The effects of illness beliefs and chemotherapy impact on quality of life in Japanese and Dutch patients with breast or lung cancer
Introduction
Over time, a diagnosis of cancer may have many consequences, for instance, surgery, radiation, chemotherapy, remission, progression, palliative care, and death. Different patients will experience different chains of events over different periods of time, and different patients may respond differently under similar circumstances. Such responses are mediated by the perceptions and beliefs that patients have about their illness. As Petrie and Weinman asserted, these beliefs are organized in cognitive models or mental representations that “directly influence the individual’s emotional response to the illness and their coping behavior ..” (1). Therefore, it is important to assess a patient’s illness beliefs. Several instruments have been designed to do so, in particular the Revised Illness Perception Questionnaire by Moss-Morris et al. (2) and the Brief Illness Perception Questionnaire (B-IPQ) by Broadbent et al. (3). These questionnaires measure eight different illness beliefs: consequences, timeline, personal control, treatment control, identity, concern, coherence, and emotional response.
Health related quality of life (HRQOL)
An important aspect of illness and illness behavior is the patient’s HRQOL. HRQOL is a multidimensional construct pertaining to the physical, mental, and social condition of the patient. Over time, many instruments have been developed to measure this construct in patients suffering from cancer. One such instrument, the Quality of Life Questionnaire of the European Organization for Research and Treatment of Cancer (the EORTC QLQ-C30; QLQ-C30, for short), has become one of the standard instruments for measuring HRQOL in patients with any form of cancer (4). The QLQ-C30 yields 15 scales that measure various aspects of HRQOL.
Illness perceptions
It is generally understood that illness beliefs and HRQOL are related (5-8). As explained by the self-regulation theory developed by Leventhal and his co-workers (9), HRQOL is influenced by the beliefs and perceptions that patients hold about their illness. Both illness perceptions and HRQOL are influenced by the information that is provided about the disease (10,11). McCorry et al. have suggested, that this interrelatedness might be clinically applied “via intervention to improve psychological well-being” (12). Possibly also HRQOL can be improved in the same way.
Cross-cultural differences
As explained by Dein, illness beliefs are influenced by culture, that is, “each culture has its own system of beliefs, perceptions, and ideas about health and illness” (13). Therefore, a relevant question is how the belief systems of patients in different cultures differ from each other.
A recent publication by Kaptein et al. (14) reported that Japanese and Dutch patients with non-small-cell lung cancer showed only few differences, both with respect to B-IPQ illness beliefs and with respect to HRQOL as measured by the QLQ-C30. Among the beliefs, the only significant cultural differences occurred on personal control (how much control do you feel you have over your illness?) and treatment control (how much do you think your treatment can help your illness?) with higher mean score for the Japanese patients. Among the QLQ-C30, the Dutch patients more often had more favorable subscale means than the QLQ-C30 international reference values (15), while the Japanese patients generally had less favorable mean scores. In addition, the mean scores on global health/QL status, emotional functioning (EF), social functioning (SF), constipation (CO), and financial difficulties (FI) of the Japanese patients were significantly less favorable than those of the Dutch patients.
In a study with Japanese and Dutch breast cancer patients by Kaptein et al. (16), the only significant difference among the B-IPQ illness beliefs occurred on concern (how concerned are you about your illness?) with a higher mean score for Japanese women. On the QLQ-C30, the Dutch patients had significantly higher means on fatigue, while the Japanese women had higher means on diarrhea and FI. Overall, the QLQ-C30 subscale means of both the Japanese and the Dutch patient were close to the international reference values (15).
In a cross-cultural study by Kleijn et al. (17) the QLQ-C30 scale quality of life (global health/QL) was the only subscale on which a significant difference between Japanese and Dutch patients with various types of cancer (stomach, lung, breast, colorectal, or prostate) occurred, with a higher mean score for the Dutch patients. Interestingly, the two groups did differ significantly on the four scales of a Japanese QL questionnaire, which “clearly contains items that reflect Japanese explanatory models about patients’ responses to cancer” (17). Such items are lacking in Western oriented ‘international’ questionnaires.
The fact that no strong cultural difference were found in the above studies, could indicate that individual differences among patients within a culture are more important than group differences between cultures. As noted above, different patients will experience different chains of events over different periods of time, therefore it is relevant to investigate individual patient differences in more detail as they develop over time.
Individual differences among patients over time
In the field of breast and lung cancer, several longitudinal studies focused on long-term psychological adjustment and distress, that is, the constellation of social, psychological, and psychiatric problems of the (ex)patients (18). The recognition that cancer survivors have different patterns of adjustment has led to the identification of (clusters of) individual trajectories, that is, different sequences of responses over time (12,19-26). Because those studies were concerned with relatively long periods after diagnosis and initial therapy of breast or lung cancer, with follow-up times of several months or even years, it is still unknown whether systematic differences among individual patient trajectories can also be delineated in the earlier phases of cancer and its therapy. Although Kaptein and his coworkers (14,16) assessed HRQOL on three occasions in the early beginning of chemotherapy regimens, they focused on the average trajectories of the patients and not on individual differences.
