was 30% vs. 55%, respectively (P=0.005). The incidence rate of new brain metastases during treatment was 12% and 3% in the HX and LX groups, respectively, which was not significantly different (P=0.16). In conclusion, application of the lapatinib plus capecitabine regimen in MBC patients with a trastuzumab-resistant tumor can more effectively control the disease compared with continuous administration of trastuzumab plus capecitabine.
In recent years, new target spots or agents appear continuously. BRCA1 was known as an antioncogene related to hereditary BC. We analyzed the mutation of BRCA1 gene in Chinese BC patients (22), 13/186 of patients showed mutation which comprised 7% of the total number of patients. Another target agent is the everolimus, an inhibitor of mTOR in PAM signaling pathway. The activation of PAM signaling pathway plays an important role in cell proliferation, metastasis and apoptosis, and it has been proved to be associated with resistance to AIs and trastuzumab. The BOLERO-1 study assessed the efficacy and safety of adding everolimus to trastuzumab and paclitaxel as first-line treatment for patients with HER2-positive advanced BC (23). Although PFS was not significantly different between groups in the full analysis population, there are 7.2 months prolongation we noted with the addition of everolimus in the HR-negative patients, which are consistent with the previously reported predefined subgroup analysis in BOLERO-3 where in patients with HR-negative, HER2-positive advanced BC derived more benefit when everolimus was added to HER2-targeted treatment in the absence of hormonal treatment.
The application of targeted therapy has made individual treatment possible, and promotes the precision treatment of BC. Although the financial burden and the lower effect of monotherapy still exist, the development of technology and the further clinical study will promote its application, especially organic combine with chemotherapy and endocrine therapy.
From tissue to liquid: the more sensitive and less invasive biopsies
In the past few decades, tumor tissue is the gold standard for clinical and investigational detection. But its development has been restricted for the major barriers exist in terms of acquisition and utility (24). Biopsies are inconvenient from a scheduling perspective, also increasing the cost of patient care. Besides, they are uncomfortable and invasive procedure for patients that often do not influence outcome. To overcome the limitations of tissue biopsies, less invasive techniques capable of capturing tumor heterogeneity and the molecular changes cancer cells when they are exposed to therapy are needed. Liquid biopsies, such as circulating tumor cell (CTC) and circulating tumor DNA (ctDNA), can in principle provide the same tumor burden and genetic information as a tissue biopsy necessary to interrogate key companion diagnostics.
As traditional biomarkers, the combination of carcinoembryonic antigen (CEA), CA153 and CA125 detection can help to predict the OS and PFS of metastasis BC (25), but the sensitivity is not high enough for serum levels so that rarely increased in patients with early or localized disease. The micrometastases originate from the seeding of tumor cells in circulation. Therefore, CTC may be a marker of distant metastasis (26). In recent years, the development of sensitive molecular biological techniques has made the isolation and counting of peripheral CTC possible. We compared the CTC and serum tumor markers, and found that the CTC is significantly related to CEA and CA125, informing that it’ may be more helpful through the joint detection. Particularly for CTC alone, its expression at baseline is an independent predictor for both PFS and OS of MBC.
However, retrospective data seem to indicate a limited prognostic value of CTCs in BC patients with newly diagnosed MBC and HER2 overexpression/amplification. Moreover, studies have shown controversial reports on the incidence of CTC detection and a predictive value in disease with enriched triple-negative breast cancers (TNBCs). To evaluate the potential utility of CTC measurements in predicting responses to anticancer therapies, including response to HER2-targeted agents, PFS, and OS in Chinese women with MBC, we conduct the CBCSG004 study (27). A total of 294 of the 300 MBC patients enrolled from six leading Chinese cancer centers were assessable. In multivariate Cox regression analyses, the baseline CTC number remained an independent prognostic factor for PFS [HR =1.93; 95% confidence interval (CI), 1.39–2.69; P<0.001] and OS (HR =3.76; 95% CI, 2.35–6.01; P<0.001). Similar results were observed for CTC counts at the first follow-up visit for both PFS (P=0.049) and OS (P<0.001). In summary, we firstly report the prognostic utility of CTC in Chinese patients with MBC, and demonstrate that the detection of CTC in patients with MBC is associated with substantial prognostic information. Additionally, in women with newly diagnosed MBC starting first-line therapy, MBC patients with HER2-positive tumors, and TNBC patients, CTC enumeration is associated with substantial prognostic information.
