CROSS and beyond: a clinical perspective on the results of the randomized ChemoRadiotherapy for Oesophageal cancer followed by Surgery Study
Introduction
Despite extensive research efforts, oesophageal cancer remains a disease with a poor prognosis. The past decades there has been an improvement in overall survival for patients with operable oesophageal cancer, however 5-year survival still rarely exceeds 40% (1). In the United States and Europe the incidence of adenocarcinoma (AC) rises steeply while the incidence of squamous cell carcinoma (SCC) is stable and worldwide still the most prevalent type of oesophageal cancer (2,3). Despite their different etiologies, SCC and AC have long been studied collectively because of shared similarities in oncogenic dysregulation (4). Only in recent years their differences in tumor biology, histology, and molecular biology have become more apparent.
In patients undergoing surgery without (neo)adjuvant therapy, 25% have irradical resected tumors (1). Locoregional recurrence rates of 30–40% have been reported in several randomized studies (1,5-7). Based on the hypothesis that improved local control will improve outcome, research has focused on the addition of different treatment modalities to surgery. Initially, two large clinical trials adding chemotherapy to surgery versus surgery alone reported conflicting data. The intergroup 0113 trial showed no benefit of neoadjuvant cisplatin plus fluorouracil, while the OEO2 trial did show a significant benefit on overall survival with neoadjuvant cisplatin and fluorouracil (8,9). Subsequently, a meta-analysis of 11 trials did not show a benefit on survival after 3 years follow-up for patients treated with neoadjuvant chemotherapy and surgery (10). A second meta-analysis confirmed the lack of significant difference in overall survival at 3 years follow-up (relative risk, 1.21; 95% CI: 0.88–1.68; P=0.25). However, at 5 years follow-up a significant difference in overall survival in favor of surgery plus chemotherapy was reported (relative risk, 1.44; 95% CI: 1.05–1.97; P=0.02) (11).
Walsh et al. were the first to report a benefit on overall survival of neoadjuvant chemoradiotherapy (CRT) in patients with AC of the esophagus compared to surgery alone (12). Patients were randomized to receive cisplatin and fluorouracil with concurrent radiotherapy followed by surgery or surgery alone. A significant benefit on overall survival was reported at 3 years of follow-up with a median OS of 16 vs. 11 months (P=0.01) in favor of the CRT followed by surgery group.
In 2011, a meta-analysis by Sjoquist et al. confirmed that patients receiving neoadjuvant CRT had a statistically significant benefit in terms of all-cause mortality compared to surgery alone with a hazard ratio of 0.78 (95% CI: 0.70–0.88; P<0.001) (13). This benefit was present in patients with SCC (HR, 0.80; 95% CI: 0.68–0.93; P<0.004), and AC (HR, 0.75; 95% CI: 0.59–0.95; P<0.02.). Most trials used a combination of a fluoropyrimidine and platinum compound. Subsequently, in 2012 the data from the Dutch ChemoRadiotherapy for Oesophageal cancer followed by Surgery Study (CROSS) trial were presented, supporting the use of neoadjuvant CRT in operable oesophageal cancer (6). The results of this trial comparing concurrent CRT consisting of 41.1 Gy with the cytotoxic agents carboplatin and paclitaxel followed by surgery with surgery alone have resulted in a paradigm shift for many clinical oncologist in treatment of oesophageal cancer. In this article we will discuss the most important features and results from the CROSS trial, its impact on clinical practice and future research.
CROSS trial design
Between March 2004 and December 2008, 368 patients with operable oesophageal cancer were included in the Dutch multicenter CROSS trial. Patients were to receive either neoadjuvant treatment with intravenous carboplatin (AUC 2 mg/mL per min) and intravenous paclitaxel (50 mg/m2 of body-surface area) and concurrent radiotherapy (41.4 Gy) followed by surgery or surgery alone. The rationale for this chemotherapy backbone was based on studies in small cell lung cancer and oesophageal cancer, showing good responses with limited toxicity (14-16).