Goals of the present study
In this article we further investigated the data collected among Japanese and Dutch patients by Kaptein and his colleagues (14,16) in a cross-cultural study of illness beliefs, HRQOL, and differences among patient trajectories during the early cycles of chemotherapy. The goals of the present study were to examine the following issues: (I) differences in illness beliefs between Japanese and Dutch patients in conjunction with their diseases (breast or lung cancer); (II) differences in HRQOL between Japanese and Dutch patients in conjunction with their diseases; (III) group (i.e., country and disease) and individual patient differences among the trajectories of HRQOL in the beginning of chemotherapy; (IV) the impact of illness beliefs and other patient characteristics on HRQOL trajectories.
Patients and methods
Patients
In this study, the data were obtained from 22 Japanese and 24 Dutch non-small-cell lung cancer patients and 21 Japanese and 22 Dutch patients with breast cancer. All patients received a chemotherapy regimen. This international research project was approved by the Medical Ethical Committee of the Leiden University Medical Centre, Leiden, The Netherlands, and by the Internal Review Board of the Saitama International Medical Centre, Hidaka City, Japan. In both institutions, patients gave informed consent. The present article is one of a series of publications on this project (14,16,27).
Questionnaires
Immediately before their first chemotherapy cycle (week 0), one week after their first chemotherapy cycle (week 1), and 8 weeks after the start of chemotherapy (week 8), patients completed the QL questionnaire of the European Organization for Research and Treatment of Cancer, the EORTC QLQ-C30 (QLQ-C30, for short) (4,28). On week 0 they completed the B-IPQ (3).
The QLQ-C30 was scored according to the manual (29). This yielded measures on global health/QL, physical functioning (PF), role functioning (RF), SF, cognitive functioning (CF), EF, and on the nine symptom scales fatigue (FA), nausea/vomiting (NV), pain (PA), dyspnoea (DY), insomnia (SL), appetite loss (AP), CO, diarrhea (DI), and FI. Using the procedure described by van der Kloot et al. (27), PF and RF were averaged to form a new scale labeled PRF (for physical and RF), thus combining two related scales into one. EF and CF were also averaged to obtain a new scale labeled PSY (for psychological functioning). All symptom scores were averaged as well, yielding a new variable SYM (for symptomatology). Subsequently, QL, SF, PRF, PSY, and SYM were reduced by categorical principal component analysis (CATPCA) (30) to two scores on two principal components, each with mean 0.0 and standard deviation (SD) 1.0. The first component can be interpreted as a generalized HRQOL dimension, the second component is considered to measure psychological well-being (PSYQOL) that is not related to physical health. We call those components GENQOL and PSYQOL, respectively. An adequate approximation of the component scores can be obtained by standardizing the values [QL + PF + RF + SF + CF + EF − (FA + NV + PA + DY + SL + AP + CO + DI + FI)/9] and [CF + EF − (QL + RF)/2], respectively (27). (Note: the part that is subtracted in the latter formula suppresses the contribution of the general QL dimension from the psychological dimension).
The B-IPQ consists of eight questions that measure eight dimensions of illness perception: consequences (how much does your illness affect your life?), timeline (how long do you think your illness will continue?), personal control (how much control do you feel you have over your illness?), treatment control (how much do you think your treatment can help your illness?), identity (how much do you experience symptoms from your illness?), concern (how concerned are you about your illness?), coherence (how well do you feel you understand your illness?), and emotional response (how much does your illness affect you emotionally? e.g., does it make you angry, scared, upset or depressed?). The responses are measured on a scale from 0 (not at all) to 10 (very much). The Dutch and Japanese versions of the B-IPQ can be found on www.uib.no/ipq.
In addition, the type of cancer and the cancer stage classification of the Union for International Cancer Control (UICC) were registered. At week 0, physicians rated the Karnofsky performance status (0% = dead, .., 100% = normal) (31).
Statistical analyses
The four goals of this study were pursued as follows. Study goal 1: in order to investigate cultural and disease differences and their interaction among illness beliefs, multivariate and univariate analyses of variance (MANOVA, ANOVA) were run with the eight belief dimensions as the dependent variables and country and disease as the independent variables. Study goal 2: in order to investigate cultural differences among HRQOL trajectories in conjunction with cancer type, the scores on the dependent variables GENQOL and PSYQOL were broken down by countries, diseases, and weeks (i.e., 0, 1 and 8). MANOVA and ANOVA were used to test for differences among the latter variables and their interaction. Study goal 3: trajectories of individual patients were graphically inspected. Study goal 4: multilevel analysis (MLA) (32) was used to investigate the relationship between illness beliefs and other patient characteristics on the one hand and individual patients’ HRQOL trajectories on the other hand.
