β-catenin and SKP2 proteins as predictors of grade and stage of non-muscle invasive urothelial bladder carcinoma
Introduction
Bladder cancer is the fourth most common cancer in men, and the eighth most common cause of cancer death (1). Approximately 70% of bladder cancers are superficial at initial diagnosis (2). Transitional tumors represent nearly 90% of bladder cancers, and range from low grade lesions that might never recur, to high grade aggressive cancers associated with high risk of recurrence and death (3).
Regulation of intercellular adhesion is settled by homotypic interaction of cadherin (a cell-cell adhesion molecule), which are attached to the cytoskeleton via various cytoplasmic proteins including beta-catenin (β-catenin) (4). β-catenin is an oncoprotein, encoded by the CTNNB1 gene, and responsible for anchoring the cadherin’s with the cytoskeleton. Loss of cadherin-mediated adhesion and activation of the Wnt/β-catenin signaling pathway are important steps in the development and progression of many neoplasms (5,6). Previous studies (7) have reported that nuclear β-catenin accumulation was significantly higher in bladder cancer and associated with poor prognosis (8).
We investigated the expression of β-catenin and SKP2 proteins in a cohort of superficial bladder cancer patients and to explore their prognostic ability through their association with pathological outcomes.
Methods
After Institutional review board approval, we retrospectively retrieved formalin-fixed paraffin-embedded specimens. Patients’ biopsy specimens were divided into two groups, the 1st with superficial bladder cancer receiving transurethral resection of bladder tumor (TURBT) and the 2nd with chronic nonspecific cystitis. We excluded patients diagnosed with invasive bladder cancer.
Chart review included demographic variables, preoperative routine laboratory tests and a negative urine culture as well as procedure-related factors.
Histopathological evaluation
Each specimen was sectioned; one to be stained with routine Hematoxylin & Eosin (H. & E.) for histopathological evaluation, and the other section was set over a positively charged slide for immunostaining.
Under light microscopy, chronic cystitis slides, stained with H. & E. were examined, for presence of dysplasia, metaplasia or specific infection, while those with superficial cancer bladder were evaluated according to the 2007 TNM classification and graded by the 2004 WHO classification. Mitotic and apoptotic tumor cells were counted using binocular Olympus CH2 light microscope (400× magnifications), and then micro-vessel density (MVD) was detected thoroughly, using (40× magnification) (9,10).
Immunohistochemical staining
Four-micrometer sections were cut from formalin-fixed, paraffin-embedded tissue followed by deparaffinization and rehydration using xylene and transferred to alcohol, respectively. Antigen retrieval was by sequential boiling for 10–20 min, in a citrate buffer (pH =6.0) then cooling at room temperature. β catenin (Thermo Fisher Scientific, UK), was applied by dropping on the tissue sections then incubated, overnight, in a humidity chamber, at room temperature. SKP2 (United States Biological, Massachusetts, USA) was applied by same technique but for incubated 30 min only. Negative control slides were prepared by omitting the primary antibody from the staining procedure. Excess reagent was discarded and the slides were rinsed twice in phosphate-buffered saline (PBS) for 5 min.
After blotting of excess buffer, 1–2 drops of biotinylated Goat-polyvalent 2ry antibody were applied (Labvision Corp., Thermo Scientific, USA).
Visualization under appropriate substrate/chromogenic, diaminobenzidine tetra hydrochloride (DAB) reagent and counter staining through Mayer’s Hematoxylin (BioGenex, Fermont, CA, USA).
Statistical analysis
Univariate analysis was performed using Wilcoxon’s signed-rank test and Fisher’s exact test comparing different variables.
Results
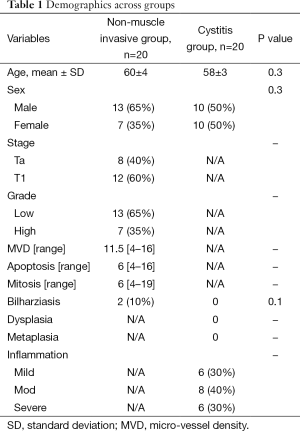
A total of 40 patients were retrospectively identified, 50% underwent bladder biopsy for a superficial cancer bladder, while the other half underwent biopsy for chronic nonspecific cystitis. Sex and age were not significantly different across the two groups (Table 1). Forty percent of non-muscle invasive bladder cancers (NMIBC) were staged as Ta, while the other 60% were T1 and regarding grading, high grade constituted 65% and the others were low grade. None of the chronic cystitis groups (control) showed any specific infections, dysplasia, or metaplasia, while about 30% were mild degree inflammation, 40% moderate degree inflammation and the rest were sever degree inflammation.
Full table
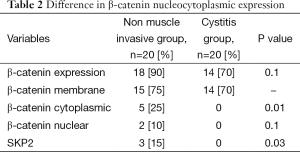
Eighteen (90%) patients of the NMIBC group showed positive β-catenin expression in comparison to 14 (70%) of the control group with (P=0.1). β-catenin-positive membranous localization, were identified in only 15/18 of NMIBC in comparison to 14/14 of control group.