In addition to enumeration, we firstly elicit that the molecular characterization of CTCs can be recognized as a valuable tool that provide real-time information to distinguish subgroups of patients who can benefit from certain types of therapy. Our center studied the predictive value of CTC HER2 expression for efficient anti-HER2 therapy in HER2-positive MBC patients (28). We proposed a CTC HER2-positive criterion, defined as >30% of CTCs over-expressing HER2. Among patients undergoing anti-HER2 therapy, those with HER2-positive CTCs had longer PFS (8.8 vs. 2.5 months, P=0.002). Among patients with HER2-positive CTCs, the median PFS for those receiving anti-HER2 therapy was significantly longer than those who were not (8.8 vs. 1.5 months, P=0.001). Notably, up to 52% (14/27) of the HER2-positive patients were CTC HER2-negative, and anti-HER2 therapy did not significantly improve the median PFS in these patients (2.5 vs. 0.9 months, P=0.499). Our findings underscore the necessity of a comprehensive CTC analysis, which may provide valuable prognostic and predictive information for optimizing individually tailored therapies in HER2-positive MBC patients.
HER2-ECD (extracellular domain) is another hotspot of liquid biopsies for the lower cost and better specificity. We evaluate the serum HER2 ECD level and its significance in advanced BC patients with different molecular subtypes (29). A total of 322 advanced BC patients were enrolled. It was found that 55.9% (19/34) Luminal A, 42.7% (44/103) Luminal B-HER2(−), 70.6% (60/85) Luminal B-HER2(+), 73.8% (45/61) HER2-enriched and 23.1% (9/39) triple-negative patients had serum concentrations of HER2 ECD at least 15 ng/ml respectively. The prevalence of elevated ECD level in patients of different molecular subtypes differed significantly (P<0.001). Tissue HER2 status, number of metastatic sites, visceral metastasis, CA15-3 and CEA levels exhibited statistically significant correlations with the prevalence of elevated serum HER2 ECD level. The serum concentrations of HER2 ECD decreased after effective targeted therapy in tissue HER2-positive patient. In conclusion, the prevalence of elevated serum levels of HER2 ECD differed significantly in advanced BC patients with different molecular subtypes. The serum HER2 ECD level may reflect both histological HER2 status and tumor burden. And the dynamic changes of ECD concentrations are somewhat correlated with the efficacies of targeted treatment.
Along with the development of genome sequence technology, the era of precision sequence of liquid biopsies is coming, particularly for the CTC and ctDNA sequence. Cell-free fragments of DNA are shed into the bloodstream by cells undergoing apoptosis or necrosis, and the load of circulating cell-free DNA correlates with tumor staging and prognosis. One of the key advantages of ctDNA analyses is the high degree of specificity offered, because mutations found in ctDNA are in essence integral agents of an individual’s cancer and are defined by their presence in tumor DNA and absence in matched normal DNA. Our team detected the cancer-related genes mutation in circulating cell free DNA by next generation deep sequencing (NGS) in HER2-positive MBC, hoping to explore the relative gene mutations associated with the anti-HER2 treatment, the initial result showed a good covering property and depth (30). However, it’s hard to get the cell morphology, immunohistochemistry and epitope characteristics via ctDNA, but can be got by the CTC sequence (31), which will be the future research direction of our center.
In conclusion, as a real time, dynamic and noninvasive technology, liquid biopsies can be a necessary and useful supplement for tumor tissue detection among variant subtypes and stages of BC. In the precision medicine era, besides the use of predicting and judging the risk of metastasis and the early prediction of the treatment efficacy, the liquid biopsies can also help the assessment of molecular heterogeneity, identification of genetic determinants for therapy, tracking of genomic evolution, and development of acquired resistance.
Stratified medicine to the precision medicine: the promising future
At the moment, stratified medicine is the dominant model. The big data from large clinical studies has promoted the standard treatment for one subtype patients. But the existence of tumor heterogeneity has made every patient individual (32). As a matter of fact, a significant proportion of BC patients are being over-treated: many patients are likely cured by locoregional therapy alone, but are enduring the side effects of unnecessary additional systemic therapies. And some patients may lose the opportunity to accept the effective therapy which has been denied by “statistical differences”. The underlying goal of improving systemic treatments of BC is to evolve from a shotgun approach of treating every patient with relatively non-specific cytotoxic chemotherapy or hormonal therapy to a rational design in which patients are treated with therapies aimed at specific molecular targets, just like the “Basket Trial” and “Umbrella Trial” (33). Basket trials are a new and evolving form of clinical trial design and are predicated on the hypothesis that the presence of a molecular marker predicts response to a targeted therapy independent of tumor histology. Basket trials have generated an enormous amount of interest because they implement a hypothesis-driven strategy incorporating precision medicine into clinical trials even for mutations that are rare or difficult to study solely within a disease-specific context. While Umbrella trials are built on a centrally performed molecular portrait and molecularly selected cohorts with matched drugs, and can include patients’ randomization and strategy validation. Both of the two trials are helpful to identify large and meaningful differences in small, molecularly selected groups of patients.