Results
Patient characteristics
Respondents with non-small-cell lung cancer were 17 male and 5 female Japanese patients (mean age and SD: 63.0±6.6 years) and 16 male and 8 female Dutch patients (mean age and SD: 63.3±9.7 years). The respondents with breast cancer were 21 Japanese women (mean age: 49.9±9.6 years) and 22 Dutch women (mean age: 46.8±7.8 years). Two Japanese patients (one lung cancer; one breast cancer) had missing data on all QLQ-C30 questions in weeks 1 and 8 and were deleted from the analyses. Seven Japanese lung cancer patients had completely missing QLQ-C30 data on week 8 and some Japanese and Dutch patients had missing data on one or on two QLQ-C30 variables on one of the three occasions. Table 1 summarizes several clinical data for each country and illness (type of cancer, UICC stage of cancer, mean Karnofsky ratings by doctors) and contains the mean scores of the patients on GENQOL and PSYQOL. Among the Japanese lung cancer patients were more patients with adenocarcinoma and fewer patients with squamous cell carcinoma than in the Dutch group. This difference was statically significant (χ2=4.763; df=1; P=0.029). The distributions of UICC cancer stage among Japanese and Dutch lung cancer patients did not differ from each other. In the breast cancer patients, lobular carcinoma did not occur in the Japanese group and occurred in only two of the Dutch patients. Both groups had 20 patients with ductal breast cancer, and thus were virtually identical. However, the distribution of UICC cancer stage in the Japanese breast cancer patients differed from that of the Dutch patients. In Japan the majority of patients had stage IIB or IIIA carcinoma, whereas the tumors of the Dutch patients were predominantly in stages I and IIA. This difference [after forming three groups (I, IIA), (IIB), and (IIIA, IIIB)] is statistically significant (χ2=7.244; df=2; P=0.027). Among the lung cancer patients, no significant differences were found between the mean age of the Japanese and the Dutch groups, nor between the mean Karnofsky scores. Among the breast cancer patients, the Japanese group did not differ significantly from the Dutch group with respect to age, but did so with respect to the mean Karnofsky score, with a higher mean for the Japanese patients (F[1.35]=27.642; P<0.001).
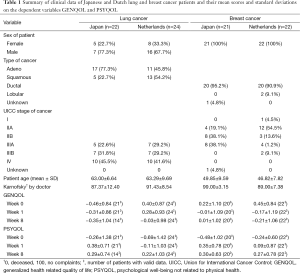
Full table
Cultural and disease differences among illness beliefs
A MANOVA was performed with countries and diseases as between-patient factors and the eight dimensions of the B-IPQ as the dependent variables. Multivariate tests indicated a significant difference between the Japanese and Dutch patients (F[8,64]=2.438; P=0.023) and a near significant difference between the country × disease combinations (F[8,64]=1.943; P=0.069). Univariate ANOVAs on the B-IPQ dimensions, which were run to interpret the multivariate differences, showed that the Japanese patients had higher mean scores than the Dutch patients on concern (8.73 vs. 7.20; P=0.006) and to a lesser extent on time line (6.84 vs. 5.75; P=0.053). The (near significant) multivariate difference among the country × disease combinations were limited to treatment control (P=0.008). On this variable, the mean of the Japanese lung cancer patients was significantly higher than that of the Dutch lung cancer patients (8.21 vs. 6.82; P=0.043), whereas the means of the Japanese and Dutch breast cancer patients did not differ significantly from each other (7.65 vs. 8.32; P=0.322).
Cultural and disease differences among HRQOL scores
A MANOVA was performed with countries and diseases as between-patient factors, weeks as within-patient factor, and GENQOL and PSYQOL as the dependent variables. The multivariate tests only showed statistically significant differences between the weeks (F[2,74]=10.206; P<0.0001). Univariate ANOVAs showed that a difference between weeks occurred both on GENQOL and PSYQOL (F=5.88, P=0.012 and F=17.88, P<0.0001, Huynh-Feldt df=1.7, 128.7) and consisted of significant differences between week 0 and week 1 (GENQOL: F[1,75]=4.72, P=0.033; PSYQOL: F[1,75]=17.05, P<0.0001). Differences between week 1 and week 8 were not statistically significant. The significant differences between week 0 and week 1 consisted of a decrease in generalized quality of life (GENQOL) and an increase in PSYQOL.
Individual differences among HRQOL trajectories
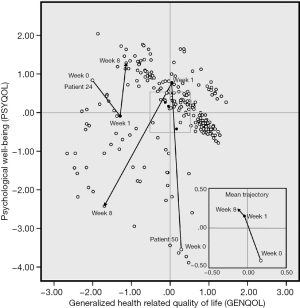
Of the 87 patients in our analyses, seven Japanese lung cancer patients had completely missing QLQ-C30 data on week 8, therefore the following results are based on 254 observations, that is, 3 observations for each of 80 patients and 2 observations for each of 7 patients. Figure 1 shows the scatter plot of GENQOL (horizontal) versus PSYQOL (vertical) of these 254 observations. Each small circle in this figure represents one patient on one of the three assessment occasions. For each patient one could draw an arrow from her or his location on week 0 to her or his location on week 1 and from there to this patient’s location on week 8. Obviously, this would yield an inextricable muddle of intersecting lines. Therefore, only two individual trajectories are shown in Figure 1, that is, the trajectories of one Japanese patient (patient 24) and one Dutch patient (patient 50), both with lung cancer. The small figure inserted in the lower right part of Figure 1 is an enlargement of the middle segment of the larger figure. The inserted figure displays the mean trajectory (i.e., the trajectory formed by the means of the weeks for all patients combined). It shows a continuing deterioration of general QL and an increase of PSYQOL over the weeks. The two individual trajectories demonstrate that substantial differences among individuals do occur.
Illness beliefs and other patient characteristics and HRQOL trajectories
In this section we address the question whether patients with different scores on a particular illness belief or other background variable follow different HRQOL trajectories (as measured by GENQOL and PSYQOL) in the period from week 0 to week 8. To answer this question, we ran several separate multilevel analyses (MLA) for longitudinal data (32) using the pooled data of all 87 patients.