Regarding the β-catenin nucleocytoplasmic expression in NMIBC group, five patients showed cytoplasmic expression and two patients were having nuclear expression ; while none showed its expression in the control group with (P=0.01, 0.1) respectively (Table 2).
Full table
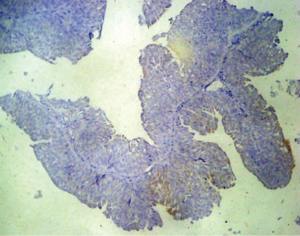
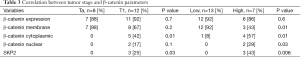
Correlation between tumor stage and β-catenin parameters did not show a statistically significant difference between Ta and T1, except for β-catenin cytoplasmic expression, found exclusively present in T1 and absent in Ta patients (42% to 0, P=0.01).
Similarly, a statistically significant difference were showed for β-catenin cytoplasmic expression, found in 4 (57%) of high grade patients and 1 (8%) of low grade patients (P=0.01) (Table 3).
Full table
SKP2 expression was exclusively present in malignant bladder group (P=0.03) and was significantly associated with higher grade tumors (P=0.006) and higher stage (P=0.03).
Discussion
Seventy percent of new transitional bladder cancer cases are classified as non-muscle invasive, including Ta, T1 tumors, and Tis (carcinoma in situ) (11). About 40–80% of NMIBCs recur within 6 to 12 months if managed with TURBT without additional therapy, and 10–25% will develop muscle invasive, regional, or metastatic disease (12).
Accurate and sensitive incorporation of pathological variables such as: the depth of invasion, histological grade, and the presence or absences of multicentric disease is important in determining management options, estimating prognosis, predicting response to treatment, and monitoring recurrence.
Unfortunately, these conventional prognostic factors are sometimes not accurate enough to predict true biologic behavior, due to failure of fulfilling the essential steps of biopsying, handling and reporting of bladder tumor specimens (13,14).
New prognostic markers are needed to individualize and strengthen the diagnostic accuracy for contemporary treatment of patients with high-risk superficial bladder cancer.
Since its discovery in the early 1990s (15), many studies demonstrated the role played by β-catenin in cell adhesion, through function in linking cadherin cell adhesion receptors to the cytoskeleton, and hence may be responsible for transmitting the contact inhibition signal that causes cells to stop dividing once the epithelial sheet is complete.
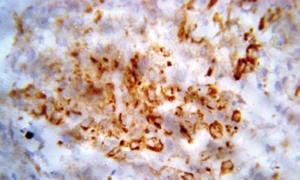
We evaluated β-catenin expression and localization in patients with non-muscle-invasive bladder cancer and correlated these findings with different prognostic factors (Figure 1).
The NMIBC group showed a greater extent of β-catenin expression 18 (90%) in comparison with 14 (70%) in the control, but didn’t reach a significant difference (P=0.1).
Recently, researchers (16) discovered significantly higher levels of nuclear β-catenin and matrix metallopeptidase 9 (MMP9) in human bladder cancer specimen with metastasis, compared to those without metastasis.
We found nucleocytoplasmic expression in 7 (35%) cases out of 18 NMIBC cases with positive β-catenin expression while none of the β-catenin positive cases of the control group with significant difference (P=0.01). similarly, Urakami et al. (7), had also found that the nuclear β-catenin accumulation was significantly higher in bladder tumor than in normal bladder mucosa.
Other reports (17) identified the detection of high nuclear β-catenin expression, prognostic for colon cancer patients with a high risk for distant metastasis.
In contrast, Khramtsov et al. (18), reported that membrane-associated β-catenin was observed in all breast cancer subtypes, and its expression decreased with tumor progression.
Regarding correlation between histopathological outcomes of NMIBC and different form of β-catenin expression, we found significant difference between cytoplasmic expression between Ta (0) and T1 (42%) cases, being more expressed in T1 patients (P=0.01) (Figure 2).
Several studies had showed also that cytoplasmic β-catenin expression was associated with a poorer prognosis in patients with cancers of breast, liver, and colon (19).
Also, we found a significant difference of nuclear and cytoplasmic β-catenin expression, between low and high grade NMIBC cases (Table 3), favoring high grade tumor for expression (P=0.01, 0.03).
SKP2 overexpression can regulate the ability of cells to migrate and invade, and may impair the excellular cytoskeleton mainly microtubules. Few studies had evaluated SKP2 expression in urothelial carcinoma (20), researchers found that SKP2 is overexpressed in bladder urothelial carcinoma and its expression is associated with poor prognosis.
Other reports had found that WIF1, a Wnt pathway inhibitor, regulates SKP2 and c-myc expression leading to growth inhibition of human invasive urinary bladder cancer cells (21).
