Neuroendocrine tumors (NETs) of unknown primary: is early surgical exploration and aggressive debulking justifiable?
Introduction
Neuroendocrine tumors (NETs) are relatively rare oncologic pathology. NETs with an unknown primary are even rarer which only accounts for less than 5% of all unknown primary gastrointestinal cancers. According to the Surveillance, Epidemiology and End Results (SEER) program data, primary tumor location could not be identified in 4,752 cases among the 35,618 (13%) NETs registered over the last 31 years (1).
NETs of unknown origin were thought to be mostly (90%) poorly differentiated and therefore more aggressive. Polish and colleagues cautioned that the search for the primary in these cases must be weighed against the need to initiate a prompt treatment (2), and NCCN guidelines recommend octreotide therapy, observations, or resection if possible only for metastatic well or moderately differentiated NETs (3). Polish and colleagues also conclude that frequently, the primary tumor site cannot be found despite comprehensive workup (2). We hypothesize that NETs of unknown primary are predominantly low grade, easily localizable surgically, amendable to timely surgical debulking and cytoreduction, which will increase survival in these patients.
Methods
The charts of all 346 surgical patients, seen in our clinic at Ochsner-Kenner between 1/2009 and 9/2012 were retrospectively reviewed to determine the incidence of unknown primary. The rate of successful primary tumor localization and resection was then recorded. Survival for these “unknown primary” patients were then compared to that of a large group of NET patients with a pre-operatively known primary from the same institutional data base. The mortality, the grade of the tumor, and the ability to locate the primary surgically were the main focus of our observation. Institutional Review Board (IRB) approval was obtained to collect the data from patient charts for the research purposes.
Results
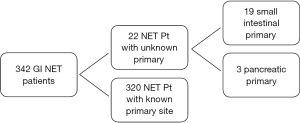
A total of 342 patients were seen during the 45-month time span (Figure 1). Twenty-two patients (6.4%) were identified with a pre-operative diagnosis of a “NET with unknown primary”. All of these patients had a biopsy proven carcinoid/neuroendocrine tumor diagnosis and all underwent surgical intervention. The patient demographics are shown in Table 1. There are 10 females and 12 males in the study group with an average age of 56. The average length between diagnosis and surgical intervention was 11 months. The primary tumor sites were identified in all 22 patients (100%)—and they were 19 small intestines (86.4%) and 3 pancreatic (13.6%). There is no difference in surgical complications or survival rate while compared to their known counterpart. All 22 patients had low-grade tumors and all are still alive as of 9/2012.
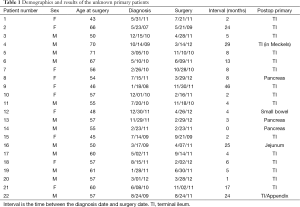
Full table
Discussion
Mid gut carcinoid tumors make up the majority of the NETs and are the most commonly occurring intestinal endocrine tumors. The incidence of mid gut carcinoid is estimated to be approximately 1.5 cases per 100,000 of the general population (4). The slow growth rate of most NETs and their nonspecific clinical presentations often allow the disease to progress undetected until late and/or while an acute clinical manifestation such as bowel obstruction or liver metastasis induced carcinoid syndrome occurs. The recently revised general goal for treating these patients is an aggressive one. The focus is now on resecting the primary tumor and regional lymph nodes in conjunction with resection or cytoreduction of the distant metastases (5). Resection of the primary tumor and the mesenteric lymph nodes can lead to a significant reduction in the tumor-related symptoms and result in a survival advantage (6). This survival advantage of the aggressive surgical cytoreduction has been extended even into patients with unresectable metastatic disease (7). Many nonsurgical treatment modalities like chemoembolization and somatostatin analogs therapy can often be adopted to complement the surgical debulking (5). This combination therapy has been proven to further enhance the survival advantage of NETs patients with advance disease (8).
“NETs of unknown primary are a rare “zebra” amongst “zebras”. Attempts were made to identify biological markers to guide the clinical approach to these minority patients. Tumor grade has become the most commonly used marker in helping direct patient management based on its prognostic value (9). In general, the consensus have always been against the surgical exploration and debulking for high grade tumors (2).
It is unclear if unknown primary NET tumors are biologically different from those of known primary sites (10). Nonetheless, they were often labeled as poorly differentiated and aggressive tumors presumptively. Some clinicians believed and claimed that having an unknown primary site is indicative of a high-grade tumor and are associated with poorer prognosis. As a result of these permissive erroneous observation and assumption, treatment for patients with metastatic NETs of unknown primary has been passive, especially for those who are clinically “stable” or asymptomatic. In rare occasion, the treatments were started but often only focused on symptomatic control and aiming toward addressing the distant disease without the attempt of identifying and removal of the primary tumor. Platinum based chemotherapy has been recommended for metastatic poorly differentiated disease (11).
Many NETs patients, especially those with unknown primary, are often deemed “unresectable” or declared to be non-surgical candidates by clinicians who are not familiar with the current array of treatments that have been developed (5). Current NCCN guidelines recommends management decision based on grade of differentiation of NET with unknown primary (12). Well differentiated NET are treated with carcinoid tumor protocol whereas poorly differentiated NET are only resected if tumor is local or loco-regional. Metastatic disease is often deemed non-surgical and only chemotherapy is recommended (3).