As a trajectory from week 0 to week 1 consists of a single straight line, it can be described by four parameters: the starting value on GENQOL where the trajectory begins, the starting value on PSYQOL where the trajectory begins, the length of the trajectory (i.e., the amount of change), and the direction of the trajectory. Each individual patient’s trajectory can be described by those four parameters. In MLA it is investigated whether these individual parameter values can be predicted on the basis of the patient’s score on a particular covariate. This amounts to a regression problem that yield sets of four predicted parameters for each observed value of the covariate, and thus to predicted trajectories for each covariate value. The corresponding regression coefficients may be tested for statistical significance. Note that the sign of a coefficient indicates whether the parameter values are positively or negatively related to the values of the covariates.
The covariates used were sex, age, cancer stage, Karnofsky rating, and illness beliefs. Preliminary analyses showed that the effects of the covariates occurred only in the first period, that is, in the trajectories from week 0 to week 1. Therefore, we have run a second set of MLAs assuming that no change occurred in the second period (i.e., week 1 to week 8).
The MLAs showed that neither sex (tested separately in the lung cancer patients and in the combined group), nor age or cancer stage, had statistically significant effects on the trajectories. However, significant effects on one or more of the parameters were found for seven B-IPQ dimensions (consequences, timeline, identity, coherence, emotional response, treatment control, and concern) and for Karnofsky rating. The only illness belief without any significant contribution was personal control.
In this section we discuss the results of our MLAs by inspection of the graphs of Figure 2 that display the relationships between the covariates and the trajectories by depicting the predicted trajectories for each covariate value. Technical and statistical details of our analyses are presented in the appendix.
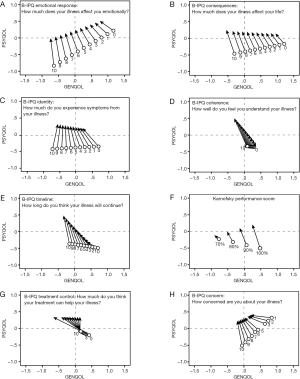
In Figure 2 (A through H; the order is in terms of effect size, see appendix) we have drawn the trajectories from week 0 to week 1 for different patient values of eight separate covariates. Each open circle marks the starting point that belongs to a particular value of the covariate and each arrow indicates the length and the direction of the corresponding (predicted) trajectory. For instance, Figure 2A contains 11 open circles that indicate the starting positions of the trajectories of patients with scores 0 to 10, respectively, on the B-IPQ dimension emotional response. The average patient with an emotional response score of 0 lies above and to the right of patients with scores 1 through 10 on emotional response. The same is true for the positions where the arrows end. This figure is an example of a covariate’s effect on the starting points only, since length and direction are virtually the same for all trajectories.
The covariate with the largest effect size or explanatory value was B-IPQ emotional response. Patients with higher emotional response scores had significantly (P=0.001) lower starting values both on GENQOL and PSYQOL. This is shown in Figure 2A, where the trajectory starting points of patients with responses 0 through 10 on emotional response are clearly ordered from right to left, that is, on week 0, patients with higher emotional responses started and ended with a lower GENQOL and lower PSYQOL. Irrespective of their emotional response scores, the patients showed the same amount and direction of change from week 0 to week 1, as indicated by the (almost) parallel lines of (almost) equal lengths in Figure 2A that represent the trajectories of patients with different scores on emotional response. For all values of emotional response, the change is in the direction of less GENQOL and more PSYQOL, with patients who experience fewer emotional responses starting and ending with more favorable HRQOL.
The covariate with the one but largest effect was B-IPQ consequences. As illustrated in Figure 2B, higher scores on consequences go together with significantly (P<0.05) lower starting points both on GENQOL and PSYQOL, although the differences among the starting points on PSYQOL are smaller than those on GENQOL. On week 0, patients who experienced more consequences from their illness started with proportionally lower scores on GENQOL and, to a lesser extent, on PSYQOL. The apparent differences in length and direction of the 11 trajectories show, were not statistically significant. As with emotional response, for all values of consequences the change is in the direction of less GENQOL and more PSYQOL, with patients who experience fewer consequences starting and ending with more favorable GENQOL.
For B-IPQ identity as well, a significant effect on the two starting points (P<0.05) was found. Figure 2C shows that on week 0, patients who experienced more symptoms from their illness had proportionally lower starting scores on GENQOL. Here, however, they had virtually the same starting values on PSYQOL. The apparent differences in direction of the 11 trajectories were not statistically significant. Thus, patients with more symptoms, both in week 0 and week 1, reported a lower GENQOL. They did not differ with respect to PSYQOL in week 0 or week 1, but their PSYQOL improved—independently of their identity score—after the first chemotherapy session.
As higher scores on B-IPQ coherence indicate that one has a better understanding of one’s illness, it is to be expected that such higher scores would coincide with higher scores on HRQOL. In Figure 2D it appears that this expectation is not correct, because the arrows for the covariate values 0 to 10 are ordered from right to left. However, the starting point differences on GENQOL are not statistically significant. Thus, statistically speaking, the arrows emerge from one and the same point. However, B-IPQ coherence did have a significant positive effect on trajectory length (P<0.05), that is, patients with higher coherence scores reported a larger positive change on PSYQOL than patients with lower coherence scores. Thus, the more insight in one’s disease, the more effect of the first treatment.
