Chinese Guidelines on the Diagnosis and Treatment of Melanoma (2015 Edition)
Introduction
Malignant melanoma is one of the most common malignancies. Its incidence grows rapidly at an annual rate of 3−5%. Although the incidence of melanoma remains low in China, it has increased rapidly, with approximately 20,000 new cases reported each year. The mortality of melanoma also has been increasing rapidly; in contrast, although the incidence of melanoma is also increasing in western countries, its mortality basically remains unchanged and does not rise along with the escalation in prevalence. Thus, there is still a wide gap between China and Western countries in the diagnosis and treatment of melanoma. Melanoma has become one of the diseases that pose a major threat to health of Chinese people. However, compared with other common malignant tumors, there is still a far way to go to achieve the standardized diagnosis and treatment of melanoma. In May 2007, the Chinese Society of Clinical Oncology (CSCO) formally established the CSCO Melanoma Panel with an attempt to promote the development of clinical oncology, facilitate the multidisciplinary standardized treatment for melanoma, advocate the active learning and application of currently available scientific evidences at home and abroad, and explore the development of Chinese guidelines on the clinical practices on melanoma. After consultations with multidisciplinary experts, the first edition of the Chinese Consensus on the Diagnosis and Treatment of Melanoma was released in 2008; in 2009, 2011, and 2013, three revisions of this consensus document were published after many multidisciplinary seminars. The past 5 years have witnessed several breakthroughs in the clinical treatment of melanoma. Melanoma has become one of the malignant tumors whose treatment patterns have changed rapidly. To adapt to the fast advances in melanoma treatment and make the clinical management of melanoma in China more standardized and internationalized, the 2015 edition of the Chinese Guidelines on the Diagnosis and Treatment of Melanoma was finalized after repeated and wide consultations with multidisciplinary experts and updated and added with much new information, with an attempt to provide the up-to-dated and reliable instructions on clinical practices based recent scientific evidences.
Updates in these guidelines (from the 2013 edition)
Epidemiology
- The global and Asian incidence and mortality of melanoma were updated (source: Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359-86);
- The incidence and mortality of melanoma in China in 2011 were updated (The 2011 data were based on the unpublished data in the China Cancer registry annual report).
Legends of the melanoma diagnosis and treatment flow chart
- The “satellites (if present)” was changed to “microsatellites (if present)”, and a new footnote c was added: definition of microsatellites: tumor nests at least 0.3 mm deep in the reticular layer, lipid membrane or vessel of the primary lesion and sized larger than 0.05 mm, highly relevant with the regional lymph node metastasis. Local microsatellites are staged as N2c (stage IIIB) if they are found during initial biopsy or extended examination of resection specimens. Patients with microsatellites need to receive sentinel lymph node biopsy (SLNB); if the result is positive, the microsatellite can be staged as N3 (stage IIIC);
- The unit of mitotic rate (MR) was changed from “mm2” to “/mm2”. For stage IA, the “mitotic rate <1 mm2” was changed to “mitotic rate 0/mm2”;
- For stages IB and II, new footnotes were added: “If palpation of local lymph nodes fails to yield satisfactory results, ultrasound or CT may be considered before SLNB; however, neither ultrasound nor CT can replace the SLNB. If a lymph node metastasis is suspected, a biopsy should be further performed.” The “ultrasound diagnostic criteria of lymph node metastasis” was added: peripheral perfusion, loss of central echoes (or, loss of ring-like enhancement), and balloon shape. The sensitivities and positive predictive values of these three methods were 77% and 52%, 60% and 65%, and 30% and 96%, and the combined sensitivity was 82% (source: Voit C, van Akkooi AC, Schäfer-Hesterberg G, et al. Ultrasound morphology criteria predict metastatic disease of the sentinel nodes in patients with melanoma. J Clin Oncol 2010;28:847-52);
- New footnote was added for stage III: “Patients with metastases <0.1 mm in sentinel node need not to receive regional lymph node dissection and the 5-year survival rate is 91%.” (source: van der Ploeg AP, van Akkooi AC, Rutkowski P, et al. Prognosis in patients with sentinel node-positive melanoma is accurately defined by the combined Rotterdam tumor load and Dewar topography criteria. J Clin Oncol 2011;29:2206-14);
- For stage IIIC tumor, new clinical trials and intratumoral drug injection were added;
- For treatment of stage IV tumor, “unresectable metastatic lesions” were discussed in two parts: mutant genes and wild-type genes.
Surgical treatment
- Resection margin: For patients with skin carcinoma in situ, the resection margin was changed from 0.5 to 0.5−1 cm;
- Principles of lymph node dissection: “It is recommended that skin tumors of the head and neck with clinically or microscopically identified lymph node metastasis in parotid gland should be treated with parotid gland resection + cervical lymph node dissection in drainage area”.
Local therapy
For patients with unresectable stage IIIC metastatic transitional lesion, new “Clinical trials” and “Intratumoral drug injection” were added.
Adjuvant therapy
The principles 2, 3, 4, and 5 of adjuvant therapy were changed as follows: “The following two conditions must be met simultaneously: (I) less than 1.5× upper limit of normal (ULN) for lactate dehydrogenase (LDH) assay; (II) lymph node extranodal invasion and/or: (i) metastasis to ≥1 parotid lymph nodes, regardless of the size of lymph nodes; (ii) metastasis to ≥2 cervical lymph nodes and/or with the size of single lymph node ≥3 cm; (iii) metastasis to ≥2 axillary lymph nodes and/or with the size of single lymph node ≥4 cm; and (iv) metastasis to ≥3 inguinal lymph nodes and/or with the size of single lymph node ≥4 cm.”
Systemic therapy
- Unresectable metastatic stage IV melanoma was discussed according to gene mutations and speed of disease progression;
- Among cytotoxic drugs, albumin-bound paclitaxel was added;
- The use of CTLA-4 monoclonal antibody plus PD-1 monoclonal antibody [ipilimumab (Ipi) + nivolumab] was added;
- Discussions on PD-1 monoclonal antibodies (nivolumab and pembrolizumab) were added.
Follow-up examinations
- The frequency of history-taking and physical examination for stage IA patients was changed from “every 3−12 months” to “every 6−12 months”; for stage III patients, the recommended chest imaging mode was changed from “X-ray or CT” to “CT” only;
- CT or PET-CT was recommended for patients with stage III/IV melanoma {source: (i) Brady MS, AKhust T, Spanknebel K, et al. Utility of preoperative [(18)]f fluorodeoxyglucose-positron emission tomography scanning in high-risk melanoma patients. Ann Surg Oncol 2006;13:525-32; (ii) Jeremy L, Alexandra S, Imogen W, et al. Surveillance imaging with FDG-PET in the follow-up of melanoma patients at high risk of relapse. J Clin Oncol 2015;33:abstr 9003}.
Special types of melanoma
- The content of head and neck MM was updated;
- The content of gastrointestinal tract MM was updated;
- The content of reproductive tract MM was updated;
- The content of uveal melanoma was updated;
- A flow chart of the management of MM was added.
Note: the update of these guidelines was based on:
- The United State National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology: Melanoma Version 3. 2015;
- American Society of Clinical Oncology (ASCO) annual meetings [2013−2015];
- CSCO annual meetings [2013−2015];
- Articles in SCI-indexed journals and Chinese-language core journals.
Evaluation of the international guidelines and consensus on melanoma diagnosis and treatment
In Western countries, melanoma in the Caucasian populations mainly arises from skin, accounting for up to 90%. Thus, the internationally available guidelines on melanoma management are mainly focused on the melanoma of skin. The guidelines mainly include: (I) The United State NCCN Clinical Practice Guidelines in Oncology: Melanoma; and (II) National Health and Medical Research Council (NHMRC) Clinical Practice Guidelines for the Management of Melanoma in Australia and New Zealand. All these guidelines involve the staging, diagnosis, treatment, and treatment of melanoma.
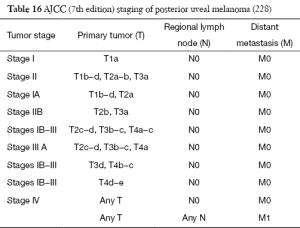
Staging of melanoma
The staging of the skin melanoma is based on the AJCC Cancer Staging Manual, 7th Edition [2010]. Except for melanoma arising from eye (conjunctiva, eyelids, and choroid), MM has no uniform staging criteria.
Diagnosis of melanoma
Both pathologic and clinical diagnostic criteria are applied in diagnosis of melanoma. The diagnostic methods include physical examination, histopathology, and imaging (including ultrasound, CT, MRI, and PET-CT). As emphasized by NHMRC, “early diagnosis can be life-saving”.
Treatment of melanoma
Surgery is the main treatment for early melanoma. The main surgical procedure is extended resection, whose scope is determined by T stage (depth of invasion). SLNB is recommended in melanoma patients with a tumor invasion depth of >0.75 mm. In patients with positive SLNB results (tumors in lymph nodes sized ≥0.1 mm) or with clinically diagnosed regional lymph node metastasis, regional lymph node dissection should be performed. Isolated limb perfusion (ILP) or isolated limb infusion (ILI) or intratumoral injection of drugs such as bacille Calmette-Guerin (BCG) vaccine, interferon, and talimogene laherparepvec (T-VEC) are recommended for patients with in-transit metastasis. Adjuvant therapy using 1-year high-dose interferon α-2b (INTRON A®) is indicated for post-operative stage IIB (or higher) patients at high risk of recurrence at a dosage of 20 million IU/m2 d1−5 ×4 w (induction phase) and 10 million IU/m2 tiw ×48 w (maintenance phase). Adjuvant radiotherapy of regional lymph nodes can increase the local control rates but has no impact on the long-term survival. Breakthroughs have been made in the management of stage IV or unresectable melanoma has in recent years. BRAF inhibitor plus MEK inhibitor, anti-CTLA-4 monoclonal antibody (ipillimumab), and anti-PD-1 monoclonal antibodies (nivolumab and pembrolizumab) have been listed as the standard treatment (the treatment protocol is developed in according with BRAF gene mutation and speed of disease progression) (category 1).
Follow-up of melanoma
Due to the lack of high-level evidences that meet the requirements of evidence-based medicine, the purpose of follow-up is to timely detect local recurrence and distant metastasis, so as to support timely surgical treatment and achieve long-term survival. Meanwhile, follow-up also helps to achieve the detection (and thus timely management) of the second primary malignant melanoma and other skin cancer other than melanoma.
Glossary
In-transit metastasis: lesions that are >2 cm from the primary tumor and located between the primary lesion and regional lymph nodes, forming cutaneous, subcutaneous, or soft tissue metastatic nodules by lymphatic dissemination.
Satellite: a metastatic nodule that is within 2 cm from the primary tumor.
Regional lymph node: The first or second lymph node stations that are involved after the metastasis of a primary lesion. Typically, the regional lymph nodes in the lower limbs (including feet) are in the ipsilateral inguinal region, whereas the regional lymph nodes in the upper limbs are the ipsilateral axillary lymph nodes.
ILP or ILI: ILP is surgical treatment for the in-transit metastasis of skin melanoma metastases. During ILP, chemotherapy drug melphalan is perfused through limb artery. Heating and oxygenation are needed during ILP, making this procedure particularly challenging. During ILI, the chemotherapy drug melphalan is infused into limb artery by interventional means, during which no oxygenation is needed, making this procedure relatively easy and repeatable.
Cloquet lymph node: The most proximal deep inguinal lymph node, Cloquet’s node, is located in or adjacent to the femoral canal beneath inguinal ligament. The first station of lower limb melanoma metastasis is often the superficial femoral lymph nodes, whereas the second station is often the deep femoral lymph nodes. Melanoma can further spread to pelvic lymph nodes (in particular the external iliac lymph nodes) via the Cloquet lymph node.
Microsatellitosis: tumor nests at least 0.3 mm deep in the reticular layer, lipid membrane or vessel of the primary lesion and sized larger than 0.05 mm, highly relevant with the regional lymph node metastasis. Local microsatellites are staged as N2c (stage IIIB) if they are found during initial biopsy or extended examination of resection specimens. Patients with microsatellites need to receive SLNB; if the result is positive, the microsatellite can be staged as N3 (stage IIIC).
Categories of evidences
Category 1
Based upon high-level evidence (e.g., randomized controlled trials), there is uniform CSCO consensus that the intervention is appropriate.
Category 2A
Based upon lower-level evidence, there is uniform CSCO consensus that the intervention is appropriate.
Category 2B
Based upon lower-level evidence, there is CSCO consensus that the intervention is appropriate.
Category 3
Based upon any level of evidence, there is major CSCO disagreement that the intervention is appropriate
All recommendations are category 2A unless otherwise noted.
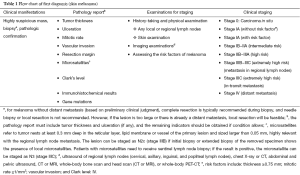
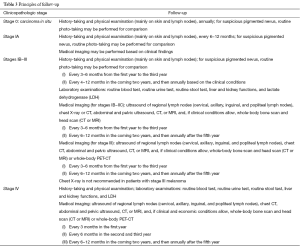
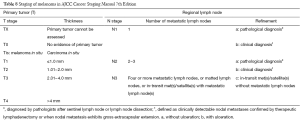
Legends of the melanoma diagnosis and treatment flow chart
The flow charts for melanoma diagnosis and treatment are shown in Figure 1, Tables 1-10.
Full table

Full table
Full table

Full table

Full table

Full table

Full table
Full table

Full table
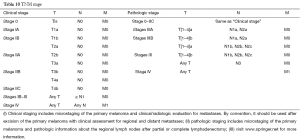
Full table
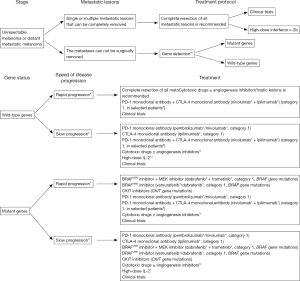
Cutaneous melanoma
Epidemiology
Melanoma has become the fastest-growing malignant tumor in recent years, with an annual growth rate of 3−5%. In 2012, there were 232,000 new cases of melanoma and 55,000 deaths worldwide (1). The incidence of melanoma was 10.2/100,000 and 9.3/100,000 in males and females in developed areas, and the mortality was 2.0/100,000 and 1.2/100,000, respectively. In contrast, the incidence of melanoma was 0.8/100,000 and 0.7/100,000 in males and females in less developed areas, and the mortality was 0.4/100,000 and 0.3/100,000, respectively. Australia and the United States have the highest incidence of melanoma. The incidence of melanoma was 28.2/100,000 and 16.8/100,000 in males and females in the United States from 2008 to 2012, and the mortality was 4.1/100,000 and 1.7/100,000, respectively. The incidence of melanoma was 58.5/100,000 and 39/100,000 in males and females in Australia in 2011, and the mortality was 9.6/100,000 and 3.5/100,000, respectively. In contrast, the incidence of melanoma was 8.6/100,000 and 8.9/100,000 in males and females in Europe, and the mortality was 2.0/100,000 and 1.3/100,000, respectively. The incidences of melanoma are relatively low in Asian countries (compared with Western countries); however, they are growing rapidly. According to the World Health Organization (WHO), the incidence of melanoma was 0.5/100,000 and 0.4/100,000 in males and females in Asia in 2012, and the mortality was 0.3/100,000 and 0.2/100,000, respectively. However, the incidence of melanoma was 0.6/100,000 and 0.5/100,000 in males and females in East Asian countries, and the mortality was 0.4/100,000 and 0.3/100,000, respectively. Melanoma incidence in China ranks fifth among the East Asian countries. The comparisons of incidence and mortality of melanoma among some countries and areas are shown in Table 11 (1).
According to Chinese Cancer Registry Annual Report (2), the total number of new melanoma cases in China was 6,505 (3,478 males and 3,027 females) in 2011, yielding an incidence of 0.48/100,000; the melanoma incidence was 0.58/100,000 in urban areas and 0.38/100,000 in rural areas. In China in 2011, there were 2,660 deaths (1,410 males and 1,250 females) due to melanoma, yielding a mortality of 0.20/100,000. The mortality was 0.23/100,000 in urban areas and 0.16/100,000 in rural areas. In addition, both incidence and mortality are higher in urban areas than in rural areas. Among patients aged 20−85 years, the incidence of melanoma is on the rise with aging (males: 0.05/100,000−3.75/100,000; females: 0.03/100,000−3.15/100,000). The incidence and mortality of melanoma in China from 2004 to 2011 are summarized in Table 12 (2-8).
In Asians and coloured populations, melanoma arising from skin accounts for 50−70%, with the acral areas including sole, toes, ends of fingers, and subungual area being the common primary sites. In a study enrolling 522 Chinese melanoma patients, tumors arising from extremities accounted for 41.8%, followed by those occurred in mucous membrane (e.g., rectum, anus, vulva, eyes, and nasopharyngeal area; accounting for 22.6%); 10% of cases had unknown primary sites. For Caucasians, melanoma arising from skin accounted for about 90%, which is commonly seen at the skin of back, chest, abdomen, and lower limbs; melanoma arising from mucous membrane and acral areas accounted for only 1−5% (9).