Study procedures
Patients aged between 18 and 75 years with a clinical stage T1N1M0 or T2–3N0–1M0 and histologically confirmed adeno or SCC were eligible (17). Past or current history of malignancy, previous treatment with chemo or radiotherapy and weight loss of more than 10% were the main exclusion criteria. An external beam radiation technique was used to deliver a total of 41.4 Gy in 23 separate fractions of 1.8 Gy, with 5 fractions per week. Chemotherapy was administered intravenously on days 1, 8, 15, 22 and 28 concurrently with radiotherapy. Toxicity was evaluated on a weekly basis according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events, version 3.0. Patients in the CRT group were operated 8–10 weeks after completion of CRT and patients in the surgery alone group were operated as soon as possible. The Mandard scoring system was used to evaluate the tumor in the resection specimen (18). Patient entered follow-up for a total of 5 years.
Analysis
A total of 178 patients in the CRT plus surgery group and 188 in the surgery alone group were included in the final (and updated) analysis. All patients in the CRT group were included into analysis based on an intention to treat principle, irrespective of the dose of neoadjuvant treatment they received. The primary end point of this study was overall survival; secondary end points included progression free survival and progression free interval. The Kaplan Meier method including log rank tests was used to asses overall survival. Univariable and multivariable Cox proportional hazard models were used for subgroup analysis.
Initial results and follow-up
Baseline characteristics of the two groups did not show significant differences between the groups. Of the 171 patients that received neoadjuvant therapy 95% completed the entire treatment. Grade 3 or worse hematological toxicity was reported in 8% and grade 3 or worse non-hematological toxicity was reported in 11% of patients receiving CRT. The most reported grade 3 toxicities were leucopenia, anorexia and fatigue. Low platelet count was the most common reason for not receiving all cycles of chemotherapy. In the CRT group 94% of patients underwent surgery, and 90% of tumors could be resected, while in the surgery alone group 99% underwent surgery and 86% of tumors were resected, indicating that CRT did not lead to an increased rate of withdrawal from surgery. Post-operative complications in the CROSS trial were higher than expected and reported in other studies, but similar in both groups. Postoperative in-hospital mortality was low (4%) and similar between both groups. Pathological complete response (pCR) was reported in 29% and a R0 resection was achieved in 148 of 161 (92%) patients in the CRT group versus 111 of 161 (69%) in the surgery alone group.
The first results reported an estimated survival benefit of 13% in favor of the CRT group (HR, 0.81; 95% CI: 0.70–0.93; P=0.002) after a minimum of 24 months follow-up, with median survival in the CRT group being 49.4 months compared to 24.0 months in the surgery alone group. Recently the results of the CROSS trial after a minimum potential follow-up of 60 months (median 84 months) have been reported by Shapiro et al. (19). These results confirmed the initial reported significant survival benefit of neoadjuvant CRT, which was demonstrated for both patients with AC and SCC. For patients with SCC median overall survival was 84 months in the CRT group and 21 months in the surgery alone group (HR, 0.48; 95% CI: 0.28–0.83). For patients with AC median OS in the CRT group was 43 months versus 27 months in the surgery alone group (HR, 0.73; 95% CI: 0.55–0.98).
At final analysis 39% of patients in the neoadjuvant CRT plus surgery group and 25% of patients in the surgery alone group were alive and disease free. In the updated analysis it was reported that nine patients in the CRT plus surgery group died of treatment related causes versus seven in the surgery alone group (19). The number needed to treat to prevent one death at 5 years is 7.1 (95% CI: 4.6–13.2). Median progression free survival was 37.7 months in the CRT plus surgery group and 16.2 months in the surgery alone group. The reported number needed to treat to prevent one progression at 5 years is 6.1 (95% CI: 4.2–10.0).
Recurrence patterns in CROSS
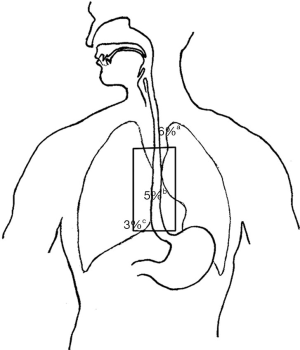
Recurrence patterns in 422 patients included in the phase 2 trial preceding CROSS and the CROSS trial have been more extensively examined by Oppedijk et al. (20). Overall recurrence rate after median 24 months follow-up was 48% in the surgery arm versus 35% in the CRT arm. Locoregional recurrence was significantly reduced by CRT (34% to 14%; P<0.001) as was the occurrence of peritoneal carcinomatosis (14% to 4%; P<0.001). The reported recurrence in relation to radiation field confirms that preoperative CRT reduces locoregional recurrence rate and only 5% of recurrences occurred within the radiation target volume (Figure 1). Nearly 85% of patients with a locoregional recurrence had synchronous (within 3 months) distant metastasis, suggesting that increasing the radiation dose or field size will not have a large effect on overall survival. Next to the improved locoregional control, a small but significant reduction in the development of hematogenous metastasis was observed in the patients receiving CRT. Whether this was due to the systemic effect of chemotherapy or because of improved locoregional control could not be determined based on these data. Multivariate analysis showed that pCR was a favorable prognostic factor for locoregional and distant recurrences.