In our study, SKP2 expression was associated with NMIBC only (P=0.03) and it was associated with poor prognostic parameters. A highly statistically significant correlation was found between the expression of SKP2 in NMIBC with higher tumor stage (T1) (P=0.03) and higher grade (P=0.006).
Conclusions
β-catenin and SKP2 expression are providing promising results for differentiating higher grade and stage non muscle invasive bladder cancers.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Jacobs BL, Lee CT, Montie JE. Bladder cancer in 2010: how far have we come? CA Cancer J Clin 2010;60:244-72. [PubMed]
- Kaufman DS, Shipley WU, Feldman AS. Bladder cancer. Lancet 2009;374:239-49. [PubMed]
- Vaidya S, Lakhey M. Urothelial tumours of the urinary bladder: a histopathological study of cystoscopic biopsies. JNMA J Nepal Med Assoc 2013;52:475-8. [PubMed]
- Takayama T, Shiozaki H, Shibamoto S, et al. Beta-catenin expression in human cancers. Am J Pathol 1996;148:39-46. [PubMed]
- Shimazui T, Schalken JA, Giroldi LA, et al. Prognostic value of cadherin-associated molecules (alpha-, beta-, and gamma-catenins and p120cas) in bladder tumors. Cancer Res 1996;56:4154-8. [PubMed]
- Osterheld MC, Bian YS, Bosman FT, et al. Beta-catenin expression and its association with prognostic factors in adenocarcinoma developed in Barrett esophagus. Am J Clin Pathol 2002;117:451-6. [PubMed]
- Urakami S, Shiina H, Enokida H, et al. Epigenetic inactivation of Wnt inhibitory factor-1 plays an important role in bladder cancer through aberrant canonical Wnt/beta-catenin signaling pathway. Clin Cancer Res 2006;12:383-91. [PubMed]
- Kastritis E, Murray S, Kyriakou F, et al. Somatic mutations of adenomatous polyposis coli gene and nuclear b-catenin accumulation have prognostic significance in invasive urothelial carcinomas: evidence for Wnt pathway implication. Int J Cancer 2009;124:103-8. [PubMed]
- Greene FL, Page DL, Fleming ID, et al., editors. AJCC Cancer Staging Manual. 6th ed. New York: Springer-Verlag, 2002.
- Eble JN, Sauter G, Epstein JI, et al., editors. World Health Organization classification of tumours: pathology and genetics of tumours of the urinary system and male genital organs. Lyon: IARC Press, 2004.
- Kirkali Z, Chan T, Manoharan M, et al. Bladder cancer: epidemiology, staging and grading, and diagnosis. Urology 2005;66:4-34. [PubMed]
- Aldousari S, Kassouf W. Update on the management of non-muscle invasive bladder cancer. Can Urol Assoc J 2010;4:56-64. [PubMed]
- Cheng L, Montironi R, Davidson DD, et al. Staging and reporting of urothelial carcinoma of the urinary bladder. Mod Pathol 2009;22 Suppl 2:S70-95. [PubMed]
- Coblentz TR, Mills SE, Theodorescu D. Impact of second opinion pathology in the definitive management of patients with bladder carcinoma. Cancer 2001;91:1284-90. [PubMed]
- McCrea PD, Turck CW, Gumbiner B. A homolog of the armadillo protein in Drosophila (plakoglobin) associated with E-cadherin. Science 1991;254:1359-61. [PubMed]
- Du Y, Wang Y, Zhang F, et al. Regulation of metastasis of bladder cancer cells through the WNT signaling pathway. Tumour Biol 2015;36:8839-44. [PubMed]
- Ormanns S, Neumann J, Horst D, et al. WNT signaling and distant metastasis in colon cancer through transcriptional activity of nuclear β-Catenin depend on active PI3K signaling. Oncotarget 2014;5:2999-3011. [PubMed]
- Khramtsov AI, Khramtsova GF, Tretiakova M, et al. Wnt/beta-catenin pathway activation is enriched in basal-like breast cancers and predicts poor outcome. Am J Pathol 2010;176:2911-20. [PubMed]
- Yu B, Yang X, Xu Y, et al. Elevated expression of DKK1 is associated with cytoplasmic/nuclear beta-catenin accumulation and poor prognosis in hepatocellular carcinomas. J Hepatol 2009;50:948-57. [PubMed]
- Kawakami K, Enokida H, Tachiwada T, et al. Increased SKP2 and CKS1 gene expression contributes to the progression of human urothelial carcinoma. J Urol 2007;178:301-7. [PubMed]
- Tang Y, Simoneau AR, Liao WX, et al. WIF1, a Wnt pathway inhibitor, regulates SKP2 and c-myc expression leading to G1 arrest and growth inhibition of human invasive urinary bladder cancer cells. Mol Cancer Ther 2009;8:458-68. [PubMed]