The male/female ratio of melanoma in China is 1.12 in China, with the median age at diagnosis being 50−55 years. Elderly patients aged ≥65 years accounted for 17.8%. Ulceration at the primary lesion can be seen in a high proportion of melanoma patients (44.8%). The primary tumor can be thick: ≥4 mm in 40.6% of patients and 1−4 mm in 44.4% of patients. Most patients were at stage II at diagnosis and the remaining patients can be at stage III (25.1%) or IV (12.8%). Survival analysis showed that stage was significantly associated with survival (P<0.001). For patients with stage I, II, III, and IV melanoma, the 5-year survival rate was 94%, 44%, 38%, and 4.6%, respectively, and the median survival was 5.00, 4.25, 2.83, and 1.42 years. The thickness of primary lesion was significantly correlated with survival: the 5-year survival rate was 92% and 43% in patients with the thickness of primary lesion being ≤1 and >4 mm, respectively. The ulceration of primary lesion also has shown certain association with survival, although the difference was not statistically significant: the 5-year survival rate in patients with ulceration and those without ulceration was 69% and 42%, respectively (P=0.08) (8). Multivariate analysis of the relationship between gene mutation and prognosis showed that both KIT gene and BRAF gene mutations are independent prognostic factors of melanoma, with the relative risk (RR) being 1.989 (95% CI: 1.263−3.131) and 1.536 (95% CI: 1.110−2.124) (P=0.003, P=0.01, respectively) (10,11).
Etiology and pathology
Excessive exposure to ultraviolet (UV) is one of the clear cause of melanoma of the skin. The sun’s UV rays can burn the skin and induce DNA mutations. Both ultraviolet A (UVA) and ultraviolet B (UVB) can induce the occurrence of melanoma. UVB is the main cause that destroys melanin cell genes and induces pathogenesis, whereas UVA can suppress certain functions of the immune system and accelerate the formation of tumors. Among Caucasian melanoma patients, the common pathogenic types (superficial spreading and nodular types) are confirmed to be associated with chronic or intermittent high-intensity UV radiation. In addition, photosensitive skin is susceptible to freckles. Individuals with a large number of ordinary nevi or dysplastic nevi or with a family history of skin cancer are believed to be at high risk of melanoma (12-14). In Asia (including China) and Africa, the primary melanoma lesions are mainly located at heel, palm, fingers, toes and subungual area that are seldom exposed to UV, and the exact etiology remains unclear. However, improper management (e.g., cutting with a knife or rope, salting, laser therapy, and cryotherapy) of pigmented nevus may include its canceration rapidly growth. Whether endocrine, chemical, and physical factors also affect the occurrence of melanoma remain unknown.
The common pathological types of melanoma include superficial spreading melanoma, nodular melanoma, lentigo maligna melanoma, and acral lentiginous melanoma. The less common types included epithelioid, desmoplastic, malignant non-pigmented nevus, balloon-like cells, spindle cells, and giant congenital melanocytic nevus. Superficial spreading melanoma is the most common type in Caucasians, whereas acral lentigo melanoma is the most common type in Asians and Africans (15-17).
Superficial spreading melanoma
Characterized by horizontal growth, the large pigmented tumor cells show pellet-like or Paget-like spread among squamous epithelium. This type is most common among Caucasians, accounting for about 70%. Usually it arises from nevus or skin pigmented spot. Generally, its appearance is irregular; its color may vary, including brown-black, pink, white, gray, or even depigmented. Its edge may become itchy. The diameter is typically larger than 0.5 cm. This type is often found at the back or in females’ lower limbs. Intermittent excessive sunlight exposure may be the main cause.
Nodular melanoma
Nodular melanoma arises from nevus and often is manifested as rapidly growing pigmented nodule (occasionally non-pigmented nodular melanoma). In some cases bleeding or ulceration may occur. The incidence of this type is about 15% among Caucasians. While it can occur at any site and any age, it is relatively more common in elderly individuals aged >60 years and in males. It is often dome-shaped, and may look like blood blister in some cases. This type is highly malignant and grows rapidly. It often has already invaded deeply in the skin at diagnosis.
Lentigo maligna melanoma
Lentigo maligna melanoma is a melanoma that is manifested as the linear or nested hyperplasia of atypical melanoma cells along the dermal-epidermal junction (which can extend to the follicle walls and sweat ducts), accompanied by severe solar lesions; meanwhile, infiltration of atypical melanocytes within dermis may also be found. This is a relatively rare type, accounting for about 10%. It often occurs at areas commonly exposed to sunlight (e.g., face) in mid-aged and elderly individuals. This type is not arising from nevus; rather, it develops after many years of sunlight exposure. In its early stage, it is manifested as irregular dark skin spots, which are often mis-diagnosed as “age pigment” or “burn spot”.
Acral lentiginous melanoma
Acral lentiginous melanoma is rarely seen in Caucasians, accounting for about 5%. MM is often classified in this type. It has not shown association with UV. However, this is the major melanoma type in Asians and Africans. It has been reported that this type accounted for 58% of all melanoma cases in Asians and 60−70% in Africans. It is often seen in palm, heel, fingers, toes, nail bed, and mucous membrane (nasopharynx, oral cavity, and female reproductive tract). This type is often misdiagnosed due to its special and hidden sites.
Along with research advances in the relationships among the molecular biology, clinical and histological features, and gene mutations of melanoma, specific types have been found to be correlated with specific gene mutation(s). New classification is more conducive to clinical applications such as staging, prognostic prediction, and treatment planning. Currently, melanoma is usually divided into four fundamental mutation types: (I) acral type; (II) mucosal type; (III) chronic sun-induced damage (CSD); and (IV) non-CSD (including the subtypes with unknown primary lesion). The CSD mainly includes head/neck and limb melanoma, because these sites are more likely to be exposed to sunlight. CSD corpuscles may be seen under high-magnification microscope. According to foreign literature, 28% of melanoma patients may develop KIT gene variations (mutations or increased copies), 10% BRAF variation, and 5% NRAS variation. The acral and mucosal types are more like to develop KIT gene variation, followed by BRAF variation. Most non-CSD patients (e.g., melanoma of trunk) have BRAF V600E mutation (60%) or NRAS mutation (20%) (18-21).
Detection of KIT gene in 502 Chinese patients with primary melanoma showed that the overall KIT gene mutation rate was 10.8%, and the gene proliferation rate was 7.4%, among which the overall mutation rate and gene proliferation rate were 11.9% and 7.3%, 9.6% and 10.2%, 20.7% and 3.4%, 8.1% and 3.2%, and 7.8% and 5.9% for the acral type, mucosal type, CSD, non-CSD, and subtypes with unknown primary lesion). The results of this study provided theoretical basis for the use of KIT inhibitors in Chinese melanoma patients. Detection of BRAF gene in 468 Chinese patients with primary melanoma showed that the BRAF mutation rate was 25.9%; specifically, the BRAF mutation rate was 17.9% and 12.5% in acral type and mucosal type, among which V600E is the most common mutation site (87.6%). The findings of this study provided theoretical basis for the use of BRAFV600 inhibitors in Chinese melanoma patients (10,11).
Pathology report
According to the revised edition of the AJCC Cancer Staging Manual, melanoma is divided into the following three categories: localized non-metastatic melanoma (stages I−II), regional metastatic melanoma (stage III), and distant metastatic melanoma (stage IV) (20,22). For patients with localized melanoma, thickness, ulceration, and MR (especially for primary lesion with a thickness of ≤1 mm) are three most important features for predicting prognosis (20).
MR, expressed in terms of mitoses per square millimeter, is an indicator of tumor proliferation. The updated AJCC staging guidelines recommend to use the “hot spot” technology to project the MR (23,24). Barnhill et al. compared the values of MR vs. ulceration in predicting the prognosis of localized melanoma; multivariate analysis of MR, ulceration, and tumor thickness showed that MR (<1, 1−6, and >6) is the most independent prognostic factor. In addition, some other studies also confirmed that MR is an important prognostic factor of skin melanoma (25-28). According to the AJCC staging system (2010 edition), patients with an MR value of ≥1 is an independent prognostic factor of poor prognosis, in particular in patients with an infiltration thickness of ≤1 mm. In these patients, MR can replace the Clark grading in differentiating stage IA and stage IB (29,30).
According to the American Academy of Dermatology, the pathology report should also include MR, vertical growth phase (VGP) (if any), tumor infiltrating lymphocytes (TIL), and degeneration (31,32). Microsatellite should also be recorded because it represents an extremely high risk of local or systemic metastasis. Patients with microsatellites should be included in stage N2c, and their prognosis is similar to stage IIIB patients. In 2013, the College of American Pathologists defined microsatellites as follows: (I) micro-metastatic lesion in the reticular layer, lipid membrane, or vessel of membrane lipid or vessel of dermis; (II) sized >0.05 mm; and (III) over 0.3 mm away from the primary lesion (33,34).
The proliferation of some melanoma cells may be difficult to diagnose. These malignancies may include atypical melanocytic proliferation, melanocytic tumor of unknown malignant potential, unspecified superficial melanocytic neoplasms, atypical Spitz tumor, and atypical cellular blue nevus. These lesions often occur in young patients. Pathologists with rich experiences should be consulted if the above conditions are suspected. For cases need to be differentiated from melanoma, the pathology report should include the prognostic factors of melanoma. For lesions that are difficult to diagnose histologically, comparative genomic hybridization (CGH) or fluorescence in situ hybridization (FISH) may be applied to detect the specific gene mutations (if condition allows). As suggested by a recent small-sample study on atypical Spitz tumor, compared with FISH, CGH can provide more detailed information and has higher sensitivity and specificity in determining the change in the copies of relevant chromosomes (35).
For stage II patients with lymph node metastasis, the number of metastatic lymph nodes and clinical features (e.g., palpable during physical examination) are key prognostic factors. For patients with positive sentinel lymph nodes (SLNs), the number of positive lymph nodes as well as the tumor load of SLNs, thickness of primary lesion, MR, ulceration, and age is the most important factors in predicting survival. For patients with clinically positive lymph nodes (physical examination or imaging shows the presence of swollen lymph nodes), the prognostic factors include the number of lymph nodes, ulceration of primary lesion, and age (36). All these information must be included in the pathology report.
For patients with distant metastasis (stage IV), the location of distant metastases is the most important prognostic factor. AJCC divided the distant metastasis into three levels: metastasis to the distant soft tissue of skin; metastasis to lung; and metastasis to other viscera except the lungs (22,23). Elevated LDH IV is also considered an independent prognostic factor in stage IV melanoma patients with poor prognosis and has been included in the AJCC staging system (37-39).
The panel recommends that the pathology report must include tumor thickness and ulceration (if any), and the remaining indicators should be obtained if condition allows.
Diagnosis
Diagnosis of melanoma is often based on typical clinical manifestations and physical examination findings. Pathology is the final criteria for the diagnosis and staging of melanoma, and thus plays a key role throughout the diagnosis, staging, treatment, and prognosis of melanoma. Immunohistochemical staining is the main auxiliary means for differentiating melanoma. While S-100, HMB-45 and vimentin is specific indicators for the diagnosis of melanoma, HMB-45 is more specific than S-100.
Clinical symptoms
Most skin melanoma arises from nevus, and the early malignant symptoms of nevus can be summarized as the following ABCDE rules:
- A—asymmetry: one half of the spot does not match the other half;
- B—border irregularity: the borders of a melanoma may be uneven, blurred, or notched. In contrast, normal moles (nevus) are round or oval in shape and have sharply defined borders;
- C—color variation: common moles are usually one color throughout. Melanomas may have several colors or an irregular pattern of colors (mainly dirty black, but also can be brown, dark brown, blue, pink, black, or even white);
- D—diameter: spots with a diameter of >5−6 mm or those that have remarkably enlarged should be evaluated. Melanomas are often larger than common moles. Biopsy is recommended for spots with a diameter larger than 1 cm. Biopsy is recommended for spots with a diameter larger than 1 cm;
- E—elevation: in some early melanomas, the tumor may be slightly elevated.
The only shortcoming of the ABCDE rule is that it does not consider the speed of melanoma progression (e.g., the remarkable change within weeks or months). The further progression of early skin melanoma can be manifested by satellites, ulceration, recurrence, regional lymph node metastasis, and in-transit metastasis. The symptoms of advanced melanoma vary due to their different metastatic sites, which mainly include lungs, liver, bones and brain. Melanoma of eye and rectum origin can easily develop liver metastasis.
Medical imaging
Medical imaging should be based on the local conditions and the patients’ economic status. Ultrasound of regional lymph nodes (cervical, axillary, inguinal, and popliteal lymph nodes), chest X-ray or CT, abdominal and pelvic ultrasound, CT, or MRI, and, if feasible, whole-body bone scan and head scan (CT or MRI) are required. PET-CT may be performed in patients with good economic status, especially those with unidentified primary lesions. PET is a more useful in detecting subclinical metastases. It is widely accepted that, for patients with early localized melanoma, PET is not sensitive in detecting the metastatic lesions and thus can not satisfactorily benefit the patients (40-42). PET/CT is more useful in stage III patients because it can help to identify lesions that can not be diagnosed by CT and display locations that can not be displayed by conventional CT (e.g., limbs) (43). In a systematic review that included 17 studies, PET had a sensitivity of 68−87% and a specificity of 92−98% in sage III/IV patients. In contrast, the sensitivity was 0−67% and the specificity was 77−100% for stage I/II patients (44). The results of other large meta analyses showed that PET/CT is superior to the conventional CT in detecting distant metastases (45).
Laboratory examinations
Laboratory examinations typically include routine blood test, liver/kidney function tests, and LDH measurement. These indicators can be used for subsequent treatment or prognosis. Although LDH is not a sensitive indicator for metastasis detection, it can guide the prognosis. No specific serum tumor marker has been available for melanoma. Therefore, tumor marker detection is not recommended for this disease.
Surgical treatment
Biopsy
Complete excisional biopsy is recommended for suspicious pigmented lesions to obtain accurate T stage. With a cutting edge of 0.3−0.5 cm, the incision shall go through the skin line (e.g., in limbs, the incision shall be along the long axis of the skin). Direct extended excision should be avoided to prevent the damaged quality of SLNB due to the change in regional lymphatic drainage. If complete resection can not be achieved in patients whose lesions are too large or the lesions are located at face, palm, sole of foot, ear, fingers, toes, or nail bed, resection of the whole cutaneuous layer where the lesion is located or puncture biopsy may be considered. If a large tumor becomes ruptured or a metastasis has been confirmed, puncture or incisional biopsy of the lesion may be performed.
Extended resection
Once early melanoma is confirmed by biopsy, extended resection of the primary lesion should be performed as soon as possible. The safe cutting edge during the extended resection should be decided according to the depth of tumor infiltration: (I) the safe cutting edge is 1 cm if the lesion thickness is ≤1.0 mm; (II) the safe cutting edge is 1−2 cm if the lesion thickness is 1.01−2.0 mm; (III) the safe cutting edge is 2 cm if the lesion thickness is >2 mm; and (IV) the safe cutting edge is 2 cm, as supported by new evidences, the lesion thickness is >4.0 mm. A European multicenter, randomized study included 936 melanoma patients with tumor thickness >2.0 mm, in whom extended resection with a cutting edge of 2 or 4 cm was performed; the results showed the overall 5-year survival rates were similar in both groups (46). These findings were similar to the previous studies (47-49). Systematic review and meta analysis also showed that a cutting edge of 2 cm is sufficient.
Sentinel lymph node biopsy (SLNB)
Andtbacka et al. (50-56) carried out retrospective analysis on seven relevant studies on SLNs and found that in melanoma patients with tumor thickness of <0.75 mm, the positive rate of SLN was only 2.7%; in patients with tumor thickness of 0.75−1.0 mm, the SN-positive rate was 6.2%. Therefore, SLNB is not recommended for stage IA or IB patients with a tumor thickness of <0.75 mm. For stage IA or IB patients with a tumor thickness of 0.75−1.0 mm and for patients with a tumor thickness of >1 mm, SLNB can be considered, which can be performed during en bloc resection or subsequently. However, the incidence of ulceration is above 60% among melanoma patients in China, and the prognoses of these patients are often poor. Thus, if a reliable invasion depth can not be detected due to the limitations of biopsy and pathological detection technology, SLNB is recommended in melanoma patients accompanied by ulceration. SLNB is helpful for N staging; lymph node dissection should be carried out promptly once positive SLNs are identified. According to a prospective study conducted by the Rotterdam Erasmus University Cancer Center (57), if the metastatic lesion in the SLN was <0.1 mm in diameter, the patients’ long-term survivals showed no significant difference with SN-negative patients. Thus, they believed that patients with low tumor load in SN did not need to receive extended lymph node dissection. An ongoing international multicenter study MSLT-II will further validate the feasibility of this treatment strategy. However, before the publishing of these clinical findings, regional lymph node dissection is still needed in SN-positive cases.
MSLT-I is an international multicenter phase III study (58), aiming to evaluate the influence of SLNB on the survival of patients with early melanoma. It found that SLNB was a very important procedure for staging; compared with the extended resection alone for primary lesions, extended resection + SLNB did not increase the disease-related survival. Compared with the control group, the SLNB group had a higher relapse-free survival (increased by 7−10%). In a retrospective analysis targeting melanomas with a medium thickness (1.2−3.5 mm), SN-positive patients had more survival benefits than patients with clinically positive regional lymph nodes (56% vs. 41.5%, P=0.04). However, such survival benefit was not found in patients with a tumor thickness larger than 3.5 mm. In addition, among SN-positive patients, patients who received immediate lymph node dissection had significantly higher survival rate than those who received delayed procedure (72% vs. 52%).
However, the MSLT-I trial did not mention the clinical relevance of SLNB for melanoma with a tumor thickness of ≤1.2 mm. Since melanoma patients with small tumor thickness generally have good prognosis, the role of SLNB has not been determined in these patients (59). A recent study (60) confirmed that melanoma patients with a tumor thickness of less than 0.75 mm had an SN-positive rate of about 2.7%; in contrast, this figure was 6.2% in melanoma patients with a tumor thickness of 0.75−1 mm. In a multicenter study enrolling 1,250 melanoma patients with a tumor thickness of <1 mm, melanoma patients with a tumor thickness of <0.75 mm had a SN-positive rate of less than 5%, which was independent of Clark level and ulceration status (61). However, the SN-positive rate increased in melanoma patients with small thickness but with at least one risk factor (ulceration Clark level IV, nodular growth, high MR, recurrence, or aged ≤40 years) (62). In melanoma patients with tumor thickness of ≤1 mm, the relationship between positive SLNs and survival is still disputed (63-67).