Matters of debate
Different approaches for SCC versus AC?
SCC and AC of the esophagus and oesophageal junction have different tumor biology. AC has a lower local response rate and more patients develop distant metastases. Several studies have shown that survival after neoadjuvant therapy is better in patients with SCC compared to AC. In Japan, where the majority of the patients has SCC standard therapy is chemotherapy followed by surgery, based on the results of large randomized controlled trials (RCTs) in the Japanese population (21,22). These trials reported a better outcome of preoperative chemotherapy in comparison to western trials. It should be noted however, in the CROSS trial a significant beneficial effect of neoadjuvant CRT on OS was seen for patients with either AC (median OS 43 vs. 27 months) or SCC (median OS 84 vs. 21 months) which persisted after long-term follow-up.
Chemotherapy or chemoradiation?
In several countries perioperative chemotherapy is the favored therapeutic strategy over neoadjuvant CRT. It should be noted, however, that CRT regimens may be less toxic than chemotherapy, while efficacy may be similar. The Medical Research Council Adjuvant Gastric Infusional Chemotherapy (MAGIC) trial randomized patients with gastric or gastro esophageal junction (GEJ) AC to receive either perioperative chemotherapy or surgery alone (7). In the MAGIC trials grade 3 of higher hematological toxicity was reported in 60% and more than half of the patients was unable to complete the adjuvant cycles of chemotherapy, while in the CROSS study 95% of the patients completed the neoadjuvant treatment. In the 2011 meta-analysis of Sjoquist an indirect comparison of chemotherapy and CRT reported a hazard ratio of 0.88 (95% CI: 0.76–1.01) in favor of CRT, with no significant differences found in perioperative mortality (13). Also the CROSS trial showed no increased perioperative mortality for use of neoadjuvant CRT (4% in both groups). This advantage in toxicity is largely attributed to the use of carboplatin and paclitaxel instead of cisplatin and 5-fluorouracil (5-FU). In a retrospective study comparing patients that received neoadjuvant CRT with either a cisplatin and fluoropyrimidine based or a carboplatin and paclitaxel based chemotherapeutic regimen, grade 3 toxicity or higher was reported in 41% of the cisplatin and fluoropyrimidine group as compared to 25% in the carboplatin and paclitaxel based group (23). The French FFCD 9901 trial comparing neoadjuvant cisplatin and 5-FU followed by surgery to surgery alone reported a perioperative mortality of 11.1% in the neoadjuvant chemotherapy group versus 3.4% in the surgery alone group (24). Two smaller prospective RCTs comparing neoadjuvant CRT followed by surgery with chemotherapy followed by surgery reported higher pCR rates and less locoregional recurrence in the CRT groups, with similar in R0 resections rates (25,26). Perioperative mortality did not differ significantly between both groups. pCR rates are a prognostic factor for improved overall survival and less loco regional recurrence contrary to R0 resection rates.
Two ongoing trials will hopefully provide a final answer on the best (neo)adjuvant regimen for both AC and SCC. The Irish Neo-AEGIS trial randomizes patients with AC of the esophagus or GEJ to receive either perioperative chemotherapy similar to the scheme used in MAGIC trial (epirubicin, cisplatin and 5-FU or capecitabine) versus neoadjuvant CRT according to CROSS (27). The Japanese NEXT trial randomizes patients with SCC between three arms (I) neoadjuvant chemotherapy with cisplatin and 5-FU; (II) neoadjuvant chemotherapy scheme including cisplatin, 5-FU and docetaxel; and (III) neoadjuvant cisplatin, 5-FU and concurrent radiotherapy with a dose of 41.4 Gy in 23 fractions (28).
Future perspectives
CROSS regimen as a backbone for targeted therapy
Because of its favorable tolerability without compromising response rates, treatment schemes based on the CROSS protocol can be used as backbone for investigating the addition of newer targeted agents.