Along with the reformation of big data handling techniques and payment mode, progresses in high throughput generation of genome, transcriptome, proteome, and interactome data offer the possibility of unprecedented high precision diagnosis at prices that become affordable. The integration of sciences through informatics and mathematical modeling constitutes a new opportunity to improve cancer therapies. Despite disappointing results partly due to incorrect or imprecise prevailing views and technology limitations, precision medicine remains an indispensable route to decrease the toxicity of cancer treatment and to increase its benefit to patients.
Acknowledgements
Funding: This project was supported by the National Natural Science Foundation of China (No. 81472477).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Jiang ZF, Zhang HQ. The status of chemotherapy in the era of classification treatment. National Medical Journal of China 2014;94:2001-3. Available online: http://zhyxzz.yiigle.com/CN112137201426/561821.htm. In Chinese.
- Jiang Z, Song S, Liu X. Paclitaxel (Tesu) as a single agent in the treatment of breast cancer. Zhonghua Zhong Liu Za Zhi 1997;19:445-7. [PubMed]
- Zhang HQ, Wang T, Bian L, et al. Cisplatin combined chemotherapy in HER-2-negative metastatic breast cancer patients with anthracyclines and taxanes treatment failure. Chinese Journal of Cancer Prevention and Treatment 2014;21:1820-4.
- Huang H, Jiang Z, Wang T, et al. Single-agent capecitabine maintenance therapy after response to capecitabine-based combination chemotherapy in patients with metastatic breast cancer. Anticancer Drugs 2012;23:718-23. [Crossref] [PubMed]
- Song ST, Tang ZM, Li GM, et al. Clinical, Pathological and outcome feature of ER positive breast cancer. China Academic Journal 1983;(4). Available online: http://www.cnki.com.cn/Article/CJFDTotal-JSYX198304018.htm. In Chinese.
- Jiang Z, Song S, Liu X. Prospective randomized trial on the efficacy of adjuvant endocrine therapy for ER-positive breast cancer patients after radical mastectomy. Zhonghua Zhong Liu Za Zhi 2001;23:420-2. In Chinese. [PubMed]
- Yu KD, Zhou Y, Liu GY, et al. A prospective, multicenter, controlled, observational study to evaluate the efficacy of a patient support program in improving patients' persistence to adjuvant aromatase inhibitor medication for postmenopausal, early stage breast cancer. Breast Cancer Res Treat 2012;134:307-13. [Crossref] [PubMed]
- Jiang ZF, Song ST, Li JY, et al. Megadose of medrysone for the treatment of metastatic breast cancer. Chinese Journal of Oncology 1995;17:71-3. [PubMed]
- Jiang H, Wang T, Zhang SH, et al. Fulvestrant for the treatment of advanced breast cancer after prior aromatase inhibitor therapy. China Oncology 2013;23:224-8.
- Robertson JF, Zefei J, Di Leo D, et al. Abstract P5-14-01: A meta-analysis of clinical benefit rates for fulvestrant 500 mg versus alternative therapies for treatment of postmenopausal, estrogen receptor-positive advanced breast cancer. Cancer Res 2016;76:P5-14-01.
- Yan M, Jiang ZF, Song ST, et al. Case report of a patient treated with zoladex combined with Arimidex. National Medical Journal of China 2003;83:1137. Available online: http://www.cqvip.com/QK/93850X/200313/9016259.html. In Chinese.
- Zhang ZQ, Jiang ZF, Song ST, et al. ZoladexTm + arimidexTm in the treatment of premenopausal patients with advanced breast cancer. Oncology Progress 2004;2:127-30.
- Liu Q, Wang T, Jiang ZF, et al. Clinical research of medical ovarian suppression combined with anastrozole in the treatment of metastatic breast cancer in premenopausal women. Cancer Research and Clinic 2012;24:392-4.
