Targeted therapy in non-small cell lung cancer: a focus on epidermal growth factor receptor mutations
Lung cancer represents the main tumoral pathology with a high mortality (1). The last ten years have seen the emergence of histology (squamous cell vs. non squamous cell) as a determining factor for the management of lung cancer. But, above all, an important proportion of patients may now benefit of a molecular characterisation of their tumoral lesions which can be treated with targeted therapy (on mutations, on fusion genes). Currently, the majority of the molecular targets concerned by this therapeutic strategy are found in tumors which are of adenocarcinoma type.
Background
The main molecular targeting of lung cancer [non-small cell lung cancer (NSCLC)] concerns mutations of epidermal growth factor receptor (EGFR). The first applied tyrosine kinase inhibitors (TKIs) like erlotinib and gefitinib have a preferential activity against activating EGFR mutations of lung cancer, these agents have been the first to open the era of targeted therapy of lung cancer in the beginning of 2000 (2). Of note, the presence of these mutations is globally at relatively low frequency in NSCLC with the occurrence in 17% of Caucasian patients and 40% of Asian patients of targetable EGFR mutations and around 6% of patients with the ALK translocation. The awaited responsiveness of tumors carrying these mutations is high with for instance 60% to 80% to TKIs hitting EGFR mutations (3). After an initial and satisfactory response to EGFR TKIs, almost all patients present a phenomenon of resistance manifested by tumoral progression evident after 9 to 12 months (4). Focused genotyping analyses performed on biopsy samples of resistant patients with acquired resistance have put the light on the EGFR T790M as a secondary mutation as responsible for the occurrence of this resistance phenomenon. This secondary mutation is occurring in almost 60% of resistant tumors (4). The mechanism of action by which the resistance is playing involves a conformational modification in the ATP pocket of the EGFR itself giving to the active site more affinity towards ATP than gefitinib or erlotinib. As a primary site of acquired resistance EGFR T790M was an evident tempting target for drug developers facing an important medical need. In principle, a drug which would impact preferentially the mutant EGFR would spare adverse events carried by the presence of WT-EGFR. Not surprisingly a multitude of drugs have been produced and tested with this property of a specific binding at the EGFR T790M site. Afatinib is among these emerging drugs showing activity on this specific form of EGFR (4). More recently (5), a 3rd generation of drugs targeting specifically T790M were made available (AZD9291, CO1686…). To summarize at this stage, most EGFR mutations concern exon 19 deletions (Del 19) and L858R mutation in exon 21, they represent globally 90% of all mutations and are linked with sensitivity to EGFR TKIs. At the opposite, lung cancers exhibiting exon 20 insertions or T790 M in exon 20 are shown to be resistant to these drugs (5).
ALK targeting with crizotinib is offering 50% to 60% of objective response rate in patients whose tumor is carrying the ALK anomaly (3). A new generation of ALK TKIs are now of clinical use with ceritinib and alectinib. These drugs allow a new phase of therapeutic gain to be obtained in cases of resistance to crizotinib (6). Work is in progress in order to identify predictive factors for a resistance to crizotinib with candidates being numerous including growth factors, kinases, interacting proteins, transcription factors but no one among this large list is emerging currently with sufficient evidence (6). A second-generation of ALK inhibitors, with ceritinib as a concrete example, can overcome several crizotinib-resistant mutations and has shown efficacy both in vitro and in vivo with the use of pertinent laboratory models of acquired resistance to crizotinib. This is consistent with recent clinical data showing an evident activity of ceritinib in patients with crizotinib-resistant disease (7).