Figure 2E shows that the starting points with respect to GENQOL differ proportionally with B-IPQ timeline (although, strictly speaking, not statistically significant; P<0.10) and that they are almost equal on PSYQOL. Moreover, the slopes of the trajectories appear to become steeper and the lengths of the trajectories longer with higher scores on timeline (P<0.10). Thus, patients who believe that their illness will last longer seem to have the largest improvement in PSYQOL after the first chemotherapy session.
In the MLA of Karnofsky performance rating we deleted the single patient who had a Karnofsky rating of 50% because we feared he might cause an outlier effect. In this analysis (see Figure 2F) patients with higher Karnofsky ratings start—as is to be expected—higher on GENQOL. Patients with lower Karnofsky scores have proportionally lower values on GENQOL but are higher on PSYQOL (P<0.01). Although the trajectory lengths of patients with lower Karnofsky scores (i.e., more serious disabilities) seem to shorten, this effect is not statistically significant. The figure shows that even in patients with relatively high Karnofsky scores improvement on PSYQOL did occur.
As higher scores on B-IPQ treatment control indicate more confidence in the treatment one receives, it is expected that such higher scores would coincide with better HRQOL. Figure 2G shows that this expectation is not met: higher covariate scores seem to go together with lower scores on GENQOL. However, this effect is not statistically significant. Statistically, all arrows emerge from one and the same point. However, a significant length and direction effect (P<0.05) was found, that is, the trajectories of patients with different perceptions of treatment control had different lengths and different slopes. Figure 2G shows that all patients, irrespective of their beliefs on treatment control, had (statistically) similar starting points both on GENQOL and PSYQOL. Their final locations on week 1 were virtually the same with respect to PSYQOL. However, with respect to GENQOL, the final locations were proportional to the treatment control beliefs. Patients who perceived less treatment control thus showed a larger decrease in GENQOL after their first chemo treatment and no change on PSYQOL.
For B-IPQ concern (Figure 2H) two significant results were found: a starting point effect (P<0.01) indicating that patients who are more concerned about their disease have lower scores on GENQOL and on PSYQOL. There is also a significant length effect (P<0.05), that is, patients with lower values on concern showed less change from week 0 to week 1 than patients who were more concerned. Thus, patients with higher scores on B-IPQ concern are lower on GENQOL and at the same time improve more on PSYQOL between week 0 and week 1. Note that the apparent differences among the slopes are not statistically significant.
Discussion
In this study we investigated cultural and disease differences with respect to illness beliefs and HRQOL of Japanese and Dutch patients with breast cancer or lung cancer. In addition we studied individual differences among patients with respect to changes in HRQOL on three assessment occasions before and during chemotherapy. We used measures that summarize EORTC QLQ-C30 responses in two dimensions: a general quality of life component (GENQOL) and a psychological component that measures PSYQOL not related to physical health (PSYQOL). We found that this type of PSYQOL was subject to changes over a relatively short period of time. Therefore, PSYQOL differs from the more stable trait-depression or trait-anxiety, and from the personality traits pessimism and optimism. The malleability of PSYQOL suggests that this dimension measures a state-like psychological characteristic.
Illness beliefs
With respect to illness beliefs, we found that, on average, the Japanese patients were more concerned about their disease and expected that their illness would last longer. This finding is not easily explained. Because the Japanese breast cancer patients, predominantly, had lower cancer stages than the Dutch patients, one would expect less concern and a shorter time line.
The fact that Japanese lung cancer patients believed that their treatment would have more effect on their illness than the Dutch patients, could suggest that Japanese patients have more confidence in the doctors’ authority.
Health related quality of life (HRQOL)
With respect to QL, we found that Japanese and Dutch patients did not differ from each other on GENQOL and PSYQOL; neither were differences found between the cancer types. We only established an occasion effect distinguishing the first assessment occasion (week 0; immediately before the start of chemotherapy) from the second and third occasions (week 1 and week 8). That the last two occasions did not differ significantly may be explained by the cyclical character of chemotherapy and its effects. The data of week 1 were collected one week after the first chemotherapy session. As chemotherapy usually is administered in cycles of 3 to 4 weeks, week 8 comes between one or two weeks after the third session. Probably, QL is then comparable to QL after the first administration because adverse events will be comparable.
The mean trajectory of all patients shows a deterioration of general HRQOL and an increase of PSYQOL over the weeks. Plausibly, the increase in PSYQOL indicates that—despite a decreased physical QL—patients were happy or relieved that something was being done against their cancer and became more hopeful. Deshields et al. (33) mentioned that increased depression and anxiety after the termination of treatment may be due to “the loss of the ‘medical safety net’, the loss of treatment as a form of ‘active coping’, diminished support of family and friends, and fear of recurrence” (33). The increased PSYQOL that we found after the beginning of chemotherapy, thus may reflect the perception of a ‘medical safety net’, a feeling of active coping by undergoing treatment, and support from one’s social environment. Fear of recurrence probably is not yet relevant. An interesting question is whether this phenomenon is an early form of “positive adjustment”, a term introduced by Boot et al. to replace some “commonly used terms [that] include ‘posttraumatic growth’, ‘benefit finding’ and ‘stress-related growth’” (19).