The recent PACT trial investigated the addition of the epidermal growth factor receptor (EGFR) inhibitor panutimumab to neoadjuvant CRT (29). Although in this trial the pCR rate did not achieve the preset rate of 40% and these negative findings were similar to other trials investigating the addition of an EGFR inhibitor, the toxicity profile in the PACT trial was more favorable in comparison with the other trials that used a docetaxel/cisplatin and 5-FU/cisplatin backbone and the addition of panutimumab did not result in an increase of perioperative morbidity and mortality (29-31).
Based on the results of the ToGA trial in patients with metastatic oesophagogastric cancer, were the addition of trastuzumab to chemotherapy consisting of a fluoropyrimidine combined with cisplatin resulted in a significant improvement of median overall survival of 2.7 months (32), human epidermal growth factor receptor 2 (HER2) targeting in oesophagogastric cancer is currently being investigated in patients treated with curative intent (33,34). The large RTOG 1010 trial randomizes patients with locally advanced oesophageal AC between CRT with a dose of 50.4 Gy along with weekly carboplatin (AUC 2) and paclitaxel (50 mg/m2) and the same regimen with concurrent and maintenance trastuzumab (NCT01196390).
Since most patients with metastasized disease eventually do develop resistance to trastuzumab, recent studies have evaluated the effect of combined HER2/HER3 targeting with trastuzumab and pertuzumab shows promising results (35,36). To explore whether this approach of combined HER2 targeting is feasible and has potential benefit in HER2 positive operable oesophageal cancer, a study on the addition of neoadjuvant trastuzumab and pertuzumab to neoadjuvant CRT with the CROSS scheme as a backbone is currently recruiting (NCT02120911).
CROSS regimen and the need for response prediction
Although neoadjuvant CRT according to the CROSS regimen has substantially improved treatment outcome, it should be noted that median overall survival still does not exceed 49 months. To further improve treatment outcome, we are currently exploring the feasibility of the addition of adjuvant chemotherapy with oxaliplatin and S-1 (a fourth generation fluoropyrimidine) in patients that received neoadjuvant CRT according to the CROSS regimen followed by surgical resection (NCT02347904).
Based on previous studies in rectal cancer, it may be hypothesized that those patients that had a good pathological response to neoadjuvant treatment may benefit most from this adjuvant chemotherapy strategy (37). However, as exemplified by the MAGIC study, adjuvant chemotherapy after neoadjuvant treatment and major surgery is hard to accomplish. Therefore, a treatment strategy incorporating all therapeutic agents before surgery may be preferable. However, in the neoadjuvant setting we would lack pathological response data to select patients and, therefore, alternative predictors of response are urgently required. Recently, in an exploratory study in 20 patients with oesophageal cancer the apparent diffusion coefficient (ADC) of tumors as measured by diffusion weighted MRI (DW-MRI) was reported to have predictive value for the histopathological response. We are currently exploring new innovative MRI techniques to improve treatment stratification for patients with oesophageal cancer (NCT02253602). The CALB 80803 will further aid in the identification of the chemotherapy backbone that achieves the best tumor response. Patients are randomized to receive either a combination of oxaliplatin, leucovorin and fluorouracil or combined carboplatin and paclitaxel. After induction chemotherapy all patients will receive concurrent radiotherapy combined with either chemotherapeutic regimen. After 6 weeks an evaluation PET/CT scan will be performed and responders will maintain the same chemotherapy regimen and non-responders will switch to the other (NCT01333033).
Finally, if we are able to correctly distinguish responding from non-responding patients, we may even explore a watchful waiting strategy after chemoradiation for truly pCR patients, i.e., omitting surgery, as has been successfully introduced for rectal cancer (38,39). Studies combining imaging and watchful waiting strategies in rectal cancer have shown good results. The PreSANO trial is currently investigating the feasibility of this approach using endoscopic ultrasound, biopsies and positron emission tomography/computerized tomography (PET/CT) as response diagnostics (NTR4834).