Mutation analyses
In France, the National Cancer Institute (INCa) is playing a preponderant role for putting at disposal and unifying the methods for the practice of molecular testing with clinical applications for the larger number possible of patients (see Table 1). The French territory is covered with regional platforms dedicated to the practice of molecular biology testing under the auspices of the INCa. Thus, the analytical need for the determination of molecular anomalies of therapeutic interest is taken into consideration and this is particularly true for lung cancer. The analysis is to be considered in its totality including not only the analytical aspect with a specific equipment but also the biological sample itself on which the analysis is applied. There is currently an evolution from the use of robust genotyping methods allowing the identification of given molecular anomalies (pyrosequencing for instance) towards the consideration of a much larger set of molecular anomalies under the form of a global genotyping realized with the use of next-generation sequencing (NGS) necessitating in the whole analysis the introduction of specialized step for data treatment. Currently the precise field of utilization of NGS between research and routine use remains to be elucidated. As said above another consideration to be paid to these molecular analyses concerns the tumoral material itself. This is particularly true in the domain of lung cancer where it is often difficult to obtain a tumoral sample in adequate conditions (access, optimal volume) when keeping also in mind the inherent problem of the intra-tumoral heterogeneity. In this context a perspective of amelioration is perceptible. This ray of hope is brought by the use of so-called “liquid biopsies” which is, practically speaking, the possibility to get tumoral DNA isolated from a blood sample. A recent work by Douillard et al. (8) is particularly illustrative on these aspects. The authors have compared, on the basis of almost one thousand of patients, the results of the analysis of EGFR mutations classically performed on the solid tumor in place (deletions exon 19 and point mutation L858R, as the most frequent ones) with those arising from tumoral DNA extracted from blood in parallel in the same patient. The authors reported an interesting high level of concordance higher than 90% for the cases in comparison (652 in total). These data have led the authors to conclude to a possible substitution plasma/tumor for the mutation analyses in lung cancer, a preference being however given to the intratumoral direct investigation if this one is feasible. Following the publication of these results, European health authorities have confirmed this possibility for the delivery of Iressa and Tarceva (EGFR TKIs on the market).

Full table
Conclusions
In total one can consider EGFR mutations in NSCLC as an illustrative example for targeted therapy in cancer care. In France this personalized treatment is made possible to a large number of patients thanks to the concrete and constant implication of the INCa. Table 2 is providing a complete list of gene mutations, all validated by the INCa, of concerns for the management of NSCLC with targeted therapy.
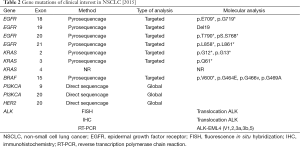
Full table
Acknowledgements
None.
Footnote
Conflicts of Interest: Honorarium from Merck Serono, Pierre Fabre Oncology, Roche, Novartis. Consulting with ONXEO, Nordic Pharma.
References
- Kerr KM, Bubendorf L, Edelman MJ, et al. Second ESMO consensus conference on lung cancer: pathology and molecular biomarkers for non-small-cell lung cancer. Ann Oncol 2014;25:1681-90. [PubMed]
- Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med 2005;353:123-32. [PubMed]
- Li T, Kung HJ, Mack PC, et al. Genotyping and genomic profiling of non-small-cell lung cancer: implications for current and future therapies. J Clin Oncol 2013;31:1039-49. [PubMed]
- Riely GJ, Yu HA. EGFR: The Paradigm of an Oncogene-Driven Lung Cancer. Clin Cancer Res 2015;21:2221-6. [PubMed]
- Kobayashi Y, Togashi Y, Yatabe Y, et al. EGFR Exon 18 Mutations in Lung Cancer: Molecular Predictors of Augmented Sensitivity to Afatinib or Neratinib as Compared with First- or Third-Generation TKIs. Clin Cancer Res 2015;21:5305-13. [PubMed]
- Wilson FH, Johannessen CM, Piccioni F, et al. A functional landscape of resistance to ALK inhibition in lung cancer. Cancer Cell 2015;27:397-408. [PubMed]
- Friboulet L, Li N, Katayama R, et al. The ALK inhibitor ceritinib overcomes crizotinib resistance in non-small cell lung cancer. Cancer Discov 2014;4:662-73. [PubMed]
- Douillard JY, Ostoros G, Cobo M, et al. Gefitinib treatment in EGFR mutated caucasian NSCLC: circulating-free tumor DNA as a surrogate for determination of EGFR status. J Thorac Oncol 2014;9:1345-53. [PubMed]