In addition to tumor thickness, other factors such as Clark level (61,63,64,66), MR (64,68), ulceration (61,69), lymphatic vessel invasion (66), VGP (70,71), and TIL (72-74) can also be used to predict the positive SLNs in patients with thin melanoma. However, some data remain controversial, and whether they can be used for predicting tumor recurrence requires further verification (75-77).
Lymph node dissection
Prophylactic lymph node dissection is not recommended. In patients with positive SLNs or in patients with radiologically or clinically confirmed regional lymph node metastasis (but without distant metastasis in stage III patients), regional lymph node dissection should be performed on the basis of extended resection. The bases of the involved lymph nodes must be completely removed, and at least ten lymph nodes in the groin and at least 15 lymph nodes in the neck and armpits must be dissected. In the groin area, if clinical examination shows that at least three superficial femoral lymph nodes are involved, iliac fossa and obturator lymph node dissection should be performed. If pelvic imaging indicates the presence of pelvic lymph node metastasis, or if intraoperative pathology of Cloquet’s node shows positive results, dissection of lymph nodes in iliac fossa and obturator should be performed (56,78).
Limb in-transit metastasis: a special type in stage III patients
Limb in-transit metastasis is manifested by the diffuse metastasis in skin, subcutaneous tissues, and soft tissues between primary lesion and regional lymph nodes. It can not be completely removed by surgery. Internationally, this type is mainly treated by ILP and ILI. ILI is a local treatment approach for the oxygen-free, low-flow infusion of chemotherapy drugs, during which arterial and venous cannulation was established to create chemotherapy pathway for infusing melphalan. It requires simple devices. Since 1992, the Sydney Melanoma Center had performed ILI in over 300 patients in ten years, and its response rate for stage III melanoma was about 80%, during which no treatment-related amputation or death was reported (79). The response rate was significantly higher in patients >70 years than in those below 70 years (91% vs. 78%, P<0.05). A recent study showed that the complete response rate reached 31% in 128 patients who had received ILI (80). In another study on ILP, higher CR rate (63%) was achieved, and meanwhile the 5-year survival reached 38% (81). Therefore, the evidence level is 2A concerning the application of ILP/ILI for treating limb in-transit metastasis.
Surgical resection may be considered in stage IV patients with solitary metastasis
Surgical resection is recommended for localized metastasis. In patients with solitary visceral metastasis, a second examination following a short observation can be considered. If there is no new metastatic lesion, surgery can be considered; the patients may be arranged to receive adjuvant therapy or enter a clinical trial after have been identified as in a tumor-free status after surgery. The SWOG9430 study found that, in patients with stage IV solitary metastasis, the postoperative median overall survival (OS) was up to 19 months and the 5-year survival rate was 20%, which was far more than the previously reported median OS (6−8 months) of patients with stage IV disease (82).
Adjuvant therapy
Postoperative prognosis varies based on the risk factors. Generally, the postoperative patients are divided into four categories according to risk factors including depth of invasion, ulceration, and lymph node metastases: (I) stage IA (low risk); (II) stage IB−IIA (intermediate risk); (III) stage IIB−IIIA (high risk); (IV) stage IIIB−IV (extremely high risk). Low-risk patients may survive for a long period of time, and the 5-year survival rate is about 95%. The post-operative 5-year survival rate is about 80% in intermediate-risk patients and ranges 10−50% in high-risk and extremely-high-risk patients. Patients at different degrees of risk should choose different adjuvant treatment. For some special types of melanoma, the treatment should be tailored. Low- and medium-dose interferon did not improve the survival when used as adjuvant treatment after the resection of high-risk melanoma. Some studies showed that it may increase the survival in relapse-free patients (83-87). The panel does not recommend the use of low- and medium-dose interferon for adjuvant treatment.
Low-risk patients
No relevant clinical trial has been conducted on low-risk melanoma patients. A retrospective study with 5-year follow-up showed that few melanoma patients with tumor thickness of <0.5 mm experienced recurrence or death. No adjuvant therapy is recommended for low-risk melanoma patients; rather, efforts should be made to prevent the occurrence of new primary lesion. Watchful waiting is recommended.
Medium- and high-risk patients
The risk of recurrence and metastasis remarkably increases in medium- and high-risk patients (exceeding 25%). Many clinical trials have been conducted in this field, which included melanoma vaccines (including whole-cell vaccines, dendritic cell vaccines, peptide vaccines, neural gangliosides vaccines, DNA vaccines, and viral vaccines), low- and medium-dose interferon, chemotherapy, bio-chemotherapy, and high-dose interferon; except for high-dose interferon α-2b, all the other treatment showed no significant difference when compared with placebo. Since many phase III randomized clinical studies have proved that high-dose interferon α-2b can prolong the recurrence-free survival (RFS) and OS, the US FDA approved the use of 1-year high-dose interferon α-2b (20 million IU/m2 d1−5 ×4 w, 10 million IU/m2 tiw ×48 w) as the adjuvant therapy for high-risk patients with recurrent melanoma. In 2011, FDA approved the use of long-acting interferon-α (for 5 years), as recommended treatment for high-risk melanoma patients. Patients with primary tumor ulcer are more likely to benefit from this new strategy. However, there is no experience of using this drug in Chinese patients.
Many randomized clinical trials have evaluated the efficacy of high-dose interferon (intravenous high-dose interferon in the first month, followed by subcutaneous injection of interferon for 11 months). The ECOG1684 study is a randomized trial that compared the efficacies of postoperative interferon α-2b vs. placebo in the treatment of stage IIB or III melanoma patients with in-transit metastasis or regional lymph node involvement (88). After 6.9 years of follow-up, the PFS and OS were significantly superior in the interferon α-2b group. After 12.6 years of follow-up, although the PFS was significantly better in interferon α-2b group, the OS showed no significant difference between interferon α-2b group and placebo group. In another follow-up trial (ECOG1690) with longer follow-up period, the high-dose interferon α-2b group also had longer PFS, although the OS showed no significant superiority (89). The E1694 trial compared the efficacies of interferon α-2b and GM2-KLH21 vaccine; during the nearly 2-year follow-up, the PFS and OS were significantly superior in the interferon α-2b group than in the GM2-KLH21 vaccine group.
Another study (E1697) evaluated the role of high-dose interferon in the adjuvant therapy. Totally 1,150 cutaneous melanoma patients (T3/any T, N1a−N2a) (90) were intravenously administered with high-dose interferon for 1 month; compared with the placebo group, there was no PFS and OS benefits in the high-dose interferon group. In another phase II clinical study designed to assess the role of the maintenance doses of interferon, 194 patients were randomly divided into two groups: in the maintenance group, intravenous high-dose interferon was applied in the first month, followed by subcutaneous injection of interferon for 11 months; in the control group, patients only received intravenous high-dose interferon in the first month. Although the 2-year relapse-free survival was similar in these two groups, the maintenance group had significantly longer 1-year OS than the control group (41.5 months vs. unreached endpoint; P=0.05) (91).
The EORTC18991 study (92) enrolled 1,256 stage III patients; after the surgery, these patients were randomized into long-acting interferon-α group and watchful observation group (for 5 years). The 5-year relapse-free survival rate was higher in the long-acting interferon-α group than in the watchful observation group (45.6% vs. 38.9%), but the OS showed no significant difference. Thus, in 2011, the US FDA approved the use of long-acting interferon-α in the adjuvant therapy of melanoma patients with lymph node involvement. Two recent large-scale phase III randomized study (EORTC1892 and EORTC18991) indicated that, after the adjuvant therapy with interferon-α, the relapse rate and mortality rate were significantly lower in primary melanoma patients with ulceration than those without ulceration, suggesting the former are more likely to benefit from interferon-α. However, its underlying clinical or biological mechanisms remain unclear (93).
Several meta analyses on the adjuvant therapy with interferon-α have shown that high-dose interferon-α can prolong relapse-free survival, although it has no definite influence on OS (94-96). One analysis reported that, 4 of 14 studies on adjuvant therapy with interferon indicated that OS was significantly improved in the interferon group, while the other studies did not find any advantage of interferon in improving OS (95,96). The panel believes that adjuvant therapy with interferon can prolong the relapse-free survival, although its influence on OS requires further investigations. The clinical use of interferon must be based on patients’ specific conditions and treatment willingness.
Extremely-high-risk patients
The adjuvant therapy for extremely-high-risk patients is still under investigation. While no standard treatment protocol has been available, high-dose interferon α-2b is still the mainstream treatment, which is similar to that in medium- and high-risk patients.
Recommended interferon dosage for Chinese melanoma patients
The standard dosage of interferon α-2b used in developed countries (20 million IU/m2 d1−5 ×4 w, 10 million IU/m2 tiw ×48 w; for one year) can be applied. In 2011, the European Journal of Cancer (EJC) published the results of a study on the use of high-dose interferon α-2b in 147 Chinese patients with melanoma; for extremely-high-risk stage IIIB-IIIC acral melanoma patients with the number of metastatic lymph nodes ≥3, the 1-year protocol (15 million IU/m2 d1−5 ×4 w + 9 million IU tiw ×48 w) can be applied; for stage IIB−IIIA high-risk acral melanoma patients, the 1-month protocol (15 million IU/m2 d1−5 ×4 w) may be applied (97).
Adjuvant radiotherapy
Melanoma generally is less sensitive to radiotherapy. In some special cases, however, radiotherapy remains an important treatment approach. The adjuvant radiotherapy of melanoma is mainly applied for lymph node dissection and for the postoperative complementary therapies of some head and neck melanomas (especially the nose melanoma), with an attempt to further increase local control rate. However, some contents in this part still lack evidences from Chinese literature.
For patients with risk factors, radiotherapy has certain role in controlling lymph node recurrence. Agrawal et al. (98) conducted the largest retrospective study on postoperative radiotherapy, in which they assessed 615 patients in terms of lymph node number, size, location, and extranodal invasion, so as to identify patients at high risk of regional lymph node recurrence. During the 5-year follow-up, local recurrence was noted in only 10.2% of patients who had received radiotherapy; in contrast, the local recurrence rate was up to 40.6% in patients who had not received radiotherapy. Multivariate analysis showed adjuvant radiotherapy could improve the local control rate (P<0.0001). Notably, radiotherapy-related diseases (in particular lymphedema) significant increased (5-year incidence: 20% vs. 13%, P=0.004). In an Australia phase III study (99), 250 patients who had undergone lymph node dissection but experienced solitary lymph node relapse were randomly arranged to receive the lymph node radiotherapy or observation. The LDH of these patients was lower than 1.5× UNL and meanwhile must meet the following conditions: parotid lymph nodes ≥1, or neck (or armpit) metastatic lymph nodes ≥2, or inguinal metastatic lymph nodes ≥3, or neck (or armpit) lymph node metastasis maximum diameter ≥3 cm, or inguinal lymph node metastasis maximum diameter ≥4 cm, or extranodal invasion. The results showed that the regional lymph node recurrence rate remarkably decreased in the adjuvant radiotherapy group (HR: 0.56; 95% Cl: 0.32−0.98; P=0.041); however, the OS might be poor in the radiotherapy group (HR: 1.37; 95% Cl: 0.94−2.01; P=0.12) and the local adverse reactions significantly increased (P=0.035) (100). Some other clinical studies compared the specific postoperative radiotherapy schedules (101-103), and the results showed that high-dose hypofraction radiotherapy was almost equally efficacious as the conventional radiotherapy.
The panel recognizes that adjuvant radiotherapy has certain value in increasing the local control rate; however, the radiotherapy-related adverse reactions and its possible effects, as shown in some studies, on lowering the OS make the application of adjuvant radiotherapy remain controversial.
Systemic treatment of unresectable stage III or metastatic melanoma
Metastatic melanoma has poor prognosis. It is estimated that the median survival is 15 months for stage M1a, 8 months for stage M1b, 6 months for bone metastasis, and 4 months for liver/brain metastasis; the overall median survival was 7.5 months, the 2-year survival rate was 15%, and the 5-year survival rate was about 5% (104). For unresectable stage III or metastatic melanoma, systemic treatment (mainly medical treatment) or clinical trial is generally recommended. Systemic treatment options include the PD-1 monoclonal antibodies, CTLA-4 monoclonal antibodies, BRAFV600 inhibitors, CKIT inhibitors, MEK inhibitors, high-dose IL-2, and chemotherapy. In recent years, notable breakthroughs have been made in the treatment of advanced melanoma. Personalized targeted therapies and targeted immune therapies are the mainstream options. In China, chemotherapy drugs remain the important treatment approaches for advanced melanoma.
Chemotherapy drugs
Dacarbazine (DTIC)
Since 1972, DTIC has been the only chemotherapy drug approved by US FDA for treating advanced melanoma. DTIC is an alkylating agent; by connecting special parts of the DNA, it can inhibit cell division and thus cause cell death. DTIC is a drug precursor and be converted into an active compound 5-(3-methyl-1-triazeno) imidazole-4-carboxamide (MTIC) in liver. This drug is intravenously administered. Since 1992, a number of randomized clinical trials (105-110) used DTIC as control groups, and over 1,000 patients have received DTIC treatment, among whom the overall response rate was 13.4%, and few patients achieved complete remission (≤5%); the median survival was 5.6−11 months. The common protocols included: 200−250 mg/m2 d1~5, repeated every three weeks; or, 800−1,000 mg/m2 d1, repeated every three weeks.
Temozolomide (TMZ)
TMZ, as a DTIC analogue, is an oral chemotherapy drug. It is converted into MTIC in vivo. Unlike DTIC, TMZ is not metabolized in the liver. TMZ can cross the blood-brain barrier (BBB), and its concentration in cerebrospinal fluid is 28−30% of that in plasma. This is particularly valuable for melanoma because the rate of brain metastases is more than 50% in the autopsy studies of melanoma patients. TMZ was initially approved for the treatment of high-grade malignant glioma; however, it can also be useful for melanoma. A European large-scale phase III clinical study (106) compared the roles of TMZ [250 mg/(m2·d), for 5 consecutive days, repeated every 4 weeks] and DTIC [200 mg/m2·d, for 5 consecutive days, repeated every 3 weeks] in treating chemotherapy-naive patients. Totally 305 patients with advanced metastatic melanoma were enrolled. The results showed that the TMZ group had higher response rate than that in DTIC group (12.2% vs. 9.4%, P=0.43) and longer PFS (1.74 vs. 1.38 months, P=0.002), whereas the OS was comparable between these two groups (7.7 vs. 6.4 months, P=0.2). Although this study did not reach its initial assumptions, the efficacy of TMZ was at least comparable with that of DTIC. The most common nonhematologic toxicities were nausea (52%), vomiting (34%), pain (34%), constipation (30%), headache (22%), and fatigue (20%). The majority of adverse reactions was mild to moderate and can be controlled. Thrombocytopenia was seen in 9% of patients in both groups; specifically, grade 3/4 thrombocytopenia was seen in 7% of patients in TMZ group and in 8% of patients in DTIC group. Treatment discontinued in 3% of patients in TMZ group and in 5% of patients in DTIC group due to bone marrow suppression. The quality of life was better in the TMZ group. In the phase III trail (E18032 trial) enrolling 859 patients (111), the modified TMZ group (150 mg/m2 d1−7q2w) was compared with the DTIC group (1,000 mg/m2 q21d); the former had significant higher response rate (14.5% vs. 9.8%, P=0.05), although the PFS and OS showed no significant difference. Since TMZ can cross the BBB, many clinical trials have evaluated the role of TMZ in treating brain metastasis. In a study published in 2007 (112), totally 179 treatment-naive patients with advanced melanoma were enrolled, among whom 52 had brain metastasis. The results showed that in patients who responded to systematic TMZ treatment, the median progression time of brain lesion was 7 months (range, 2−15 months), and the median survival of patients with brain metastasis was 5.6 months. Thus, this study demonstrated that TMZ had sustained effectiveness in controlling brain metastasis; in most patients with small brain metastases, radiotherapy may be postponed or may not be required. In a phase II clinical study published in 2006 (113), 117 patients with brain metastases received TMZ (200 mg/m2 for 5 consecutive days, repeated 28 days, for 1 year or until unable to tolerate) as first-line treatment, among whom 25% had more than four metastatic lesions. The results showed that the overall response rate was 7% (CR in 1 case and PR in 7 cases), SD 29%, and median PFS 3.5 months. There are also many studies on the roles of TMZ combined with other drugs (mainly, the combinations of TMZ with interferon or thalidomide) (114). Notably, many clinical trials on the combination with thalidomide were terminated prematurely because such combination increased the risk of developing blood clots. Currently, the combination of TMZ with thalidomide has been forbidden in the treatment of brain metastases of melanoma patients at high risk of thrombosis.
Platinum-based anti-tumor drugs
Platinum-based anti-tumor drugs have also shown certain efficacies in treating melanoma. The cisplatin monotherapy can achieve a response rate of 10−20%; however, the duration of treatment effectiveness is relatively short, lasting only 3 months. Generally, a dose of <80 mg/m2 will decrease the response rate, whereas a dose of ≥150 mg/m2 does not increase the response rate. The common toxicities include kidney toxicity, ear toxicity, neurotoxicity, vomiting, and bone marrow toxicity. Three phase II clinical trials (115-117) investigated the efficacies of carboplatin in treating metastatic melanoma and found that the response rate was similar between carboplatin and cisplatin. The main toxicity of carboplatin is bone marrow suppression, and the dose-limiting toxicity is thrombocytopenia.
Taxanes
The taxane compounds include paclitaxel, a diterpene compound extracted from taxus plants, and docetaxel, a compound extracted and synthesized from the needles of European yew tree. As a novel anti-microtubule agent, paclitaxel can maintain the stability of tubulin and suppress mitosis by inhibiting polymerization of tubulin to form microtubules. Many phase I/II clinical trials have explored the roles of taxanes in the treatment of advanced melanoma (118-123). It was found that paclitaxel monotherapy could achieve a response rate of 12−30%. The common protocols include: 175 mg/m2, every 3 weeks; or 90 mg/m2, weekly. The common toxicities include neutropenia, neurotoxicity, and fatigue.