Conclusions
The results of the CROSS trial have contributed to the current understanding that preoperative CRT has a beneficial effect on overall survival for patients with operable AC or SCC by improving local control. In addition, it has proven to be a treatment with low toxicity causing less perioperative mortality than more toxic fluorouracil and platinum containing schemes. The CROSS regimen can be considered a safe and effective backbone for targeted therapy. Local recurrence patterns confirm that the preoperative radiation schedules are currently used at their optimum for establishing locoregional control. Personalized treatment strategies for providing a tumor tailored therapy should now be the focus of research, building on the effective base of multimodality treatment.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Kelsen DP, Ginsberg R, Pajak TF, et al. Chemotherapy followed by surgery compared with surgery alone for localized esophageal cancer. N Engl J Med 1998;339:1979-84. [PubMed]
- Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11. Lyon, France: International Agency for Research on Cancer; 2013. Accessed October 10, 2014. Available online: http://globocan.iarc.fr
- Brown LM, Devesa SS. Epidemiologic trends in esophageal and gastric cancer in the United States. Surg Oncol Clin N Am 2002;11:235-56. [PubMed]
- Marsman WA, Tytgat GN, ten Kate FJ, et al. Differences and similarities of adenocarcinomas of the esophagus and esophagogastric junction. J Surg Oncol 2005;92:160-8. [PubMed]
- Bosset JF, Gignoux M, Triboulet JP, et al. Chemoradiotherapy followed by surgery compared with surgery alone in squamous-cell cancer of the esophagus. N Engl J Med 1997;337:161-7. [PubMed]
- van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074-84. [PubMed]
- Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006;355:11-20. [PubMed]
- Medical Research Council Oesophageal Cancer Working Group. Surgical resection with or without preoperative chemotherapy in oesophageal cancer: a randomised controlled trial. Lancet 2002;359:1727-33. [PubMed]
- Kelsen DP, Winter KA, Gunderson LL, et al. Long-term results of RTOG trial 8911 (USA Intergroup 113): a random assignment trial comparison of chemotherapy followed by surgery compared with surgery alone for esophageal cancer. J Clin Oncol 2007;25:3719-25. [PubMed]
- Urschel JD, Vasan H, Blewett CJ. A meta-analysis of randomized controlled trials that compared neoadjuvant chemotherapy and surgery to surgery alone for resectable esophageal cancer. Am J Surg 2002;183:274-9. [PubMed]
- Malthaner R, Fenlon D. Preoperative chemotherapy for resectable thoracic esophageal cancer. Cochrane Database Syst Rev 2003.CD001556. [PubMed]
- Walsh TN, Noonan N, Hollywood D, et al. A comparison of multimodal therapy and surgery for esophageal adenocarcinoma. N Engl J Med 1996;335:462-7. [PubMed]
- Sjoquist KM, Burmeister BH, Smithers BM, et al. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol 2011;12:681-92. [PubMed]
- Choy H, Akerley W, Safran H, et al. Multiinstitutional phase II trial of paclitaxel, carboplatin, and concurrent radiation therapy for locally advanced non-small-cell lung cancer. J Clin Oncol 1998;16:3316-22. [PubMed]
- Choy H, Devore RF 3rd, Hande KR, et al. A phase II study of paclitaxel, carboplatin, and hyperfractionated radiation therapy for locally advanced inoperable non-small-cell lung cancer (a Vanderbilt Cancer Center Affiliate Network Study). Int J Radiat Oncol Biol Phys 2000;47:931-7. [PubMed]
- Lau D, Leigh B, Gandara D, et al. Twice-weekly paclitaxel and weekly carboplatin with concurrent thoracic radiation followed by carboplatin/paclitaxel consolidation for stage III non-small-cell lung cancer: a California Cancer Consortium phase II trial. J Clin Oncol 2001;19:442-7. [PubMed]
- Sobin LH, Wittekind C. TNM classification of malignant tumours. 6th edition. New York: Wiley-Liss, 2002.