Individual differences among QL trajectories and their covariates
Using MLA to explore the trajectories of individual patients in the two-dimensional space of GENQOL and PSYQOL we discovered that those trajectories are subject to large variations among the patients with regard to starting points, directions, and lengths of movement. The most important variations occurred in the period of week 0 to week 1. We have attempted to explain these variations by including—one at a time—age, sex, cancer stage, Karnofsky rating, and the eight dimensions of the IPQ-B, consequences, timeline, personal control, treatment control, identity, concern, coherence and emotional response. Because the most important effects occurred in the period of week 0 to week 1, we have run all MLAs assuming that there were no differences between week 1 and week 8.
Significant covariations were found between the trajectories of the patients and their scores on the B-IPQ variables consequences, timeline, treatment control, concern, and emotional response, and on Karnofsky rating. Notwithstanding the differences, there are two common aspects among the effects of the explanatory variables. Firstly, the dominant direction is a decrease in general QL together with an improvement in PSYQOL. Secondly, patients with more dismal scores on some of the covariates (emotional response, consequences, identity, timeline, Karnofsky rating) have lower starting points on GENQOL and, in some cases (emotional response, consequences, concern), lower starting points on PSYQOL. In some cases (coherence, timeline, concern), patients with higher covariate scores show larger improvements on PSYQOL. In the case of coherence, it seems that the more one has insight in one’s disease, the more effect of the first treatment. With timeline and concern we can only speculate that patients with more gloomy beliefs are more susceptible to external influences, such as the benevolent expectations of chemotherapy.
Clinical relevance
It is generally recognized that illness beliefs and HRQOL are related and that illness perceptions have an influence on HRQOL (5-8). McCorry et al. suggested that this relationship could be clinically applied by offering “illness perception-based interventions” (12) to patients. In an earlier publication we have argued that “although doctors are used to discuss laboratory values, imaging results, and medication with the patient [they should also systematically review] the patient’s HRQOL during consultation” (27). On the basis of our present results, we recommend that well before cancer treatment is started, the patient’s illness beliefs are assessed. Such an assessment could be used in an attempt to create an optimal starting position for subsequent chemotherapy by influencing a patient’s illness beliefs, either by changing those beliefs—within the limits of realism—from grim to more confident, or by reinforcing already positive beliefs. Hopefully, this would improve the patient’s QL, both psychologically and physically. Such a psychological preparation would fit in the multimodal prehabilitation process advocated by Silver and Baima (34).
Conclusions
The present study, again, indicated that cultural differences between Japanese and Dutch cancer patients with respect to HRQOL and illness beliefs are not very strong nor very comprehensive. Greater concern among the Japanese patients is the clearest difference, but why this difference exists is a matter of speculation. Inspection of the individual differences among Japanese and Dutch cancer patients suggests that differences among individual patients are more important than cultural factors. This conclusion is supported by the fact that several individual illness beliefs explain the QL trajectories of individual patients. However, where the illness beliefs significantly explained some of the trajectory variations, the remaining parts of the patient variances were still substantial (see appendix). More and different explanatory variables are needed to account for the differences among the patients. Here one could think of coping style and other personality characteristics and of hospital, doctors, and other care related factors.
The major contributions of this paper are the demonstration that (I) systematic patient variations do exist and are substantial; (II) that they can be investigated (e.g., by means of MLA methodology); and (III) that illness beliefs are related to short term changes in HRQOL during the first chemotherapy cycles. The latter findings should be confirmed in future studies, as the present investigation has some limitations. A relatively small number of patients (even though they came from two countries with large cultural differences) and two forms of cancer were studied on only three occasions during chemotherapy. Moreover, a limited set of explanatory variables was employed. Future studies with more measurement occasions, different variables, different patients, and more types of cancer will tell us whether the present results can be generalized. Such studies should not necessarily be newly executed. Supposedly, many existing data sets on HRQOL in oncology could be reanalyzed with the scope and methodology of the present study.
Acknowledgements
The authors gratefully acknowledge all persons, patients included, who have contributed to the design and data collection of this international research project. We want to thank Dr. Tineke Willemsen for her constructive criticism and invaluable suggestions for improving the manuscript.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Supplementary
As stated in the main text, an individual’s trajectory from week 0 to week 1 is completely characterized by four parameters: two starting points (on the two dimensions GENQOL and PSYQOL, resp.) as well as by length and direction of the trajectory line. In order to perform our MLAs, a somewhat different characterization of a trajectory was necessary. Here, the beginning of a trajectory was quantified by (I) the average of the starting points on GENQOL and PSYQOL; and (II) the difference between these two values. Similarly, length and direction were quantified by; (III) the average value of the changes on GENQOL and PSYQOL, and (IV) the difference between these two change values. We denote these four parameters by the terms starting point average, starting point difference, average length, and differential length, respectively. Because there are huge differences between the trajectories of the individual patients, all four parameters vary between individuals. In our MLAs we investigated whether the individual parameters can be predicted on the basis of several explanatory variables. For instance: do patients with low Karnofsky scores have lower starting points on GENQOL and PSYQOL, and/or do they have longer or shorter trajectories, with or without different directions?