Albumin-bound paclitaxel (nab-paclitaxel)
Nab-paclitaxel, a novel nano-particle encapsulated paclitaxel, is a novel anti-tumor compound. It uses soluble human albumin to coat paclitaxel and deliver the drug into tumor cells. Tumor cells will secrete an SPARC protein to harvest protein in cellular junctions. The nano-particles of nab-paclitaxel are then bound to tumor cells via the SPARC protein and finally enter the tumor cells, releasing cytotoxic drugs to kill tumor cells. This design helps to avoid the potential safety problems associated with the use of polyoxyethylene castor oil as solvent during the administration of conventional paclitaxel. Meanwhile, it also improves the distribution of paclitaxel in human body, enhances the unique activities of the drug when targeting and penetrating the tumor tissue. It makes the drug highly concentrated in tumor tissue and reduces its retention in blood. As a result, nab-paclitaxel can achieve better efficacy and have less impact on normal tissues. The standard dosage of nab-paclitaxel is as follows: 260 mg/m2, repeated every three weeks; optimized protocol: 100−150 mg/m2, once weekly. A multicenter randomized phase III clinical trial (124) compared the safety and effectiveness of nab-paclitaxel (Abraxane) vs. chemotherapy drug DTIC in treatment-naive patients with stage IV metastatic melanoma. Totally 529 patients randomly received Abraxane (150 mg/m2, once weekly, for 3 consecutive weeks, repeated every 4 weeks) (n=264) or DTIC (1,000 mg/m2, once every 3 weeks) (n=265). Among these treatment-naive patients with metastatic melanoma, Abraxane significantly increased median PFS (4.8 vs. 2.5 months; HR: 0.792; 95% Cl: 0.631−0.992; P=0.044); however, the OS showed no significant difference between these two groups (12.8 vs. 10.7 months, P=0.09). In the Abraxane group, toxicities with an incidence of ≥10% included neurotoxicity (25% vs. 0%) and decreased neutrophils (20% vs. 10%). In the Abraxane group, the median time required for improvement in neuropathy was 28 days.
Nitrosoureas
With the structure of P-chloro-ethyl nitrosourea, nitrosoureas have a broad spectrum of antitumor activity. These drugs have strong lipophilicity and can easily cross the BBB to enter cerebrospinal fluid. Thus, they have been widely applied in the treatment of brain tumors and other tumors of the central nervous system. Its main side effect is the delayed and cumulative myelosuppression. The most widely used nitrosourea is fotemustine; in Europe it has been approved for the treatment of metastatic melanoma. Many clinical trials have shown that it can achieve a response rate of about 22%. In addition, the lipid-soluble fotemustine has been confirmed to be effective for 25% of brain metastases. In a phase III clinical trial (107) comparing fotemustine (100 mg/m2 every week, for 3 weeks) and DTIC (250 mg/m2 daily, for 5 consecutive days, repeated every 4 weeks), 229 patients with advanced melanoma were enrolled. The responsive rate was 15.2% in fotemustine group and 6.8% in DTIC group (P=0.053). The median control duration of brain metastasis was 22.7 months in fotemustine group and 7.2 months in DTIC group. The main toxicities included delayed myelosuppression and gastrointestinal toxicities.
Personalized targeted therapies
Imatinib (Kit inhibitor)
The largest clinical study on drugs against KIT mutation was a phase II clinical trial conducted in China (125). Totally 43 advanced melanoma patients with KIT gene mutation or amplification from multiple centers across China received imatinib therapy; the results showed that the 6-month PFS rate was 36.6%, and the median PFS was 3.5 months. Compared with patients with other exon mutations, patients with mutation in exon 11 or 13 had longer median PFS; in addition, patients with multiple CKIT variations had longer PFS than those with single CKIT variation (but without significant difference). Among these patients, 10 patients (23.3%) achieved PR, 13 patients (30.2%) achieved SD, whereas 20 were found to have progressive disease (PD). Although it was not as effective as BRAFV600E inhibitors, this drug was found to be more promising when compared with other factors that still lack definite efficacies: the 1-year survival rate reached 51% and the median OS was 14 months; the OS of patients who had achieved PR or SD was 15 months, which was significantly different from that in PD patients (P=0.036). Therefore, the panel recommends the use of imatinib in advanced melanoma patients with CKIT mutation or amplification (category 2).
BRAFV600 inhibitors and MEK inhibitors
About half of Caucasians with metastatic melanoma have intracellular BRAF gene mutation (126). Vemurafenib is a specific inhibitor of BRAF gene mutation (127). In a phase III randomized clinical trial, 675 treatment-naive metastatic melanoma patients with BRAFV600E gene mutation were randomized into vemurafenib group and DTIC group (128). Compared with DTIC, vemurafenib could significantly prolong OS and PFS (risk ratio of death: 0.37; risk ratio of death or disease progression: 0.26; P<0.001). The 6-month survival rate was 84% and 64%, respectively, in these two groups. Skin complications were the most common adverse reactions in the vemurafenib group, in which 18% of patients developed squamous cell carcinoma of the skin or keratosis acanthoma and thus required surgical resection; in addition, 12% of patients suffered from grade 2−3 skin photosensitive reactions. Joint pain is the most common non-skin adverse reaction (21%). Based on this study, the US FDA approved the use of vemurafenib for treating metastatic or unresectable melanoma with BRAFV600E gene mutation in August 2011. According to a study participated by 132 non-treatment-naive patients, vemurafenib achieved an OS rate of 53% and a median survival of 15.9 months (129). Secondary skin lesions were seen in 26% of patients.
Two other BRAF inhibitors have also been approved by the US FDA. A phase III clinical trial (130) compared the roles of dabrafenib and DTIC in treating melanoma patients with BRAFV600E mutation. Totally 250 patients with stage IV melanoma or with unresectable stage III melanoma were enrolled in this study, with the primary endpoint being PFS. The results showed that the PFS was 5.1 months in dabrafenib group and 2.7 months in DTIC group (HR: 0.3; 95% CI: 0.18−0.51; P<0.001). In patients treated with dabrafenib, the incidence of grade 2 or higher toxicities was 53%, whereas grade 3−4 toxicities were rare. The most common adverse reactions were adverse skin reactions, fever, fatigue, joint pain, and headache. Compared with vemurafenib, dabrafenib-associated cutaneous squamous cell carcinoma or keratosis acanthoma were less seen. In contrast, fever was more common (11%). During the treatment of 172 patients with BRAF-mutant melanoma with brain metastasis (131), the response rate was 39% in treatment-naive patients and 31% in non-treatment-naive patients.
In the MAP signaling pathway, MEK1 and MEK2 are located in the downstream of BRAF gene. Trametinib is an orally administered inhibitor of MEK1 and MEK2. In a phase III randomized clinical trial, 322 metastatic melanoma patients with BRAFV600E/K gene mutation were randomized into trametinib group and chemotherapy group (132). Compared with the chemotherapy group, the trametinib group had significantly improved PFS (4.8 vs. 1.5 months; HR: 0.45; 95% CI: 0.33−0.63; P<0.001) and 6-month OS rate (81% vs. 67%; HR: 0.54; 95% CI: 0.32−0.92; P<0.01). The most common adverse reactions were adverse skin reactions, diarrhea, and peripheral edema. Unlike BRAF inhibitors, trametinib is associated with fewer secondary skin lesions. In a phase II clinical trial, the objective response rate was lower in trametinib group than in BRAF inhibitor group (133). Compared with BRAF inhibitor, trametinib achieved lower response rate in treatment-naive patients (22% vs. 48−50%) (128,133,134).
Combined targeted therapy
Although BRAFV600E inhibitors can achieve relatively high initial response rates, about half of patients who are using BRAFV600E inhibitor monotherapy will experience PD within 6 months. A phase III clinical trial enrolled 247 cases of BRAFV600E-mutant patients with advanced melanoma and evaluated the safety and efficacy of the combination of BRAF inhibitor and MEK inhibitor (135). Patients were randomized into two groups: dabrafenib monotherapy group and dabrafenib + trametinib group. The results showed that the response rate (76% vs. 54%, P=0.03) and PFS (9.4 vs. 5.8 months; HR: 0.39; 95% CI: 0.25−0.62; P<0.001) were significantly superior in dabrafenib + trametinib group than in dabrafenib monotherapy group. In addition, the incidence of secondary cutaneous squamous cell carcinoma significantly reduced (7% vs. 19%), although the proportion of fever dramatically increased (71% vs. 26%) in the combination group. In the 2015 ASCO annual meeting, the results of coBRIM study, which investigated the values of vemurafenib + MEK inhibitor (cobimetinib), was updated (136): till January 2015, after 14 months of follow-up, the PFS was 7.2 months in the vemurafenib + placebo group and 12.3 months in the vemurafenib + cobimetinib group, indicating that the combination group had significantly lower risk of progression. Vemurafenib, dabrafenib, and trametinib have not been licensed in China. However, the BRAFV600E variation rate approaches 26% among Chinese melanoma patients (11); although it is not as high as in Caucasians (about 50%), it is still meaningful for the management of Chinese melanoma patients. Therefore, in these guidelines we recommend the use of these drugs for BRAFV600E-mutant patients (category 1).
Immunotherapy/immune targeted therapy
CTLA-4 monoclonal antibody (ipilimumab, Ipi)
In a phase III study on treatment-naive patients, both Ipi monotherapy group and Ipi + DTIC group had significantly higher OS than the control group. Among the non-treatment-naive patients, the OS was 10.1 months in Ipi group and was only 6.5 months in the control group (administered with gp100 vaccine only) (P=0.003) (137). Among the treatment-naive patients, the Ipi group also had significantly increased OS than the control (DTIC) group (11.2 vs. 9.1 months, P<0.001) (138). Notably, Ipi will cause severe immune-mediated toxicities. Therefore, special caution must be taken during its usage, and any possible toxicity must be closely observed. Ipi has not been licensed in China.
PD-1 monoclonal antibodies (pembrolizumab and nivolumab)
The US FDA has approved the use of PD-1 monoclonal antibody as second-line treatment in Ipi- and BRAF inhibitor-resistant melanoma patients. The panel believes that pembrolizymab and nivolumab have higher response rates than liplimumab and have fewer side effects; thus, these two drugs should be considered in the first-line treatment. In a large phase I clinical trial, pembrolizumab achived an overall response rate of 38%, although the median duration has not been reached (139). In patients who experienced PD despite Ipi treatment, the use of pembrolizuma achieved an overall response rate of 38%, although the median duration has not been reached (140). In another large-scale phase III clinical trial targeting treatment-naive patients with a wild-type BRAF genotype, the 1-year survival rate (73% vs. 42%), median PFS (5.1 vs. 2.2 months), and ORR (40% vs. 14%) were significantly superior in nivolumab group than in DTIC group (141). Both pembrolizumab and nivolumab can cause immune-mediated toxicities; although grade 3/4 toxicities are fewer than those treated with Ipi, close monitoring is still needed. The common adverse events (incidence >20%) include nausea, skin rashes, itching, coughing, diarrhea, decreased appetite, constipation, and joint pain. When severe immune-mediated pneumonia, colitis, hepatitis, hypophysitis, nephritis, and/or thyroid dysfunction occur, steroid therapy may be considered. For patients with a history of hypophysitis following the use of Ipi, hormone replacement therapy should be applied firstly before the initiation of pembrolizumab treatment.
CTLA-4 monoclonal antibody + PD-1 monoclonal antibody
In the 2015 ASCO annual meeting, the results of a clinical trial on the combination of PD-1 monoclonal antibody (nivolumab) and CTLA-4 monoclonal antibody (Ipi) were reported. Totally 142 patients with advanced or unresectable melanoma were randomized (at a ratio of 2:1) into Ipi (3 mg/kg, q3w ×4 w) + nivolumab (1 mg/kg), followed by maintenance treatment with nivolumab (3 mg/kg, every two weeks) or Ipi (3 mg/kg q3w ×4 w) + placebo (every two weeks). The primary endpoint was ORR, and the secondary endpoint was PFS. It was found that the ORR was 60% in the combination group and 11% in monotherapy group; in addition, the complete response rate was 12% and 0% and the PFS was 8.9 and 4.7 months in these two groups, respectively (P=0.0012). Subgroup analysis showed patients with poorer prognosis benefited more from combination therapy: The response rate was 53% vs. 0% and 62% vs. 25% in patients with elevated LDH and in stage M1c patients. Unfortunately, the grade 3/4 toxicities significantly increased in the combination group (51% vs. 20%). Except that patients with endocrine diseases needed additional replacement treatment, these toxicities can be improved by immunosuppressants (e.g., prednisone). In addition, the efficacy was similar in the combination and monotherapy groups among patients with high programmed cell death receptor ligand-1 (PD-L1) expression; in contrast, the combination group had significantly higher efficacy than monotherapy group among patients with low PD-L1 expression.
Interleukin-2 (IL-2)
IL-2 has complex biological effects. It mainly exerts its anti-tumor effect by enhancing CTL and NK cell lysis. As shown in some foreign studies (142-145), high-dose IL treatment (600,000−720,000 IU/kg, intravenous injection, repeated every 8 hours, 8−14 injections per cycle, with two cycles in each treatment course) attained a CR rate of 6% and a PR rate of 16−20%, with a median duration of efficacy of 8.9 months. Up to 44% of patients who had responded to the treatment remained alive in the 6th year. However, the majority of patients could not tolerate such high dose, and the dosage was reduced in most patients. Intravenously infused high-dose IL is more toxic than subcutaneously or intravenously injected low-dose IL; under the same dose, continuous infusion is more toxic than dripping. IL-2 treatment can be accompanied by flu-like symptoms such as fever, chills, muscle pain and fatigue; it specific toxicity is capillary leak syndrome, which is manifested as systemic edema, weight gain, pulmonary edema, pleural effusion, and ascites. Capillary leak syndrome-associated hypovolemia may cause reduced blood perfusion in kidney, gastrointestinal tract, heart, and brain, resulting in oliguria, ischemia, and ultimately dysfunction of the body. Due to the significant toxicity of high-dose IL-2, lower doses of IL-2 have been used with an attempt to reduce toxicity. However, although the toxicity was lowered after dose reduction, the response rate (often below 5%) and duration of efficacy often dropped accordingly. In a Chinese phase II clinical trial that treated advanced melanoma with recombinant human IL-2 (146), Chinese patients with advanced melanoma received high-dose IL-2 treatment, with ORR being 8.3%, PFS <2 months, PR 8.3%, CR 0, which were significantly lower than those reported in foreign literature. Although FDA has approved the use of IL-2 for the treatment of metastatic melanoma, the optimal dose and efficacy-predicting factors remain unclear and need to be addressed in multicenter randomized controlled trials.
Anti-angiogenic targeted therapy
Recombinant human endostatin injection (endostar)
As the most effective angiogenesis inhibitor ever known, endostatin can specifically inhibit endothelial cell proliferation and markedly suppress tumor growth and metastasis. However, endostatin is a protein preparation, with high costs and unstable nature. Excitingly, based on Folkman’s idea and previous basic research, Professor Luo et al. synthesed the recombinant human vascular endostatin, which successfully resolved many technical problems (e.g., protein refolding) and enabled the commercialized production and clinical application of recombinant human endostatin. Endostatin monotherapy has anti-tumor activity; in addition, it can exert remarkable synergistic effects when used in combination with the conventional chemotherapy and radiotherapy. In a multicenter, double-blind, randomized phase II clinical trial conducted in Chinese stage IIIc or IV melanoma patients who were treated with recombinant human vascular endostatin (endostar) or placebo + DTIC in the first-line treatment (147), totally 110 patients were equally randomized into group A (DTIC 250 mg/m2 d1−5 + placebo d1−14) and group B (DTIC 250 mg/m2 d1−5 + endostar 7.5 mg/m2 d1−14), with 21 days as one cycle. Among these 110 patients, 0.9% was in stage M1a, 32.1% in stage M1a, 44.6% in stage M1b, and 23.2% in stage M1c. In group A and group B, the objective response rate was 3.7% vs. 8.9% and the disease control rate (DCR) was 33.3% vs. 53.6% (P=0.051). The median PFS was 1.5 vs. 4.5 months (HR: 0.58; 95% CI: 0.38−0.89; P=0.013). The median OS was 8.0 vs. 12.0 months (HR: 0.52; 95% CI: 0.33−0.82; P=0.005). The 1-year survival rate was 22.5% vs. 49.7%, and the 2-year survival rate was 14.3% vs. 22.2%. These two groups had similar toxicities, and the overall treatment tolerance was good. Thus, compared with DTIC monotherapy, endostar + DTIC as the first-line treatment could significantly improve PFS and OS in patients with advanced melanoma, with good treatment tolerance. Thus, the panel recommends the use of this combination in the first-line treatment of advanced melanoma.