- Jiang H, Zhang SH, Du M, et al. An exploratory study of Fulvestrant in premenopausal women with advanced,hormone-positive breast cancer. Practical Oncology Journal 2013;27:140-3.
- Jiang ZF, Song ST. New method and strategy of the endocrine therapy. Chinese Journal of Oncology 2003;25:410-1. In Chinese.
- Jiang ZF, Xu BH, Song ST, et al. Basic consensus of the endocrine therapy of breast cancer. Chinese Journal of Oncology 2006;28:238-9. In Chinese. [PubMed]
- Jiang Z, Wang X. Consideration and discussion on ten hot issues of endocrine therapy for breast cancer. Zhonghua Wai Ke Za Zhi 2015;53:895-900. [PubMed]
- Yang LF, Song ST, Li XB, et al. Expression of c-erbB2 protein and its relation to prognosis in 284 primary breast cancer patients. Zhonghua Zhong Liu Za Zhi 2006;28:294-7. [PubMed]
- Sun Y, Li LQ, Song ST, et al. Result of phase II clinical trial of herceptin in advanced Chinese breast cancer patients. Zhonghua Zhong Liu Za Zhi 2003;25:581-3. [PubMed]
- Xue J, Jiang Z, Qi F, et al. Risk of trastuzumab-related cardiotoxicity in early breast cancer patients: a prospective observational study. J Breast Cancer 2014;17:363-9. [Crossref] [PubMed]
- Bian L, Wang T, Zhang SH, et al. Ki-67 index as a prognostic factor of subsequent lapatinib-based therapy in HER2-positive metastatic breast cancer with resistance to trastuzumab. Cancer Biol Ther 2014;15:365-70. [Crossref] [PubMed]
- Lai C, Jiang Z, Song S. Mutation BRCA1 gene in 186 breast cancer patients. Zhonghua Zhong Liu Za Zhi 2001;23:483-5. [PubMed]
- Hurvitz SA, Andre F, Jiang Z, et al. Combination of everolimus with trastuzumab plus paclitaxel as first-line treatment for patients with HER2-positive advanced breast cancer (BOLERO-1): a phase 3, randomised, double-blind, multicentre trial. Lancet Oncol 2015;16:816-29. [Crossref] [PubMed]
- Diaz LA Jr, Bardelli A. Liquid biopsies: genotyping circulating tumor DNA. J Clin Oncol 2014;32:579-86. [Crossref] [PubMed]
- Qian L, Liu Q, Liu Y, et al. Expression of circulating tumor cells in metastatic breast cancer patients and its relationship with multiple serum tumor markers. Practical Oncology Journal 2012;26:220-4.
- Cristofanilli M, Budd GT, Ellis MJ, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med 2004;351:781-91. [Crossref] [PubMed]
- Jiang ZF, Cristofanilli M, Shao ZM, et al. Circulating tumor cells predict progression-free and overall survival in Chinese patients with metastatic breast cancer, HER2-positive or triple-negative (CBCSG004): a multicenter, double-blind, prospective trial. Ann Oncol 2013;24:2766-72. [Crossref] [PubMed]
- Liu Y, Liu Q, Wang T, et al. Circulating tumor cells in HER2-positive metastatic breast cancer patients: a valuable prognostic and predictive biomarker. BMC Cancer 2013;13:202. [Crossref] [PubMed]
- Zhou J, Liu Y, Wang T, et al. Serum HER2 ECD level and its clinical significance in advanced breast cancer patients with different molecular subtypes. Zhonghua Yi Xue Za Zhi 2014;94:1384-7. [PubMed]
- Qi F, Bian L, Zhang SH, et al. Noninvasive Detection of Mutation-Based Circulating Cell-Free DNA by Amplicon Next-Generation Deep Sequencing in Breast Cancer. Letters in Biotechnology 2015;26:389-92.
- Lohr JG, Adalsteinsson VA, Cibulskis K, et al. Whole-exome sequencing of circulating tumor cells provides a window into metastatic prostate cancer. Nat Biotechnol 2014;32:479-84. [Crossref] [PubMed]
- Jiang ZF. Decision making for breast cancer: From the individualized treatment to precision medicine plan. Chinese Journal of Practical Surgery 2015;(7):697-700.
- Carels N, Spinassé LB, Tilli TM, et al. Toward precision medicine of breast cancer. Theor Biol Med Model 2016;13:7. [Crossref] [PubMed]