These questions are answered by four coefficients that indicate: (I) how the starting point average on GENQOL and PSYQOL changes for different values of the covariate; (II) how the starting point difference on GENQOL and PSYQOL changes for different values of the covariate; (III) how the average length of the trajectory changes for different values of the covariate; and (IV) how the differential length, that is, the difference in displacement on GENQOL and PSYQOL, respectively, changes for different values of the covariate. Each of those coefficients indicates the amount of change per unit of the covariate.
In a graphical representation of the MLA results, as in Figure 2, an average starting point effect occurs when the starting points (the white circles) lie on a straight line with an angle of almost 45° with both axes. A starting point difference effect would show up by this line running more horizontally or vertically. An average length effect would be reflected by trajectories getting shorter or longer in proportion with the covariate scores. A differential length effect goes together with different directions of the trajectories.
In MLA the four trajectory parameters are used to specify several models: (I) a basic model in which each parameter is assumed to be constant for all patients; (II) an individual differences model in which all parameters are allowed to vary over patients; and (III) several explanatory models in which the patient parameters are modeled on the basis of the patient scores one or more covariates. Each model can be evaluated by its (lack of) statistical fit, the deviance. Different (nested) models can be compared by evaluating the difference, Δdeviance, between their respective fit values. The statistic Δdeviance follows a χ2-distribution with up to four degrees of freedom (i.e., the number of covariate coefficients). In addition, each separate effect coefficient is also tested for significance (Singer & Willet, 2003). Moreover, the covariate’s importance can be assessed by comparing the variances of the individual patients’ trajectory parameters after and before including the covariate in the analysis. If the after-variance is substantially smaller than the before-variance, the covariate obviously has an effect.
In the first MLA (of the basic model) we assumed there were no individual patient differences at all, that is, that all individual trajectories would be undistinguishable. In the second analysis (of the individual differences model) the four parameters for starting points, length, and direction were allowed to vary between patients. The results showed that the individual differences in the data were substantial, as witnessed by a large Δdeviance between the first and the second analysis (Δdeviance=175.778; df=15; P<0.0005) and by the fact that all patient variances of the four parameters were statistically significant at P<0.01.
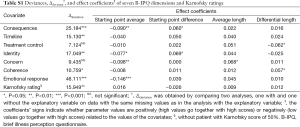
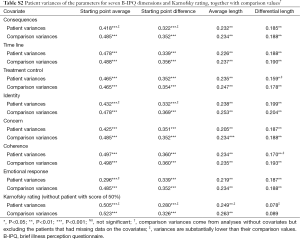
Additional MLAs were performed by including—one at a time—the explanatory variables sex, age, cancer stage, Karnofsky rating, and eight illness beliefs. The effects of including such explanatory variables or covariates are expressed by four coefficients that indicate: (I) how the starting point average on GENQOL and PSYQOL increases for different values of the covariate; (II) how the starting point difference is changed by different values of the covariate; (III) how the average length of the trajectory is changed per unit of the covariate; and (IV) how the differential length, that is, the difference in displacement on GENQOL and PSYQOL, respectively, changes per unit of the covariate. The numerical outcomes of our analyses are displayed in Table S1 (the coefficients of the covariates) and Table S2 (the variances of the patients’ parameters).
Full table
Full table
As Table S1 shows, seven of the covariates used had an overall significant effect on the trajectories; the coefficients in this table indicate which particular parameters were influenced. We have used the latter results to interpret the graphs of Figure 2. In two cases, B-IPQ treatment control and B-IPQ concern, there was no overall significance of the covariate, although several separate coefficients did reach statistical significance and were used in our interpretations.
The most striking feature of Table S2 is that, with the exception of Karnofsky scores, all patient variance remain statistically significant after inclusion of the covariates. This indicates that more explanatory variables are needed to explain the (large) individual differences among the trajectories. Even though still significant, we notice several substantial drops in some of the patient variances after inclusion of the covariates (i.e., in consequences, treatment control, identity, coherence, emotional response, Karnofsky rating). In general these drops corroborate the conclusions we drew on the basis of the coefficients in Table S1.
References
- Petrie KJ, Weinman J. Why illness perceptions matter. Clin Med (Lond) 2006;6:536-9. [PubMed]
- Moss-Morris R, Weinman J, Petrie KJ, et al. The Revised Illness Perception Questionnaire. Psychol Health 2002;17:1-16.
- Broadbent E, Petrie KJ, Main J, et al. The brief illness perception questionnaire. J Psychosom Res 2006;60:631-7. [PubMed]
- Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A quality of life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993;85:365-76. [PubMed]
- Chilcot J. The importance of illness perception in end-stage renal disease: associations with psychosocial and clinical outcomes. Semin Dial 2012;25:59-64. [PubMed]
- Foxwell R, Morley C, Frizelle D. Illness perceptions, mood and quality of life: a systematic review of coronary heart disease patients. J Psychosom Res 2013;75:211-22. [PubMed]
- Kaptein AA, Scharloo M, Helder DJ, et al. Representations of chronic illness. In: Cameron L, Leventhal H, editors. The self-regulation of health and illness behavior. New York: Routledge, 2003:97-118.
- Scharloo M, Baatenburg de Jong RJ, Langeveld TP, et al. Illness cognitions in head and neck squamous cell carcinoma: predictiong quality of life outcome. Support Care Cancer 2010;18:1137-45. [PubMed]
- Cameron L, Leventhal H, editors. The self-regulation of health and illness behavior. New York: Routledge, 2003.