Bevacizumab
Bevacizumab is a recombinant humanized monoclonal IgG1 antibody that can selectively bind with vascular endothelial growth factor (VEGF-A) and prevent its binding with endothelial cell surface receptors (FIt-1 and KDR); by doing so, it can reduce the bioactivity of VEGF and decrease tumor angiogenesis. As a result, many small tumor blood vessels can be rapidly removed and degraded. Bevacizumab can also lead to the closing the endothelial windows and cell-cell junctions that increase the VEGF-dependent vascular permeability and thus decrease vascular permeability; thus, it can lower the pressure inside tumor tissue and thus facilitate the delivery of chemotherapy drugs into tumor tissue. Studies show that melanomas in later stages have more angiogenic factors and richer blood supply. These findings support the application of bevacizumab in treating melanoma. Several phase II clinical trials (148-150) reported the efficacies of bevacizumab-based combination regimens. In the BEAM study, 214 treatment-naive patients were randomized (at a ratio of 2:1) into paclitaxel + carboplatin + bevacizumab group or paclitaxel + carboplatin + placebo group; the results showed that paclitaxel + carboplatin + bevacizumab group had longer PFS (5.6 vs. 4.2 months; HR: 0.78; P=0.16) and OS (12.3 vs. 9.2 months; HR: 0.79; P=0.19), although the differences were not statistically significant. In another phase II clinical trial, the 2-week bevacizumab protocol in combination with carboplatin + paclitaxel (weekly) was applied in 53 patients seeking retreatment; the results showed that the median PFS and OS were 6 and 12 months, respectively. However, the incidences of ≥3 grade hematologic toxicities remarkably increased: neutrophils decreased by up to 53%, white blood cells by 38%, and platelets by 11%. Up to 31 patients suffered from over 40 bleeding events, including grade 2 bronchopulmonary bleeding disorders and fatal central nervous system bleeding in two cases.
Management of melanoma metastases at special locations
Management of the liver metastasis of melanoma
Liver metastasis may occur in 50−80% of patients with advanced melanoma, in particular in those with melanoma arising from mucosal tissues such as choroid, nose, and rectum. The efficacy of systemic chemotherapy is poor; once liver metastasis occurs, there is limited chance for treatment and the prognosis is extremely poor. The median survival is 2−6 months and the 1-year survival rate is 13% even after active treatment. The degree of hepatic metastases progression often determines the survival of patients. Its impact on survival is even larger than the primary tumor or metastases to other organs. The application of transcatheter arterial chemoembolization for liver metastasis from melanoma was first reported in 1988. Compared with systemic chemotherapy, hepatic arterial infusion chemotherapy (HAIC) can achieve higher drug concentration in tumor tissue and reduce the toxicities (e.g., bone marrow suppression) of systemic drugs. In a retrospective study on the hepatic metastasis of ocular melanoma reported by the MD Anderson Cancer Center, USA (151), the efficacies of systemic chemotherapy, arterial infusion chemotherapy, and HAIC were compared; it was found that the response rate of systemic chemotherapy was below 1%, whereas platinum-based arterial infusion chemotherapy is the only therapeutic approach that can improve the survival (with a response rate of 36%), which was significantly superior to the other two therapeutic approaches. A domestic study (152) showed that HAIC could achieve a local control rate of 50% for liver metastasis.
Management of the brain metastasis of melanoma
Brain metastasis is also common in melanoma patients. It has been reported that (153) the incidence of brain metastasis of malignant melanoma (BMM) is 8−46%, whereas autopsy has shown that two thirds 2/3 of melanoma patients had brain metastasis. BMM is the end stage of melanoma; the disease progresses rapidly and often is the main cause of death. Surgical resection remains an important treatment for BMM. The reported median survivals after surgery ranged 5−6.7 months. Although surgical treatment is effective, its indications are quite limited. It is believed that surgical treatment is only suitable for solitary BMM; for multiple tumors, however, surgical treatment has limited value. Surgery itself has a certain degree of risk; furthermore, the spread of tumor to meninges and cerebral ventricle system following surgical resection makes the condition even more challenging. Increased intracranial pressure as well as obstructive hydrocephalus and difficult-to-control epilepsy caused by solitary and large space-occupying mass should be treated with surgery. Postoperative radiotherapy is recommended to timely kill the residual lesion and any subclinical foci that may already spread. Radiotherapy is generally recommended for multiple brain metastases. No effective treatment has been available for multiple diffuse BMM. Whole-brain radiotherapy (WBRT) remains a treatment in clinical settings. Literature in the past decades has shown that the median survival after WBRT was only 2−4.4 months, with few cases being reported. Stereotactic radiosurgery (SRS), also known as Gamma knife), was formally introduced in clinical practice in 1960s and has become widely applied since 1990s. Using a stereotactic technique, SRS projects a single large dose of rays onto the clearly defined intracranial lesion, making the lesion exposed to high-dose irradiation, whereas the radiation on normal tissue around the lesion dramatically decreases. Similar to surgical treatment, SRS also can control disease condition and improve neurological symptoms in patients with solitary or a small number of brain metastases. The main advantages of SRS include: minimally invasive; easy to perform; well tolerated by patients; and with relatively few complications. Therefore, SRS has wider indications than surgery for brain tumors. In addition, SRS is often used in combination with surgery and conventional radiotherapy and thus serves as an effective approach for patients with multiple metastases with varied sizes at different locations. Xie et al. (154) retrospectively analyzed the clinical data of 30 patients with brain metastases of melanoma who were treated in the Gamma Knife Center of Air Force General Hospital from 2006 to 2010 and found that, after a mean follow-up period of 15.5 months, the OS rate was 53.3%, the 12-month OS rate was 20%, and the median survival was 6.25 months.
Management of the bone metastasis of melanoma
The management of the bone metastasis of melanoma is similar to the management of the bone metastasis of other tumors. The treatment is mainly based on the metastasis location (weight-bearing bone or not) and symptoms, and the main treatment goal is to reduce the incidence of bone events and relieve the pain. Isolated bone metastases can be treated with surgery, followed by local radiotherapy if appropriate. In addition to systemic treatment, patients with multiple bone metastases should also be provided with local therapies including surgery, bone cement, and local radiotherapy. Routine use of bisphosphonates may reduce incidence of bone events. Pain drugs may be added in patients with pain. The treatment protocol for patients with spinal cord compression depends on the general status of the patients. Surgical decompression and postoperative radiotherapy may be applied in patients with relatively good prognosis and low tumor load; radiotherapy alone, however, may be considered in patients with poor general conditions. Radiotherapy is often indicated for relieving bone pain and for treatment after internal fixation.
Note
The recommendations proposed in these guidelines are just for reference. The specific individualized treatment must be determined based on the clinicians’ judgment and other factors including the local health care conditions, professional level as well as patients’ needs, desires, and expectations.
Flow chart of the management of mucosal melanoma (MM)
Flow charts of the management of MM were shown in Tables 13,14.

Full table

Full table
Head and neck mucosal melanomas (MMs)
Epidemiology
MM is a race-related disease, with its incidence remarkably higher in colored populations than in Caucasians. Head and neck MMs account for 25−30% of all MMs. Nasal cavity, paranasal sinuses, and nasopharyngeal areas are the most commonly involved areas, and MMs in these areas account for 0.3−2% of all malignant melanoma, 3.5−7% of nasal, paranasal, and nasopharyngeal tumors, and 70−80% of head and neck MMs (155,156).
Primary nasal, paranasal, and nasopharyngeal malignant melanomas arise from ectoderm and are transformed from melanocytes in the respiratory mucosa; nasal cavity is the most common onset site, followed by maxillary sinus, ethmoid sinus, and sphenoid sinus. Malignant melanoma of the nasal cavity is more likely to occur in individuals aged 50−80 years (mean 65 years), with a male/female ratio of 1:1−2:1 (155).
Most head and neck MM are always in their advanced stage when diagnosed, and their origins are difficult to confirm. Melanoma in the skull base is often associated with cribellum, orbital contents, and dura mater involvement (157). Lymph node metastasis rate ranges 6−22%, and nasal, paranasal, and nasopharyngeal MMs often spread to lungs and brain (158-160).
Genes
Few large-scale genetic studies have been conducted on MM. Many foreign studies with small sample sizes (161-163) found that the BRAF gene mutation rate was 0−10% in MM patients, KIT gene mutation rate was 5−27%, and NRAS gene mutation rate was 13−22%.
Recent studies have also found variations of p16INK4A in MM. Suzuki et al. detected 12 patients with head and neck MM and found gene mutations or loss of heterozygosity in six cases. Another study on primary nasal MM showed that 55.2% of MM patients had p16INK4A depletion (164). Gene testing for over 100 Chinese MM patients showed that the mutation rates of KIT, BRAF, and NRAS genes were was 19.2%, 12.5%, and 9.2%, respectively (10,11). Multivariate analysis showed KIT mutation was an independent prognostic factor of MM, with a hazard ratio of 2.696 (95% CI: 1.204−6.038) (P=0.016).
Diagnosis and staging
Pre-operative evaluation
In addition to routine staging examinations (e.g., head and chest CT, abdominal and pelvic CT or ultrasound, and bone scan), the scope of primary lesion and cervical lymph node metastasis (if any) must be carefully evaluated. Generally, endoscopy, enhanced CT, and (or) MRI can be used to determine primary tumorus range, CT can be used for observing bone damage, and MRI may be used for observing soft tissue involvement, particularly brain involvement. Ultrasound and enhanced CT have similar values in assessing cervical lymph nodes. These techniques should be used flexibly in different hospitals. The nasal mucosa has rich blood supply; therefore, strip biopsy deep inside the nasal cavity should be avoided to prevent tumor spread during the filling/compressing maneuvers following bleeding. Preoperative biopsy should be avoided for melanotic MM. Intraoperative frozen-section examination is recommended; however, it is highly depended on the skills and experiences of the pathologists. For highly suspicious amelanotic MM, preoperative biopsy combined with intraoperative frozen-section examination is recommended. The head and neck mucosal lymphatic drainage is very complicated; in particular, the upper neck has pharyngeal lymph chain, which is composed of rich lymph node tissues. Therefore, it is extremely difficult to locate the SLNs of nasal, paranasal, and nasopharyngeal MMs, and SLNB is not recommended as a routine test in these patients.
Preoperative staging
No widely recognized staging system of head and neck MM has been available. AJCC, Kish, and TNM staging systems are often used, among which the AJCC staging system (7th edition) [2009] is most commonly applied:
- Stage IIIA: T3aN0M0;
- T3: mucosal lesion;
- T4a: mid-term lesion involving deep tissue, bone or cartilage;
- T4b: advanced lesion involving the brain, dura mater, skull, and posterior cranial nerves (IX, X, XI, and XII), masseteric space, internal carotid artery, prevertebral space, or mediastinal structures;
- N1: regional lymph nodes;
- M1: distant metastasis;
- Stage IVA: T4aN0M0, T3N1M0, T4aN1M0;
- Stage IVB: T4b, any N, M0;
- Stage IVC: any T, any N, M1;
- Note: different from conventional staging method, this tumor staging approach has no T1 and T2, emphasizing that this tumor is highly malignant.
Multidisciplinary treatment (MDT)
MDT strategies including complete preoperative evaluation, reasonable surgical procedure and resection scope, and necessary postoperative therapies are recommended. While surgical treatment is preferred for early-stage head and neck MM, MDT is emphasized for advanced disease. The principles of MDT are as follows (165-167):
Patients with stage III MM: the primary lesion must be thoroughly removed; radiotherapy of the primary lesion or close follow-up is recommended after the surgery;
Patients with stage T4aN0 MM: the primary lesion must be thoroughly removed; radiotherapy of the primary lesion is recommended after the surgery;
Patients with stage T3−T4aN1 MM: the primary lesion must be thoroughly removed; regional cervical lymph node dissection should be performed in patients with regional lymph node metastasis, and additional radiotherapy should be performed on the primary lesion and neck after surgery;
Stage IVB: patients should be encouraged to enter clinical trial or receive palliative radiotherapy or systemic therapy;
Stage IVC: patients should be encouraged to enter clinical trial or receive systemic therapy; or, hospice care should be provided.
Pre-operative evaluation
The essential preoperative evaluation includes the range of primary lesion, lymph node metastasis, and distant metastasis, among which whether the primary is resectable and whether there is any distant metastasis is core links of the whole treatment protocol. Unresectable primary lesion or distant metastasis is contraindications of surgery.
- Primary lesion assessment: for head and neck MM, enhanced CT and/or enhanced MRI should be performed to judge the range of tumor. Invasion of skull or localized meninges by nasal, paranasal, or nasopharyngeal tumor is not a contraindication of surgery. When the tumor invades local brain tissue, the resectability of the tumor and any possible complications following the resection should be evaluated together with neurosurgeons. If the tumor is surrounded by large cervical vessels, these vessels may be peeled off or directly ligated; or, reconstruction of the internal carotid artery may be performed. However, the complications following direct ligation and high reconstruction of the internal carotid artery must be taken into consideration;
- Local metastasis evaluation: ultrasonography and enhanced CT have similar values in cervical lymph node evaluation. It is recommended that each hospital may flexibly apply the relevant rules according to their specific conditions;
- Distant metastasis evaluation: head and chest CT, abdominal ultrasound or enhanced CT/MRI, and whole-body bone scan may be applied. If economic conditions permit, PET-CT full is recommended because it can clarify systemic metastasis and correct local lymph node staging.
Surgical treatment
Surgical treatment includes the resection of primary lesion and local metastasis; surgical treatment is preferred for stage T3 and T4a head and neck MM.
Resection of primary tumor
For the resection of early nasal, paranasal, and nasopharyngeal MM, both the conventional nasal lateral wall dissection approach and the endoscopic approach can achieve the complete resection of the tumor. The endoscopic surgery is superior because it allows the surgeon to find the microsatellites and the nearby pigmented spots and meanwhile it is featured by small surgical trauma and quick postoperative recovery.
Appropriate indications must be selected for endoscopic resection. Endoscopic operation must meet the basic principles (e.g., en bloc resection, no local squeezing, etc.) of open surgical resection of malignant tumors.
Appropriate patients must be selected during endoscopic surgery. The general treatment principles include en bloc resection, no local squeezing, and ensuring a negative margin. The range of mucosal resection of lesions includes normal mucosa 1.5−2 cm away from the tumor border (including satellites). Some MM patients may also have mucosal pigmentation, which can also be removed if the pigmentation is localized. If the pigmentation is diffuse and can not be removed, it should be closely followed. The ranges of the deep resection of lesions vary; generally, whether the resection is complete is judged according to the results of intraoperative frozen-section examination. For nasal, paranasal, and nasopharyngeal MM, the tumor bed is often bony and thus the resection margin can not be learned by intraoperative frozen-section examination. In these cases, the safe resection margin will be the adjacent anatomic area with normal imaging findings around the tumor. If the tumor has invaded the mandibular periosteum, partial, horizontal, or vertical resection of the mandibula may be performed (168), with the cutting edge 2 cm away from the tumor.
Patients tend to have a poor prognosis if one of the following factors exists (169): (I) already in clinical stage T4b, and only palliative resection is feasible; (II) the internal pterygoid muscle is diffusely involved; or, the intracranial lesion invades the pterygopalatine fossa; (III) the lesion damages the skull and involves the intracranial tissues; (IV) the lesion involves the nasopharynx or invades the eustachian tube and bilateral pharyngeal recess; (V) the lesion invades the internal carotid artery; that is, radiology shows that the tumor surrounds the internal carotid artery by over 270°; and (VI) the lesion invades neck skin, mediastinal structures, prevertebral fascia, or cervical spine; or, there is subcutaneous metastasis.
Management of cervical lymph nodes
Nasal, paranansal, and nasopharyngeal MMs have a low rate of cervical lymph node metastasis. In principle, prophylactic dissection should not be performed in these patients (169-171). Close follow-up is recommended. MMs arising from oral cavity, throat, and esophagus have a rate of lymph node metastasis. Conventional functional or radical lymphadenectomy is recommended for clinically or radiographically confirmed metastasis; if there is no clinically or radiographically confirmed metastasis, SLNB or prophylactic cervical lymphadenectomy may be routinely performed (171,172).
Management of local or regional recurrence
For patients with local recurrence, if the local re-staging does not reach R-T4b, surgical resection can still be considered. For patients with regional recurrence (i.e., localized lymph node recurrence), if there is neither relapse of primary lesion nor distant metastasis, functional or radical cervical lymphadenectomy may be considered.
Other treatment options
Radiotherapy
(I) Adjuvant radiotherapy: Postoperative adjuvant radiotherapy can improve the local tumor control rates; however, no high-level evidence has indicated that postoperative radiation therapy can prolong survival.
The indications include: irradiation of the tumor bed (with a safety margin of 2−3 cm) for T3−T4aN0−1 and T3−4aN0 after radical resection; irradiation of the tumor bed (with a safety margin of 2−3 cm) (172-174) and irradiation of the cervical lymph node drainage areas (11,175) for T3−4aN1.
Irradiation may be initiated within 6 weeks after surgery. The radiotherapy dosages were as follows: high-risk areas (lymph node number ≥2, diameter ≥3 cm, lymph node extranodal invasion, and local recurrence after lymph node dissection): 60−66 Gy/6−6.5 weeks; intermediate-/low-risk areas (with suspicious subclinical lesion): 44−50 Gy/4−5 weeks.
(II) Palliative radiotherapy of the primary lesion: The main purpose of palliative radiotherapy is to reduce symptoms and improve the quality of life.
Patients with stage IVB/C head and neck MMs should be encouraged to participate in clinical trials or receive palliative radiotherapy of the primary lesion: high-risk areas (primary lesion and metastases to lymph nodes): 66−70 Gy/6−7 weeks; intermediate-/low-risk areas (with suspicious subclinical lesion): 44−50 Gy/4−5 weeks. The dosage and radiotherapy plan should be adjusted according to the patients’ physical performance and the presence of any metastasis.
(III) Principles of palliative radiotherapy for distant metastasis: (i) Radiotherapy of metastasis: palliative pain relief, or prevention of pathological fracture: 30 Gy/10 f, or 6−8 Gy/1 f;
(ii) Brain metastasis (stereotactic treatment is preferred; if the number of metastatic lesions is >5 and the diameters ≥3 cm, WBRT may be considered);
(iii) The role of whole brain radiotherapy following the resection of brain metastasis remains controversial and needs to be tailored. The relevant randomized clinical studies are now underway.
Adjuvant chemotherapy
The main purpose of adjuvant chemotherapy is to prolong RFS and OS.