The results from our study have revealed and confirmed our hypothesis that NETs of unknown primary are predominantly low grade and the primary site can be easily identified by a surgeon with experience. A study from Oregon Health & Science University (OHSU) found that laparoscopic surgical exploration had the highest diagnostic sensitivity (79%) in locating unknown primary NET as compared to radiological imaging, endoscopy or colonoscopy (13). Wang et al. were able to successfully locate 86.7% of their GI NET with unknown primary on surgical exploration (14). Molecular fingerprinting techniques via next-generation sequencing, polymerase chain reaction (PCR), immunohistochemistry etc. have been increasingly used for not only finding disease characteristics but also predicting the location of tumors of unknown primary. Although promising, molecular profiling is still in infancy owing to lack of substantial data (15). Molecular fingerprinting is expensive and the routine adaptation preoperatively for tumor localization will further increase the cost of care to NETs patients.
Our data also shows that the surgical outcomes of patients with distant metastasis from an unknown primary appear to be no worse than that of the known group as previously reported (8). The survival for our NETs patients overall have also been proven to be better than that reported and this survival advantage has been attributed to our multi-disciplinary approach and adaptation of a timely aggressive surgical debulking (8). Therefore, we advocate, NET patients with unknown primary who are low risk surgical candidate be aggressively debulked surgically in a timely fashion without the utilizations of any additional costly and low yield pre-op localization studies.
We recognize the limitation of our study, being a retrospective, one with a short follow-up and a small cohort. It is further hampered by the fact that a survival curve couldn’t be generated at this time due to the indolent nature of low grade NETs in combination with a short follow-up.
A future longitudinal study with a larger cohort and a longer follow up time will be needed to further endorse our current recommendation in dealing with NETs patients of unknown primary.
Conclusions
Unknown primary NETs are not associated with a poor prognosis as previously reported. Early surgical exploration with aggressive debulking is indicated for the treatment of these patients, as for their known counterparts. All NETs patients with an unknown primary who are low risk surgical candidates should be explored timely.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Yao JC, Hassan M, Phan A, et al. One hundred years after "carcinoid": epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol 2008;26:3063-72. [PubMed]
- Polish A, Vergo MT, Agulnik M. Management of neuroendocrine tumors of unknown origin. J Natl Compr Canc Netw 2011;9:1397-402. [PubMed]
- NCCN Clinical Practice Guidelines in Oncology. Neuroendocrine Tumors, Version 1.2015. Available online: .abstracthttp://www.jnccn.org/content/13/1/78
- Mamikunian G, Vinik AI, O’Dorisio TM, et al. Neuroendocrine Tumors: A Comprehensive Guide to Diagnosis and Management. 4th ed. Inglewood: Inter Science Institute, 2009. Available online: http://www.interscienceinstitute.com/docs/Neuroendocrine-Tumors-4th-Edition.pdf
- Joseph S, Wang YZ, Boudreaux JP, et al. Neuroendocrine tumors: current recommendations for diagnosis and surgical management. Endocrinol Metab Clin North Am 2011;40:205-31. x. [PubMed]
- Hellman P, Lundström T, Ohrvall U, et al. Effect of surgery on the outcome of midgut carcinoid disease with lymph node and liver metastases. World J Surg 2002;26:991-7. [PubMed]
- Givi B, Pommier SJ, Thompson AK, et al. Operative resection of primary carcinoid neoplasms in patients with liver metastases yields significantly better survival. Surgery 2006;140:891-7; discussion 897-8. [PubMed]
- Boudreaux JP, Wang YZ, Diebold AE, et al. A single institution's experience with surgical cytoreduction of stage IV, well-differentiated, small bowel neuroendocrine tumors. J Am Coll Surg 2014;218:837-44. [PubMed]
- Spigel DR, Hainsworth JD, Greco FA. Neuroendocrine carcinoma of unknown primary site. Semin Oncol 2009;36:52-9. [PubMed]
- Abbruzzese JL, Abbruzzese MC, Hess KR, et al. Unknown primary carcinoma: natural history and prognostic factors in 657 consecutive patients. J Clin Oncol 1994;12:1272-80. [PubMed]
- Nakakura EK, Venook AP, Bergsland EK. Systemic and regional nonsurgical therapy--what is the optimal strategy for metastatic neuroendocrine cancer? Surg Oncol Clin N Am 2007;16:639-51. x. [PubMed]
- Klimstra DS, Modlin IR, Coppola D, et al. The pathologic classification of neuroendocrine tumors: a review of nomenclature, grading, and staging systems. Pancreas 2010;39:707-12. [PubMed]
- Massimino KP, Han E, Pommier SJ, et al. Laparoscopic surgical exploration is an effective strategy for locating occult primary neuroendocrine tumors. Am J Surg 2012;203:628-31. [PubMed]
- Wang SC, Parekh JR, Zuraek MB, et al. Identification of unknown primary tumors in patients with neuroendocrine liver metastases. Arch Surg 2010;145:276-80. [PubMed]
- Massard C, Loriot Y, Fizazi K. Carcinomas of an unknown primary origin--diagnosis and treatment. Nat Rev Clin Oncol 2011;8:701-10. [PubMed]