- Mandard AM, Dalibard F, Mandard JC, et al. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer 1994;73:2680-6. [PubMed]
- Shapiro J, van Lanschot JJ, Hulshof MC, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol 2015;16:1090-8. [PubMed]
- Oppedijk V, van der Gaast A, van Lanschot JJ, et al. Patterns of recurrence after surgery alone versus preoperative chemoradiotherapy and surgery in the CROSS trials. J Clin Oncol 2014;32:385-91. [PubMed]
- Ando N, Iizuka T, Ide H, et al. Surgery plus chemotherapy compared with surgery alone for localized squamous cell carcinoma of the thoracic esophagus: a Japan Clinical Oncology Group Study--JCOG9204. J Clin Oncol 2003;21:4592-6. [PubMed]
- Ando N, Kato H, Igaki H, et al. A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907). Ann Surg Oncol 2012;19:68-74. [PubMed]
- Blom RL, Sosef MN, Nap M, et al. Comparison of two neoadjuvant chemoradiotherapy regimens in patients with potentially curable esophageal carcinoma. Dis Esophagus 2014;27:380-7. [PubMed]
- Mariette C, Dahan L, Mornex F, et al. Surgery alone versus chemoradiotherapy followed by surgery for stage I and II esophageal cancer: final analysis of randomized controlled phase III trial FFCD 9901. J Clin Oncol 2014;32:2416-22. [PubMed]
- Stahl M, Walz MK, Stuschke M, et al. Phase III comparison of preoperative chemotherapy compared with chemoradiotherapy in patients with locally advanced adenocarcinoma of the esophagogastric junction. J Clin Oncol 2009;27:851-6. [PubMed]
- Burmeister BH, Thomas JM, Burmeister EA, et al. Is concurrent radiation therapy required in patients receiving preoperative chemotherapy for adenocarcinoma of the oesophagus? A randomised phase II trial. Eur J Cancer 2011;47:354-60. [PubMed]
- Keegan N, Keane F, Cuffe S, et al. ICORG 10-14: Neo-AEGIS: A randomized clinical trial of neoadjuvant and adjuvant chemotherapy (modified MAGIC regimen) versus neoadjuvant chemoradiation (CROSS protocol) in adenocarcinoma of the esophagus and esophagogastric junction. J Clin Oncol 2014;32 Suppl:abstr TPS4145.
- Nakamura K, Kato K, Igaki H, et al. Three-arm phase III trial comparing cisplatin plus 5-FU (CF) versus docetaxel, cisplatin plus 5-FU (DCF) versus radiotherapy with CF (CF-RT) as preoperative therapy for locally advanced esophageal cancer (JCOG1109, NExT study). Jpn J Clin Oncol 2013;43:752-5. [PubMed]
- Kordes S, van Berge Henegouwen MI, Hulshof MC, et al. Preoperative chemoradiation therapy in combination with panitumumab for patients with resectable esophageal cancer: the PACT study. Int J Radiat Oncol Biol Phys 2014;90:190-6. [PubMed]
- Lockhart AC, Reed CE, Decker PA, et al. Phase II study of neoadjuvant therapy with docetaxel, cisplatin, panitumumab, and radiation therapy followed by surgery in patients with locally advanced adenocarcinoma of the distal esophagus (ACOSOG Z4051). Ann Oncol 2014;25:1039-44. [PubMed]
- Dahan L, Mariette C, Ychou M, et al. Neoadjuvant chemoradiotherapy with 5-fluorouracil-cisplatin combined with cetuximab in patients with resectable locally advanced esophageal carcinoma: A prospective phase I/II trial (FFCD-PRODIGE 3)—Preliminary phase II results. J Clin Oncol 2012;30;abstr 4091.
- Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010;376:687-97. [PubMed]
- Safran H, Dipetrillo T, Akerman P, et al. Phase I/II study of trastuzumab, paclitaxel, cisplatin and radiation for locally advanced, HER2 overexpressing, esophageal adenocarcinoma. Int J Radiat Oncol Biol Phys 2007;67:405-9. [PubMed]
- Safran H, DiPetrillo T, Nadeem A, et al. Trastuzumab, paclitaxel, cisplatin, and radiation for adenocarcinoma of the esophagus: a phase I study. Cancer Invest 2004;22:670-7. [PubMed]
- Baselga J, Cortés J, Kim SB, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med 2012;366:109-19. [PubMed]
- Gianni L, Pienkowski T, Im YH, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol 2012;13:25-32. [PubMed]
- Collette L, Bosset JF, den Dulk M, et al. Patients with curative resection of cT3-4 rectal cancer after preoperative radiotherapy or radiochemotherapy: does anybody benefit from adjuvant fluorouracil-based chemotherapy? A trial of the European Organisation for Research and Treatment of Cancer Radiation Oncology Group. J Clin Oncol 2007;25:4379-86. [PubMed]
- Habr-Gama A, Perez RO, Nadalin W, et al. Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: long-term results. Ann Surg 2004;240:711-7; discussion 717-8. [PubMed]
- Maas M, Beets-Tan RG, Lambregts DM, et al. Wait-and-see policy for clinical complete responders after chemoradiation for rectal cancer. J Clin Oncol 2011;29:4633-40. [PubMed]