In a randomized phase II study (176) on the postoperative adjuvant therapy in MM patients, which was conducted by the Tumor Hospital of Peking University, patients were randomized into three groups: watchful observation group (group A), high-dose interferon α-2b treatment group (group B: 15 million IU/m2 d1−5 ×4 w, 9 million IU tiw ×48 w) and TMZ + DDP combined chemotherapy group (group C: TMZ 200 mg/m2 d1−5 + DDP 75 mg/m2, in 2 days). The main endpoints were RFS, OS, and safety. Totally 189 patients were enrolled, with a median follow-up of 26.8 months. It was found that the median RFS was 5.4, 9.4 and 20.8 months, respectively, in groups A, B, and C, and the estimated median OS was 21.2, 40.4 and 48.7 in these three groups. Compared with groups A and B, group C had significantly longer RFS (P<0.001) and OS (P<0.01), with tolerable toxicities.
Systemic treatment
Systemic treatment should be the mainstream treatment for unresectable IVB/IVC MMs.
(I) CTLA-4 monoclonal antibody: CTLA-4 is a member of the CD28:B7 immunoglobulin family and is lowly expressed on the surface of naive T cells and regulatory T cells (Tregs). After the naive T cell surface receptors receive stimulation, CTLA-4 in serosa competes with CD28 B7 to bind B7 and ultimately shut off T-cell signal transduction (177). Ipi, an antibody against CTLA-4, can block the effect of CTLA-4 to enhance the functions of T cells and thus induce anti-tumor immunity.
In a retrospective study reported by Micheal et al., among 33 patients with unresectable or metastatic MM, the use of Ipi achieved immune-related complete response in 1 case, immune-related partial response (PR) in 1 case, immune-related stable disease in 5 cases, and immune-related disease progression in 23 cases; the OS (beginning from Ipi treatment) was 6.4 months (178). A recent study in Italy suggested that Ipi may be a treatment option for MM patients who had previously received other treatment. A total of 855 patients [including 71 patients (8%) with metastatic MMs] were enrolled in this study. The response rate was 12% (partly in the mucosal subgroup); the immune-related DCR was 36%, and the PFS and OS were 4.3 and 6.4 months, respectively, which showed no significant difference among different melanoma subtypes (179).
(II) PD-1 monoclonal antibody: PD-1 exerts its inhibitory activity during the effector phase of T cells. PD-1 and its ligands B7-H1 and B7-DC (PD-L1 and PD-L2) interact with each other in the peripheral tissues (including the tumor microenvironment), during which it induces tumor cell apoptosis by down-regulating T cell effector functions. PD-L1 may be a possible mechanism via which melanomas evades the endogenous immune response (180). PD-1 monoclonal antibodies (nivolumab and pembrolizumab) have been approved as the first-line treatment of advanced melanoma (139,181); however, their efficacies in treating metastatic MM still need to be further evaluated in subsequent clinical trials. The panel recommends the use of Ipi, nivolumab, and pembrolizumab as the first-line treatment for advanced MMs.
(III) Personalized targeted therapies: Please refer to the relevant contents in skin melanoma.
(IV) Angiogenesis inhibitors: Angiogenesis provides nutrients and oxygen to the rapidly growing tumor and provides channels for tumor metastasis. Angiogenesis-related receptors and ligands are often overexpressed in melanoma, which is associated with disease progression and prognosis. Some clinical trials have reported the outcomes of the combinations of angiogenesis inhibitors and conventional cytotoxic drugs in treating advanced MMs.
VEGF is highly expressed in melanoma. Bevacizumab is a monoclonal antibody that can selectively bind VEGF and block its binding with receptors. It has been widely applied in the treatment of metastatic melanoma (182-185). In a randomized, double-blind phase II study (BEAM study), 214 metastatic melanoma patients were randomized into carboplatin + paclitaxel + bevacizumab (CPB) group or placebo group (CP) at a proportion of 2:1. In the MM subgroup, the death risk decreased by 76% in the CPB group (HR: 0.24; 95% CI: 0.05−1.27). Currently, a study on the combination of CPB as the first-line treatment of MM was ongoing in China.
Endostar®, a typical anti-angiogenic agent, is a 20-kDa C-terminal fragment derived from type XVIII collagen. A phase II trial evaluated the efficacy of Endostar® combined with DTIC in the treatment of BRAF and C-KIT wild-type metastatic melanoma. Totally 110 patients (including 16 MM patients) were enrolled. Compared with the DTIC monotherapy group, the combination group had better tolerance and improved PFS and OS among metastatic melanoma patients (147).
(V) Chemotherapy: Metastatic MM is relatively insensitive to chemotherapy. In a retrospective study conducted in the Republic of Korea, 95 patients with metastatic melanoma (including 3 cases of skin melanoma, 37 cases of acral melanoma, 28 cases of MM, and 7 cases of ocular melanoma) were enrolled to receive DTIC chemotherapy. The overall response rate was 26.3% (20% for MM patients), with an OS of 12.1 months (186). This was similar to the findings in Caucasians (187,188).
Paclitaxel + carboplatin can be a reasonable option for metastatic MM patients who had previously received multiple cycles of treatment. A retrospective study in the Republic of Korea compared the effectiveness of paclitaxel + carboplatin in the salvage chemotherapy for metastatic MM patients who had failed in DTIC-based chemotherapy. Of 32 metastatic MM patients who had previously received multi-line treatment, 10 (31.3%) had MMs, and all these patients had received a mean of three DTIC-based chemotherapy protocols. The median PFS was 2.53 months in all patients, and 21.9% of them achieved PR. No significant difference was observed between MM patients and cutaneous melanoma patients, indicating that paclitaxel combined with carboplatin may also be a reasonable option for metastatic MM patients who have received multi-line treatment (189).
The role of new chemotherapy drugs (e.g., albumin-bound paclitaxel) in combination with carboplatin in treating metastatic MMs remain unclear. While chemotherapy still has a low response rate in treating MM, it can still be an appropriate treatment option. Chemotherapy combined with angiogenesis inhibitors may be a reasonable option for advanced MMs.
Prognosis
The 5- and 10-year survival rates of patients with nasal, paranasal, or nasopharyngeal malignant melanoma are 20% and 28% (190). Tumors with deep infiltration, necrosis, and vascular involvement often predict poor prognosis. The rate of lymph node metastasis is low. Tumor size has small influence on survival. Both large and small tumors can be associated with distant metastasis (155). The most common metastatic sites are the lungs and brain. In patients with distant metastasis, the median survival time was 7.1 months (191). However, no data based on large sample have reported the prognosis of patients with malignant melanoma in oral cavity, pharynx, hypopharynx, or larynx.
Follow-up
Frequency of outpatient re-examinations
Every three months within 18 months after surgery; every 6 months within 18 months−3 years after surgery; every 12 months within 3−5 years after surgery; and every 12 months after 5 years. The purpose of re-examination is to rule out local recurrence, local and regional lymph node recurrence, and distant metastasis, with the distant metastasis being the focus of each re-examination.
Detection items
Monitoring of local recurrence: nasal endoscopy should be performed during each visit. Nasal, paranasal, and nasopharyngeal imaging should be performed every 6 months to learn if there is any local recurrence. Since this is a repeated procedure, enhanced MRI is recommended to minimize radiation exposure.
Monitoring of regional recurrence: neck ultrasound should be performed during each visit. When necessary, enhanced CT or enhanced MRI may be performed to learn if there is any lymph node metastasis.
Monitoring of distant metastasis: head and chest CT, bone scan, and abdominal ultrasound/CT should be performed every year; if necessary, whole-body PET-CT may be performed to judge if there is any systemic metastasis. If any symptom of distant metastasis is suspected during the follow-up, in-depth examination should be immediately performed locally; or, whole-body PET-CT may be performed.
Gastrointestinal malignant melanomas
Epidemiology
Anorectal malignant melanoma (AMM) is the most common gastrointestinal melanoma, and melanomas arising from stomach or small intestine are extremely rare. Hereafter in this section we will focus on AMM. It has been reported that AMM accounts for 0.5−2% of all malignant melanomas (192,193) and 23.8% of MMs. Meanwhile, its incidence has shown an increasing trend. The median onset age was 60 years. The number of female patients is slightly larger than that of male patients. The most common metastatic site of AMM is liver, followed by lungs, brain, and bone (194).
Risk factor
Unlike cutaneous melanoma, the risk factors of AMM remain unclear. Exposure to UV, one of the main risk factors of cutaneous melanoma, is not correlated with the occurrence of AMM.
Clinical manifestations
AMM is often located near the dentate line, hidden, with insidious onset. Clinically it is mainly manifested as bloody stool, although other symptoms may also include perianal itching or pain, anal neoplasm, tenesmus, and change in bowel habits . Once metastasis occurs, the patient may suffer from fatigue, weight loss, anemia, groin or pelvic mass, or even intestinal obstruction. Colonoscopy may reveal black protruding Lesions, which may also be amelanotic changes in some cases. Clinically AMM may be misdiagnosed as other conditions such as hemorrhoid, skin tag or polyp.
Diagnosis and staging
Pathological examination should be performed for suspected AMM. The staging examinations include digital rectal examination, colonoscopy, endoscopic ultrasound, CT, MRI, and PET-CT, which can be used for assessing the tumor invasion depth, regional lymph node metastasis, and distant metastasis. Notably, MRI is a standard method for evaluating lesion area (195).
At present there are no uniform standards of AMM staging. The AJCC TNM staging method is not entirely applicable to AMM. A more commonly used staging method for AMM is as follows: stage I (local), the tumor is localized in anorectal wall or perianal skin; stage II (regional), with regional lymph node metastasis; and stage III (disseminated), the tumor becomes unresectable. The staging criteria proposed by Falch et al. (196,197) can also be useful: stage I, the tumor is localized in anorectal wall and does not reach the muscular layer; stage II, the tumor is still localized but has invaded the muscular layer of the anorectal wall; stage III, the tumor becomes locally advanced and/or with regional lymph node metastasis; and stage IV, with distant metastasis (194).
Surgery
Surgical resection is the main treatment for AMM. Radical resection is mainly used in patients with stage I patients and a small proportion of stage II patients. Stage III patients can only receive palliative surgery under special conditions. Current evidences do not support the use of prophylactic inguinal lymph node dissection (198).
How to select an appropriate surgical procedure is the main dispute. R0 resection is the primary goal of surgical resection. The main surgical procedures include abdominoperineal resection (APR) and wide local excision (WLE). APR can achieve better local control and negative resection margin; also, it enables the dissection of mesenteric lymph nodes. However, it requires a large surgical scope; in addition, it can not spare anal sphincter and thus impairs the patients’ quality of life. APR can also be applied in patients with intestinal obstruction and those requiring a salvage surgery. WLE requires a cutting edge of ≥10 mm. The outcomes of these two procedures do not differ significantly. If R0 resection can be achieved, WLE should be the first choice (199,200). Therefore, multiple factors including the possibility of R0 resection, risk of local recurrence, and quality of life should be considered during the selection of a surgical resection procedure.
Adjuvant chemotherapy and systemic treatment
Please refer to the “Head and neck mucosal melanoma (MM)”.
Prognosis
AMMs are highly malignant and prone to recurrence and distant metastasis. The prognosis is associated with tumor stage. It has been reported that the 5-year survival rates of AMM patients were below 20%, and the median survival time ranged 8−19 months (194). The mean survival time of patients with localized AMM was only 34 months even after treatment, and the median survival of patients who had experienced recurrence or metastasis was below 10 months (201). Therefore, early diagnosis and treatment is extremely important. Surgery remains the most important and effective treatment method. The management of AMM patients with metastatic lesions may refer to “Metastatic cutaneous melanoma”.
Urogenital melanomas
Epidemiology
Primary urogenital melanoma accounts for about 16% of MMs. It mainly occurs in the vulva, vagina, cervix, and uterus of femal patients (202), whereas melanomas occurring in urethra and bladder are relatively rare. Vulvar melanoma is the most common urogenital melanoma, and it is also the second most common type of vulvar cancer (only after squamous cell carcinoma). It is mainly seen in postmenopausal women aged 60−70 years.
Clinical manifestations
Vulvar melanoma is clinically manifested as vulvar nodules, bleeding, itching, and local pigmentation, which can also be accompanied by ulceration and pain. While it can occur at any site of vulva, it is more likely to occur in a smooth mucosa such as the medial side of major lips, minor lips, clitoris, and vaginal orifice, followed by the junction (e.g., major lips) between hairy skin and smooth skin; finally, the hairy skin (e.g., pubes) (203). The vagina and cervix melanomas are mainly manifested as vaginal bleeding or mass. Although genital melanoma is uncommon, attention should be paid to the presence of any of the above symptoms or the suspious pigmented lesions found during routine gynecological examinations. Local excision is advisable for biopsy.
Staging
There are no uniform staging standards for genital melanoma. The AJCC staging criteria for cutaneous melanoma (7th Edition) may be applied. According to the depth of tumor invasion, ulceration, and suspicious lymph nodes, Tasseron et al. [1992] divided the patients into two categories: low-risk, the depth of tumor invasion is below 3 cm, no ulceration; and no clinically suspicious lymph node; high-risk, the depth of tumor invasion is above 3 cm, with ulceration; and/or with clinically suspicious lymph node(s) (204). In addition, since the clinical manifestations and metastasis pathways of cervical, vaginal, and vulvar melanomas are similar to those of other malignancies in these locations, some authors have proposed to use the International Federation of Gynecology and Obstetrics (FIGO) staging systems for genital tumors instead.
Surgery
Surgical treatment for vulvar melanoma may refer to the treatment strategies for vulvar cancer. The scope of operation and handling of the lymph nodes remain controversial. The main surgical procedures include WLE, radical resection of the vulva, and bilateral inguinal lymph node dissection. An increasing number of evidences have shown that radical resection of the vulva plus and bilateral inguinal lymph node dissection can not improve the OS; thus, local extended resection should be performed when the tumor invasion depth is below 1 cm (205). According to Irivin et al., the resection margin is 1cm when the thickness of vulvar melanoma is <1 mm; the resection margin is 2 cm when the thickness of vulvar melanoma is 1−4 mm; the resection margin should be at least 1cm in depth, extending from subcutaneous fat to muscle fascia (206).
In recent years, the role of SLNB in the surgical treatment of vulvar melanoma has been confirmed; thus, it has gradually become a routine procedure in the management of vulvar melanoma. In patients in whom the lymph node is not palpable, tumor thickness >1 mm, or in patients in whom the tumor thickness is <1 mm but there are poor prognostic factors such as ulceration, high MR, microsatellites, vascular invasion, and Clark level IV, radioactive colloids or organic blue dyes can be injected at the primary lesion to locate the SLNs, followed by biopsy. Lymph node dissection should be performed in patients with positive SNs (207).
Recurrence and metastasis of vulvar melanoma are common. The site of recurrence is often associated with surgical procedure: patients who have undergone local resection tend to experience local recurrence, and patients who have received radical resection are more likely to suffer from distant metastasis. Scheistroen et al. reported 43 cases of melanoma of the vulva, the postoperative recurrence rate reached 63% (208). Patients with local recurrence or solitary distant metastasis still require surgical resection. Patients with unresectable or extensive metastases, the prognosis is extremely poor, even after radiotherapy or drug therapy.
Adjuvant chemotherapy and systemic treatment
Please refer to the “Head and neck mucosal melanoma (MM)” and “Metastatic cutaneous melanoma”.
Prognosis
The prognosis of genital melanoma is poor due to high local recurrence and distant metastasis rates. The reported 5-year survival rates ranged 27−60% (209). The main prognostic factors include tumor thickness, ulceration, and resection margin (negative or not). Early diagnosis can improve survival. Therefore, when carrying out skin examinations for elderly women, the medical staff should also encourage them to undergo gynecological examinations to screen the genital melanoma.
Uveal melanomas (UM) in eyes
Epidemiology
UM is the most common primary intraocular malignancy in adults. While UM can originate from the intermediate uveitis (including iris, ciliary body, and choroid) of eye wall, over 90% of UMs are choroidal tumors (210). Although ocular melanoma accounts for only 5% of all melanomas, it is the second most common type of melanoma (only after cutaneous melanoma) (211).
It has been reported that the incidence of UM ranges 4.3/1,000,000−10.9/1,000,000. According to Surveillance, Epidemiology, and End Results, a program under the US National Institutes of Health (NIH), the overall mean incidence of UM is 4.3/1,000,000, which is higher in males (4.9/1,000,000) than in females (3.7/1,000,000) (212). Kivelä (213) estimated that there was 6,679−7,095 new UM cases worldwide annually. UM is prone to occur in light-skinned populations. The reported UM incidences are as follows (every 1,000,000): Africans, 0.31; Asians, 0.38; Hispanics, 1.67; non-Hispanic Caucasians, 6.02 (214). Currently there is no statistical data on the incidence of UM among Chinese populations.
Biological characteristics
UM differs from cutaneous melanoma completely in terms of clinical feature and biological behaviors (215). For example, UM mainly spread via blood stream; in contrast, cutaneous melanoma often spread via lymphatic vessels. About half of the UM patients eventually develop hematogenous metastasis, with liver metastasis being the most common form. Without proper treatment, most patients with metastasis will die within a short period of time (6−8 months) (216). According to the Collaborative Ocular Melanoma Study (COMS), a large multicenter study participated by 43 clinical ophthalmic centers in North America, the median survival was only 3.6 months among UM patients with confirmed metastasis, and the 5-year cumulative survival rate was only 1% (217).
UM can be associated with multiple cytogenetic abnormalities (e.g., chromosome 3-monomer deletion and chromosome 8 gain) (218,219). The typical molecular abnormally of UM is GNAQ/11 mutation (220-222), whereas BRAF and NRAS mutations are rare; in contrast, the most prominent mutations in cutaneous melanoma are BRAF, CKIT, and NRAS mutations (223,224).