- Husson O, Mols F, van de Poll Franse LV. The relation between information provision and health-related quality of life, anxiety and depression among cancer survivors: a systematic review. Ann Oncol 2011;22:761-72. [PubMed]
- Husson O, Thong MS, Mols F, et al. Illness perceptions in cancer survivors: what is the role of information provision? Psychooncology 2013;22:490-8. [PubMed]
- McCorry NK, Dempster M, Quinn J, et al. Illness perception clusters at diagnosis predict psychological distress among women with breast cancer at 6 months post diagnosis. Psychooncology 2013;22:692-8. [PubMed]
- Dein S. Explanatory models of and attitudes towards cancer in different cultures. Lancet Oncol 2004;5:119-24. [PubMed]
- Kaptein AA, Yamaoka K, Snoei L, et al. Illness perceptions and quality of life in Japanese and Dutch patients with non-small-cell lung cancer. Lung Cancer 2011;72:384-90. [PubMed]
- Scott NW, Fayers PM, Aaronson NK, et al. EORTC QLQ-C30 reference values. 2008. Retrieved October 6, 2014. Available online: http://groups.eortc.be/qol/sites/default/files/img/newsletter/reference_values_manual2008.pdf
- Kaptein AA, Yamaoka K, Snoei L, et al. Illness perceptions and quality of life in Japanese and Dutch women with breast cancer. J Psychosoc Oncol 2013;31:83-102. [PubMed]
- Kleijn WC, Ogoshi K, Yamaoka K, et al. Conceptual equivalence and health-related quality of life: an exploratory study in Japanese and Dutch cancer patients. Qual Life Res 2006;15:1091-101. [PubMed]
- Holland J, Watson M, Dunn J. The IPOS new international standard of quality cancer care: integrating the psychosocial domain into routine care. Psychooncology 2011;20:677-80. [PubMed]
- Boot JS, Holcombe C, Salmon P. Positive adjustment to breast cancer: development of a disease-specific measure and comparison of women diagnosed from 2 weeks to 5 years. Psychooncology 2010;19:1187-94. [PubMed]
- Deshields T, Tibbs T, Fan MY, et al. Ending treatment: the course of emotional adjustment and quality of life among breast cancer survivors immediately following radiation therapy. Support Care Cancer 2005;13:1018-26. [PubMed]
- Donovan KA, Gonzalez BD, Small BJ, et al. Depressive symptom trajectories during and after adjuvant treatment for breast cancer. Ann Behav Med 2014;47:292-302. [PubMed]
- Dunn LB, Cooper BA, Neuhaus J, et al. Identification of distinct depressive symptom trajectories in women following surgery for breast cancer. Health Psychol 2011;30:683-92. [PubMed]
- Helgeson VS, Snyder P, Seltman H. Psychological and physical adjustment to breast cancer over 4 years: identifying distinct trajectories of change. Health Psychol 2004;23:3-15. [PubMed]
- Lam WW, Bonanno GA, Mancini AD, et al. Trajectories of psychological distress among Chinese women diagnosed with breast cancer. Psychooncology 2010;19:1044-51. [PubMed]
- Murray SA, Kendall M, Grant E, et al. Patterns of social, psychological, and spiritual decline toward the end of life in lung cancer and heart failure. J Pain Symptom Manage 2007;34:393-402. [PubMed]
- Wang XS, Fairclough DL, Liao Z, et al. Longitudinal study of the relationship between chemoradiation therapy for non-small-cell lung cancer and patient symptoms. J Clin Oncol 2006;24:4485-91. [PubMed]
- van der Kloot WA, Kobayashi K, Yamaoka K, et al. Summarizing the fifteen scales of the EORTC QLQ-C30 Questionnaire by five aggregate scales with two underlying dimensions: a literature review and an empirical study. J Psychosoc Oncol 2014;32:413-30. [PubMed]
- Kobayashi K, Takeda F, Teramukai S, et al. A cross-validation of the European Organization for Research and Treatment of Cancer QLQ-C30 (EORTC QLQ-C30) for Japanese with lung cancer. Eur J Cancer 1998;34:810-5. [PubMed]
- Fayers PM, Aaronson NK, Bjordal K, et al. The EORTC QLQ-C30 Scoring Manual. 3rd ed. Brussels: European Organization for Research and Treatment of Cancer, 2001.
- Linting M, Meulman JJ, Groenen PJ, et al. Nonlinear principal components analysis: Introduction and application. Psychol Methods 2007;12:336-58. [PubMed]
- Karnofsky DA, Abelmann WH, Craver LF, et al. The use of the nitrogen mustards in the palliative treatment of carcinoma. Cancer 1948;1:634-56.
- Singer JD, Willett JB. Applied longitudinal data analysis. New York: Oxford University Press, 2003.
- Deshields T, Tibbs T, Fan MY, et al. Differences in patterns of depression after treatment for breast cancer. Psychooncology 2006;15:398-406. [PubMed]
- Silver JK, Baima J. Cancer prehabilitation: An opportunity to decrease treatment-related morbidity, increase cancer treatment options, and improve physical and psychological health outcomes. Am J Phys Med Rehabil 2013;92:715-27. [PubMed]