Diagnosis and staging
The combination of indirect ophthalmoscopy, ultrasound, and MRI can achieve accurate clinical diagnosis of UM. COMS reported that the clinical diagnostic accuracy was higher than 99% for UM (225). Based on the maximum base diameter and height of the tumors, COMS divides UM into three types: small, medium, and large (Figure 2) (226,227).
The posterior UM staging in AJCC staging system (7th Edition) is shown in Tables 15 and 16. With the AJCC stage I UM as the benchmark, the metastasis/mortality rate is 3 times in stage III patients and 9−10 times in stage III patients; it is even higher in stage IV patients (228-230).

Full table
Prognostic indicators
Detecting haplotype chromosome 3 in UM tissue and typing UM by gene expression profiling (GEP) have been widely recognized as useful approaches for predicting prognosis in UM patients.
Cytogenetic tests: 50% of UM patients with haploid chromosome 3 will die within 3 years. Patients with diploid chromosome 3 accompanied by chromosome 8Q amplification will have extremely poor prognosis (218,219).
Molecular genetic tests: GEP can distinguish two tumors with markedly different metastatic potentials. The metastasis risk is small (<5%) for type 1 but can be extremely high (90%) for type 2 cells (231-233).
For patients with high risk of metastasis, close whole-body examinations should be performed to achieve the early identification of tiny metastases and thus grasp the chance of local resection; or, these patients may be encouraged to join new drug trials. For patients with low risk of metastasis, excessively frequent medical examinations should be avoided to increase their quality of life.
Treatment
Local treatment in eyes
In the COMS study, patients with medium UM were assigned randomly to enucleation group and 125I brachytherapy group; the unadjusted estimated 5-year survival rates were 81% and 82%; there was no clinically or statistically significant difference in survival rates overall. Five-year rates of death with histopathologically confirmed melanoma metastasis were 11% and 9% following enucleation and brachytherapy, respectively (234). This study provided key evidences for the local treatment in eyes for UM patients. The treatment methods can be chosen based on tumor size and location. The eye and even vision should be saved with the premise that it will not decrease the survival rate.
Radiotherapy with scleral surface applicator
This is the treatment of choice in most eye centers in developed countries. It is a brachytherapy, with a metal disc containing radioactive particle (125I or 106Ru) placed on the local scleral surface. Brachytherapy is recommended for small and medium UM.
Enucleation
Enucleation can be considered if the tumor is localized in eyeball. It is recommended for large UM patients whose eyeball is painful and visionless or has no light perception.
Exenteration
Exenteration may be performed in patients in whom the large tumor has penetrated the eyeball and reached the orbital cavity.
Other treatments
Other treatments include proton beam radiotherapy, stereotactic radiotherapy, and local excision of tumors.
Systemic treatment
No large randomized clinical study has demonstrated that systemic treatment can prolong the OS of patients with metastatic UM. Chemotherapy, hepatic artery embolization and chemotherapy, immunotherapy, targeted immune therapy, and individualized molecularly targeted therapy have been attempted in patients with metastatic UM; however, most of these attempts were reported only in non-randomized uncontrolled studies with small sample sizes or in stage I and II clinical trials (235,236). New and more effective treatments are urgently needed for metastatic UM. Participating in clinical trials is the preferred strategy for patients with metastatic UM.
MEK inhibitors
Based on the GNAQ/11 mutation, which is commonly seen in UM patients, and the activation of MAPK pathway, which is directly triggered by GNAQ/11 mutation, the MEK inhibitors have been widely recognized as promising agents in the treatment of UM. In 2014, Carvajal et al. reported the results of a randomized, multicenter, open-label phase II trial participated by 15 clinical centers in the United States and Canada, comparing the efficacies of selumetinib vs. DTIC in treating 120 patients with metastatic UM. It was found that the median PFS was 7 weeks in DTIC group and 15.9 weeks in selumetinib group. The median OS was 9.1 months in DTIC group and 11.8 months in selumetinib group. No objective response was achieved in DTIC group, and the objective response rate was 49% in selumetinib group. They concluded that, compared with DTIC, selumetinib might help to increase PFS and response rate but did not increase OS (237). A multicenter, randomized, double-blind, phase III clinical trial that further evaluate the role of selumetinib combined with DTIC in treating metastatic UM is still ongoing (238).
Ipilimumab (Ipi)
In a multicenter phase II clinical trial reported by DeCOG in 2015, after the metastatic UM patients were orally administered with Ipi at a dosage of 3 mg/kg for 4 weeks, the median OS was 6.8 months (95% CI: 3.7−8.1) and the median PFS was 2.8 months (95% Cl: 2.5−2.9). The authors concluded that Ipi has limited role in treating metastatic UM (239).
PD-1
The relevant clinical trial is still ongoing, and the results worth waiting for.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359-86. [Crossref] [PubMed]
- Chinese Cancer Registry Annual Report 2014 (unpublished data).
- National Office of Tumor Prevention and Treatment & Ministry of Health Disease Prevention and Control Bureau. Chinese Cancer Registry Annual Report 2004. Beijing: Peking Union Medical College Press, 2008.
- Zhao P, Chen WQ, editors. Chinese Cancer Registry Annual Report 2008. Beijing: Military Medical Science Press, 2009.
- Zhao P, Chen WQ, editors. Chinese Cancer Registry Annual Report 2009. Beijing: Military Medical Science Press, 2010.
- Zhao P, Chen WQ, editors. Chinese Cancer Registry Annual Report 2010. Beijing: Military Medical Science Press, 2011.
- Zhao P, Chen WQ, editors. Chinese Cancer Registry Annual Report 2011. Beijing: Military Medical Science Press, 2012.
- He J, Chen WQ, editors. Chinese Cancer Registry Annual Report 2012. Beijing: Military Medical Science Press, 2012.
- Chi Z, Li S, Sheng X, et al. Clinical presentation, histology, and prognoses of malignant melanoma in ethnic Chinese: A study of 522 consecutive cases. BMC Cancer 2011;11:85. [Crossref] [PubMed]
- Kong Y, Si L, Zhu Y, et al. Large-scale analysis of KIT aberrations in Chinese patients with Melanoma. Clin Cancer Res 2011;17:1684-91. [Crossref] [PubMed]
- Si L, Kong Y, Xu X, et al. Prevalence of BRAF V600E mutation in Chinese melanoma patients: large scale analysis of BRAF and NR AS mutations in a 432-case cohort. Eur J Cancer 2012;48:94-100. [Crossref] [PubMed]
- Dasgupta A, Katdare M. Ultraviolet radiation-induced cytogenetic damage in white,hispanic and black skin melanocytes: a risk for cutaneous melanoma. Cancers (Basel) 2015;7:1586-604. [Crossref] [PubMed]
- Swalwell H, Latimer J, Haywood RM, et al. Investigating the role of melanin in UVA/UVB- and hydrogen peroxide-induced cellular and mitochondrial ROS production and mitochondrial DNA damage in human melanoma cells. Free Radic Biol Med 2012;52:626-34. [Crossref] [PubMed]
- Reed JA, Shea CR. Lentigo maligna: melanoma in situ on chronically sun-damaged skin. Arch Pathol Lab Med 2011;135:838-41. [PubMed]
- Elder DE. Pathology of melanoma. Clin Cancer Res 2006;12:2308s-11s. [Crossref] [PubMed]
- Elder DE, Jucovy PM, Tuthill RJ, et al. The classification of malignant melanoma. Am J Dermatopathol 1980;2:315-20. [Crossref] [PubMed]
- Harmelin ES, Hocombe RN, Goggin JP, et al. Acral lentiginous melanoma. J Foot Ankle Surg 1998;37:540-5. [Crossref] [PubMed]
- Cancer Genome Atlas Network. Genomic Classification of Cutaneous Melanoma. Cancer Genome Atlas Network. Cell 2015;161:1681-96. [Crossref] [PubMed]
- High WA, Robinson WA. Genetic mutations involved in melanoma: a summary of our current understanding. Adv Dermatol 2007;23:61-79. [Crossref] [PubMed]
- Curtin JA, Busam K, Pinkel D, et al. Somatic activation of KIT in distinct subtypes of melanoma. J Clin Oncol 2006;24:4340-6. [Crossref] [PubMed]
- Curtin JA, Fridlyand J, Kageshita T, et al. Distinct sets of genetic alterations in melanoma. N Engl J Med 2005;353:2135-47. [Crossref] [PubMed]
- Balch CM, Geshenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol 2009;27:6199-206. [Crossref] [PubMed]
- Edge SB, Carducci M, Byrd DR, editors. AJCC Cancer Staging Manual. 7th ed. New York: Springer-Verlag, 2009.
- Piris A, Mihm MC, Duncan LM. AJCC melanoma staging update: impact on dermatopathology practice and patient management. J Cutan Pathol 2011;38:394-400. [Crossref] [PubMed]
- Azzola MF, Shaw HM, Thompson JF, et al. Tumor mitotic rate is a more powerful prognostic indicator than ulceration in patients with primary cutaneous melanoma: an analysis of 3661 patients from a single center. Cancer 2003;97:1488-98. [Crossref] [PubMed]
- Francken AB, Shaw HM, Thompson JF, et al. The prognostic importance of tumor mitotic rate confirmed in 1317 patients with primary cutaneous melanoma and long follow-up. Ann Surg Oncol 2004;11:426-33. [Crossref] [PubMed]
- Gimotty PA, Elder DE, Fake DL, et al. Identification of high-risk patients among those diagnosed with thin cutaneous melanomas. J Clin Oncol 2007;25:1129-34. [Crossref] [PubMed]
- Thompson JF, Soong SJ, Balch CM, et al. Prognostic significance of mitotic rate in localized primary cutaneous melanoma: an analysis of patients in the multi-institutional American Joint Committee on Cancer melanoma staging database. J Clin Oncol 2011;29:2199-205. [Crossref] [PubMed]
- Paek SC, Griffith KA, Johnson TM, et al. The impact of factors beyond Breslow depth on predicting sentinel lymph node positivity in melanoma. Cancer 2007;109:100-8. [Crossref] [PubMed]
- Sondak VK, Taylor JM, Sabel MS, et al. Mitotic rate and younger age are predictors of sentinel lymph node positivity: lessons learned from the generation of a probabilistic model. Ann Surg Oncol 2004;11:247-58. [Crossref] [PubMed]
- Bichakjian CK, Halpern AC, Johnson TM, et al. Guidelines of care for the management of primary cutaneous melanoma. American Academy of Dermatology. J Am Acad Dermatol 2011;65:1032-47. [Crossref] [PubMed]
- Sober AJ, Chuang TY, Duvic M, et al. Guidelines of care for primary cutaneous melanoma. J Am Acad Dermatol 2001;45:579-86. [Crossref] [PubMed]
- College of American Pathologists. Protocol for the examination of specimens from patients with melanoma of the skin. 2013. Available online: Melanoma-13protocol-3300.pdthttp://www.cap.org/apps/docs/committees/cancer/cancer-protocols/2013/Skin
- Harrist TJ, Rigel DS, Day CL Jr, et al. “Microscopic satellites” are more highly associated with regional lymph node metastases than is primary melanoma thickness. Cancer 1984;53:2183-7. [Crossref] [PubMed]
- Raskin L, Ludgate M, Iyer RK, et al. Copy number variations and clinical outcome in atypical spitz tumors. Am J Surg Pathol 2011;35:243-52. [Crossref] [PubMed]
- Balch CM, Gershenwald JE, Soong SJ, et al. Multivariate analysis of prognostic factors among 2313 patients with stage III melanoma: comparison of nodal micrometastases versus macrometastases. J Clin Oncol 2010;28:2452-9. [Crossref] [PubMed]
- Balch CM, Soong SJ, Gershenwald JE, et al. Prognostic factors analysis of 17 600 melanoma patients: validation of the American Joint Committee on Cancer melanoma staging system. J Clin Oncol 2001;19:3622-34. [PubMed]
- Neuman HB, Patel A, Ishill N, et al. A single-institution validation of the AJCC staging system for stage IV melanoma. Ann Surg Oncol 2008;15:2034-41. [Crossref] [PubMed]
- Weide B, Elsasser M, Buttner P, et al. Serum markers lactate dehydrogenase and S100B predict independently disease outcome in melanoma patients with distant metastasis. Br J Cancer 2012;107:422-8. [Crossref] [PubMed]
- Clark PB, Soo V, Kraas J, et al. Futility of fluorodeoxyglucose F-18 positron emission tomography in initial evaluationofpatients with T2 to T4 melanoma. Arch Surg 2006;141:284-8. [Crossref] [PubMed]
- Maubec E, Lumbroso J, Masson F, et al. F-18 fluorodeoxy-D-glucose positron emission tomography scan in the initial evaluation of patients with a primary melanoma thicker than 4mm. Melanoma Res 2007;17:147-54. [Crossref] [PubMed]
- Wagner JD, Schauwecke D, Davidson D, et al. Inefficacy of F-18 fIuorodeoxy-D-gIucose-positron emission tomography scans for initial evaluation in early-stage cutaneous melanoma. Cancer 2005;104:570-9. [Crossref] [PubMed]
- Brady MS, Akhurst T, Spanknebel K, et al. Utility of preoperative [(18)]f fluorodeoxyglucose-positron emission tomography scanning in high-risk melanoma patients. Ann Surg Oncol 2006;13:525-32. [Crossref] [PubMed]
- Schröer-Gunther MA, Wolff RF, Westwood ME, et al. F-18-fluoro-2-deoxyglucose positron emission tomography(PET) and PET/computed tomography imaging in primary staging of patients with malignant melanoma: a systematic review. Syst Rev 2012;1:62. [Crossref] [PubMed]
- Xing Y, Bronstein Y, Ross MI, et al. Contemporary diagnostic imaging modalities for the staging and surveillance of melanoma patients: a meta-analysis. J Natl Cancer Inst 2011;103:129-42. [Crossref] [PubMed]
- Gillgren P, Dzewiecki KT, Niin M, et al. 2-cm versus 4-cm surgical excision margins for primary cutaneous melanoma thicker than 2mm: a randomized multicenter trial. Lancet 2011;378:1635-42. [Crossref] [PubMed]
- Balch CM, Soong SJ, Smith T, et al. Long-term results of a prospective surgical trial comparing 2cm vs 4cm excision margins for 740 patients with 1-4mm melanomas. Ann Surg Oncol 2001;8:101-8. [PubMed]
- Balch CM, Urist MM, Karakousis CP, et al. Efficacy of 2-cm surgical margins for intermediate-thickness melanomas (1 to 4mm). Results of a multi-institutional randomized surgical trial. Ann Surg 1993;218:262-7; discussion 267-9. [Crossref] [PubMed]
- Thomas JM, Newton-Bishop J, A’Hern R, et al. Excision margins in high-risk malignant melanoma. N Engl J Med 2004;350:757-66. [Crossref] [PubMed]
- Albertini JG, Elston DM, Libow LF, et al. Mohs micrographic surgery for melanoma: a case series,a comparative study of immunostains,an informative case report,and a unique mapping technique. Dermatol Surg 2002;28:656-65. [PubMed]
- Testori A, Mozziuo N. Surgical techniques of melanoma and sentinel node biopsy. Semin Oncol 2002;29:328-35. [Crossref] [PubMed]
- Mocellin S, Hoon DS, Pilati P, et al. Sentinel lymph node molecular ultrastaging in patients with melanoma: a systematic review and meta-analysis of prognosis. J Clin Oncol 2007;25:1588-95. [Crossref] [PubMed]
- Keijzer R, Bril H, van der Loo EM, et al. Important prognostic significance of a sentinel-node biopsy in patients with malignant melanoma. Ned Tijdschr Geneeskd 2004;148:884-8. [PubMed]
- Morton DL, Thompson JF, Alistair JC, et al. Sentinel-node biopsy or nodal observation in melanoma. New Engl J Med 2006;355:1307-17. [Crossref] [PubMed]
- Lock-Andersen J, Horn J, Sjostrand H, et al. Prognosis after sentinel node biopsy in malignant melanoma. Ugeskr Laeger 2006;168:2457-62. [PubMed]
- Mann GB, Coit DG. Does the extent of operation influence the prognosis in patients with melanoma metastatic to inguinal nodes. Ann Surg Oncol 1999;6:263-71. [Crossref] [PubMed]
- Van der Ploeg AP, Van Akkooi AC, Rutkowski P, et al. Prognosis in patients with sentinel node-positive melanoma is accurately defined by the combined Rotterdam tumor load and Dewar topography criteria. J Clin Oncol 2011;29:2206-14. [Crossref] [PubMed]
- Morton DL, Thompson JF, Cochran AJ, et al. Final trial report of sentinel-node biopsy versus nodal observation in melanoma. N Engl J Med 2014;370:599-609. [Crossref] [PubMed]
- Thompson JF, Shaw HM. Sentinel node mapping for melanoma: results of trial and current applications. Surg Oncol Clin N Am 2007;16:35-54. [Crossref] [PubMed]
- Andtbacka RH, Gershenwald JE. Role of sentinel lymph node biopsy in patients with thin melanoma. J Natl Compr Canc Netw 2009;7:308-17. [PubMed]
- Han D, Zager JS, Shyr Y, et al. Clinicopathologic predictors of sentinel lymph node metastasis in thin melanoma. J Clin Oncol 2013;31:4387-93. [Crossref] [PubMed]
- Mitteldorf C, Bertsch HP, Jung K, et al. Sentinel Node Biopsy Improves Prognostic Stratification in Patients with Thin (pT1) Melanomas and an Additional Risk Factor. Ann Surg Oncol 2014;21:2252-8. [Crossref] [PubMed]
- Wright BE, Scheri RP, Ye X, et al. Importance of sentinel lymph node biopsy in patients with thin melanoma. Arch Surg 2008;143:892-9; discussion 899-900. [Crossref] [PubMed]
- Ranieri JM, Wagner JD, Wenck S, et al. The prognostic importance of sentinel lymph node biopsy in thin melanoma. Ann Surg Oncol 2006;13:927-32. [Crossref] [PubMed]
- Mozzillo N, Pennacchioli E, Gandini S, et al. Sentinel node biopsy in thin and thick melanoma. Ann Surg Oncol 2013;20:2780-6. [Crossref] [PubMed]
- Wong SL, Brady MS, Busam KJ, et al. Results of sentinel lymph node biopsy in patients with thin melanoma. Ann Surg Oncol 2006;13:302-9. [Crossref] [PubMed]
- Murali R, Haydu LE, Quinn MJ, et al. Sentinel lymph node biopsy in patients with thin primary cutaneous melanoma. Ann Surg 2012;255:128-33. [Crossref] [PubMed]
- Kesmodel SB, Karakousis GC, Botbyl JD, et al. Mitotic rate as a predictor of sentinel lymph node positivity in patients with thin melanomas. Ann Surg Oncol 2005;12:449-58. [Crossref] [PubMed]
- Yonick DV, Ballo RM, Kahn E, et al. Predictors of positive sentinel lymph node in thin melanoma. Am J Surg 2011;201:324-7; discussion 327-8. [Crossref] [PubMed]
- Bedrosian I, Faries MB, Guerry DT, et al. Incidence of sentinel node metastasis in patients with thin primary melanoma (<or=1 mm) with vertical growth phase. Ann Surg Oncol 2000;7:262-7. [Crossref] [PubMed]
- Oliveira Filho RS, Ferreira LM, Biasi LJ, et al. Vertical growth phase and positive sentinel node in thin melanoma. Braz J Med Biol Res 2003;36:347-50. [Crossref] [PubMed]
- Taylor RC, Patel A, Panageas KS, et al. Tumor-infiltrating lymphocytes predict sentinel lymph node positivity in patients with cutaneous melanoma. J Clin Oncol 2007;25:869-75. [Crossref] [PubMed]
- Azimi F, Scolyer RA, Rumcheva P, et al. Tumor-infiltrating lymphocyte grade is an independent predictor of sentinel lymph node status and survival in patients with cutaneous melanoma. J Clin Oncol 2012;30:2678-83. [Crossref] [PubMed]
- Venna SS, Thummala S, Nosrati M, et al. Analysis of sentinel lymph node positivity in patients with thin primary melanoma. J Am Acad Dermatol 2013;68:560-7. [Crossref] [PubMed]
- Fontaine D, Parkhill W, Greer W, et al. Partial regression of primary cutaneous melanoma: is there an association with sub- clinical sentinel lymph node metastasis. Am J Dermatopathol 2003;25:371-6. [Crossref] [PubMed]
- Morris KT, Busam KJ, Bero S, et al. Primary cutaneous melanoma with regression does not require a lower threshold for sentinel lymph node biopsy. Ann Surg Oncol 2008;15:316-22. [Crossref] [PubMed]
- Cecchi R, Pavesi M, Buralli L, et al. Tumour regression does not increase the risk of sentinel node involvement in thin melanomas. Chir ltal 2008;60:257-60. [PubMed]
- Strobbe LJ, Jonk A, Hart AA, et al. Positive iliac and obturator nodes in melanoma: survival and prognostic factors. Ann Surg Oncol 1999;6:255-62. [Crossref] [PubMed]
- Thompson JF, Hunt JA, Shannon KF, et al. Frequency and duration of emission after isolated limb perfusion for melanoma. Arch Surg 1997;132:903-7. [Crossref] [PubMed]
- Beasley GM, Caudle A, Petersen RP, et al. A multi-institutional experience of solated limb infusion: defining response and toxicity in the US. J Am Coll Surg 2009;208:706-15; discussion 715-7. [Crossref] [PubMed]
- Boesch CE, Meyer T, Waschke L, et al. Long-term outcome of hyperthermic isolated limb perfusion (HILP) in the treatment of locoregionally metastasized malignant melanoma of the extremities. Int J Hyperthermia 2010;26:16-20. [Crossref] [PubMed]
- Petersen RP, Hanish SI, Haney JC, et al. Improved survival with pulmonary metastasectomy: an analysis of 1720 patients with pulmonary metastatic melanoma. J Thorac Cardiovasc Surg 2007;133:104-10. [Crossref] [PubMed]
- Cascinelli N, Belli F, Mackie RM, et al. Effect of long-term adjuvant therapy with interferon alpha-2a in patients with regional node metastases from cutaneous melanoma: a randomized trial. Lancet 2001;358:866-9. [Crossref] [PubMed]
- Eggermont AM, Suciu S, Mackie R, et al. Post-surgery adjuvant therapy with intermediate doses of interferon a If a 2b versus observation in patients with stage llb/lll melanoma (EORTC 18952): randomized controlled trial. Lancet 2005;366:1189-96. [Crossref] [PubMed]
- Grob JJ, Dreno B, de la Salmoniere P, et al. Randomised trial of interferon a-2a as adjuvant therapy in resected primary melanoma thicker than 1.5mm without clinically detectable node metastases. Lancet 1998;351:1905-10. [Crossref] [PubMed]
- Hancock BW, Wheatley K, Harris S, et al. Adjuvant interferon in high-risk melanoma: the AIM HIGH Study- United Kingdom Coordinating Committee on Cancer Research randomized study of adjuvant low-dose extended-duration interferon Alfa-2a in high-risk resected malignant melanoma. J Clin Oncol 2004;22:53-61. [Crossref] [PubMed]
- Pehamberger H, Soyer HP, Steiner A, et al. Adjuvant interferon alfa-2a treatment in resected primary stage II cutaneous melanoma: the Eastern Cooperative Oncology Group Trial EST 1684. J Clin Oncol 1998;16:1425-9. [PubMed]
- Kirkwood JM, Strawderman MH, Ernstoff MS, et al. Interferon alfa-2b adjuvant therapy of high-risk resected cutaneous melanoma: the Eastern Cooperative Oncology Group Trial EST 1684. J Clin Oncol 1996;14:7-17. [PubMed]
- Kirkwood JM, Ibrahim JG, Sondak VK, et al. High- and low-dose interferon alfa-2b in high-risk melanoma: first analysis of intergroup trial E1690/S9111/C9190. J Clin Oncol 2000;18:2444-58. [PubMed]
- Agarwala SS, Lee SJ, Flaherty LE, et al. Randomized phase III trial of high-dose interferon alfa-2b(HDI)for 4 weeks induction only in patients with intermediate- and high-risk melanoma (Intergroup trial E 1697)[abstract]. J Clin Oncol 2011;29:abstr 8505.
- Payne MJ, Argyropoulou K, Lorigan P, et al. Phase II pilot study of intravenous high-dose interferon with or without maintenance treatment in melanoma at high risk of recurrence. J Clin Oncol 2014;32:185-90. [Crossref] [PubMed]
- Eggermont AM, Suciu S, Santinami M, et al. Adjuvant therapy with pegylated interferon alfa-2b versus observation alone in resected stage III melanoma: final results of EORTC 18991, a randomised phase III trial. Lancet 2008;372:117-26. [Crossref] [PubMed]
- Eggermont AM, Suciu S, Testori A, et al. Ulceration of primary melanoma and responsiveness to adjuvant interferon therapy: Analysis of the adjuvant trials EORTC18952 and EORTC18991 in 2,644 patients[abstract]. J Clin Oncol 2009;27:abstr 9007.
- Ascierto PA, Gogas HJ, Grob JJ, et al. Adjuvant interferon a If a in malignant melanoma: an interdisciplinary and multinational expert review. Crit Rev Oncol Hematol 2013;85:149-61. [Crossref] [PubMed]
- Mocellin S, Pasquali S, Rossi CR, et al. Interferon alpha adjuvant therapy in patients with high-risk melanoma: a systematic review and meta-analysis. J Natl Cancer Inst 2010;102:493-501. [Crossref] [PubMed]
- Petrella T, Verma S, Spithoff K, et al. Adjuvant interferon therapy for patients at high risk for recurrent melanoma: an updated systematic review and practice guideline. Clin Oncol (R Coll Radiol) 2012;24:413-23. [Crossref] [PubMed]
- Mao L, Si L, Chi Z, et al. A randomised phase II trial of 1 month versus 1 year of adjuvant high-dose interferon a-2b in high risk acral melanoma patients. Eur J Cancer 2011;47:1498-503. [Crossref] [PubMed]
- Agrawal S, Kane JM 3rd, Guadagnolo BA, et al. The benefits of adjuvant radiation therapy after therapeutic lymphadenectomy for clinically advanced,high-risk, lymph node-metastatic melanoma. Cancer 2009;115:5836-44. [Crossref] [PubMed]
- Burmeister BH, Henderson MA, Ainslie J, et al. Adjuvant radiotherapy versus observation alone for patients at risk of lymph-node field relapse after therapeutic lymphadenectomy for melanoma: a randomised trial. Lancet Oncol 2012;13:589-97. [Crossref] [PubMed]
- Henderson MA, Burmeister B, Ainslie J, et al. Adjuvant radiotherapy after lymphadenectomy in melanoma patients: Final results of an intergroup randomized trial (ANZMTG 0.1.02/ TROG 02.01)[abstract]. J Clin Oncol 2013;31:abstr 9001.
- Beadle BM, Guadagnolo BA, Ballo MT, et al. Radiation therapy field extent for adjuvant treatment of axillary metastases from malignant melanoma. Int J Radiat Oncol Biol Phys 2009;73:1376-82. [Crossref] [PubMed]
- Chang DT, Amdur RJ, Morris CG, et al. Adjuvant radiotherapy for cutaneous melanoma: comparing hypofractionation to conventional fractionation. Int J Radiat Oncol Biol Phys 2006;66:1051-5. [Crossref] [PubMed]
- Sause WT, Cooper JS, Rush S, et al. Fraction size in external beam radiation therapy in the treatment of melanoma. Int J Radiat Oncol Biol Phys 1991;20:429-32. [Crossref] [PubMed]
- Garbe C, Eigentler TK, Keilholz U, et al. Systematic review of medical treatment in melanoma: current status and future prospects. Oncologist 2011;16:5-24. [Crossref] [PubMed]
- Falkson CI, Ibrahim J, Kirkwood JM, et al. Phase III trial of dacarbazine versus dacarbazine with tamoxifen versus dacarbazine with interferon alfa-2b and tamoxifen in patients with metastatic malignant melanoma:an Eastern Cooperative Oncology Group study. J Clin Oncol 1998;16:1743-51. [PubMed]
- Middleton MR, Grob J, Aaronson N, et al. Randomized phase III study of temozolomide versus dacarbazine in the treatment of patients with advanced metastatic malignant melanoma. J Clin Oncol 2000;18:158-66. [PubMed]
- Avril MF, Aamdal S, Grob JJ, et al. Fotemustine compared with dacarbazine in patients with disseminated malignant melanoma: a phase III study. J Clin Oncol 2004;22:1118-25. [Crossref] [PubMed]
- Bedikian AY, Millward M, Pehamberger H, et al. Bcl-2 antisense (oblimersen sodium) plus dacarbazine in patients with advanced melanoma:the Oblimersen Melanoma Study Group. J Clin Oncol 2006;24:4738-45. [Crossref] [PubMed]
- Legha SS, Ring S, Eton O, et al. Development of a biochemotherapy regimen with concurrent administration of cisplatin,vinblastine,dacarbazine,interferon alfa,and interleukin-2 for patients with metastatic melanoma. J Clin Oncol 1998;16:1752-9. [PubMed]
- Chapman PB, Einhorn LH, Meyers ML, et al. Phase III multicenter randomized trial of the Dartmouth regimen versus dacarbazine in patients with metastatic melanoma. J Clin Oncol 1999;17:2745-51. [PubMed]
- Tentori L, Graziani G. Recent approaches to improve the antitumor efficacy of temozolomide. Curr Med Chem 2009;16:245-57. [Crossref] [PubMed]
- Boogerd W, de Gast GC, Dalesio O. Temozolomide in advanced malignant melanoma with small brain metastases: can we withhold cranial irradiation. Cancer 2007;109:306-12. [Crossref] [PubMed]
- Schadendorf D, Hauschild A, Uguel S, et al. Dose-intensified bi-weekly temozolomide in patients with asymptomatic brain metastases from malignant melanoma: a phase II DeCOG/ADO study. Ann Oncol 2006;17:1592-7. [Crossref] [PubMed]
- Krown SE, Niedzwiecki D, Hwu WJ, et al. Phase II study of temozolomide and thalidomide in patients with metastatic melanoma in the brain: high rate of thromboembolic events (CALGB 500102). Cancer 2006;107:1883-90. [Crossref] [PubMed]
- Rao RD, Holtan SG, Ingle JN, et al. Combination of paclitaxel and carboplatin as second-line therapy for patients with metastatic melanoma. Cancer 2006;106:375-82. [Crossref] [PubMed]
- Agarwala SS, Keilholz U, Hogg D, et al. Randomized phase III study of paclitaxel plus carboplatin with or without sorafenib as second-line treatment in patients with advanced melanoma. J Clin Oncol 2007;525:8510.
- Hauschild A, Agarwala SS, Trefzer U, et al. Results of a phase III,randomized,placebo- controlled study of sorafenib in combination with carboplatin and paclitaxel as second-line treatment in patients with unresectable stage III or stage IV melanoma. J Clin Oncol 2009;27:2823-30. [Crossref] [PubMed]
- Wiernik PH, Einzig AI. Taxol in malignant melanoma. J Natl Cancer Inst Monogr 1993;15:185-7. [PubMed]
- Wiernik PH, Schwartz EL, Einzig A, et al. Phase I trial of taxol given as a 24-hour infusion every 21 days: responses observed in metastatic melanoma. J Clin Oncol 1987;5:1232-9. [PubMed]
- Legha SS, Ring S, Papadopoulos N, et al. A phase II trial of taxol in metastatic melanoma. Cancer 1990;65:2478-81. [Crossref] [PubMed]
- Einzig AI, Hochster H, Wiernik PH, et al. A phase II study of taxol in patients with malignant melanoma. Invest New Drugs 1991;9:59-64. [Crossref] [PubMed]
- Walker L, Schalch H, King DM, et al. Phase II trial of weekly paclitaxel in patients with advanced melanoma. Melanoma Res 2005;15:453-9. [Crossref] [PubMed]
- Bedikian AY, Plager C, Papadopoulos N, et al. Phase II evaluation of paclitaxel by short intravenous infusion in metastatic melanoma. Melanoma Res 2004;14:63-6. [Crossref] [PubMed]
- Hersh E, Del Vecchio M, Brown M, et al. Phase 3, randomized,open-label,multicenter trial of nab-paclitaxel (nab-P) versus dacarbazine(DTIC) in previously untreated patients with metastatic malignant melanoma (MMM). Pigment Cell Melanoma Res 2012;25:863.
- Guo J, Si L, Kong Y, et al. A phase II, open label,single-arm trial of imatinib mesylate in patients with metastatic melanoma harboring c-kit mutation or amplification. J Clin Oncol 2011;29:2904-9. [Crossref] [PubMed]
- Long GV, Menzies AM, Nagrial AM, et al. Prognostic and Clinicopathologic Associations of Oncogenic BRAF in metastatic Melanoma. J Clin Oncol 2011;29:1239. [Crossref] [PubMed]
- Flaherty KT, Puzanov I, Kim KB, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med 2010;363:809-19. [Crossref] [PubMed]
- Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med 2011;364:2507-16. [Crossref] [PubMed]
- Sosman JA, Kim KB, Schuchter L, et al. Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. N Engl J Med 2012;366:707-14. [Crossref] [PubMed]
- Hauschild A, Grob JJ, Demidov LV, et al. Phase III,randomized,open-label,multicenter trial (BREAK-3) comparing the BRAF kinase inhibitor dabrafenib (GSK2118436) with dacarbazine (DTIC) in patients with BRAFV600E-mutated melanoma J Clin Oncol 2012;30:LBA8500. [abstract].
- Long GV, Trefzer U, Davies MA, et al. Dabrafenib in patients with Val600Glu or Val600Lys BRAF-mutant melanoma metastatic to the brain (BREAK-MB): a multicentre, open-label, phase 2 trial. Lancet Oncol 2012;13:1087-95. [Crossref] [PubMed]
- Flaherty KT, Robert C, Hersey P, et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med 2012;367:107-14. [Crossref] [PubMed]
- Kim KB, Kefford R, Pavlick AC, et al. Phase II study of the MEK1/MEK2 inhibitor Trametinib in patients with metastatic BRAF-mutant cutaneous melanoma previously treated with or without a BRAF inhibitor. J Clin Oncol 2013;31:482-9. [Crossref] [PubMed]
- Hauschild A, Grob JJ, Demidov LV, et al. Dabrafenib in BRAFmutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet 2012;380:358-65. [Crossref] [PubMed]
- Flaherty KT, Infante JR, Daud A, Combined BRAF, Inhibition MEK, et al. in Melanoma with BRAF V600 Mutations. N Engl J Med 2012;367:1694-703. [Crossref] [PubMed]
- Pavlick AC, Tawbi H, Carvajal R, et al. Extended follow-up results of phase lb study (BRIM7) of vemurafenib(VEM) with cobimetinib (COBI) in BRAF-mutant melanoma. J Clin Oncol 2015;33:abstr 9020.
- Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711-23. [Crossref] [PubMed]
- Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med 2011;364:2517-26. [Crossref] [PubMed]
- Hamid O, Robert C, Daud A, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med 2013;369:134-44. [Crossref] [PubMed]
- Robert C, Ribas A, Wolchok JD, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumabrefractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet 2014;384:1109-17. [Crossref] [PubMed]
- Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 2015;372:320-30. [Crossref] [PubMed]
- Rosenberg SA, Yang JC, Topalian SL, et al. Treatment of 283 consecutive patients with metastatic melanoma or renal cell cancer using high-dose bolus interleukin 2. JAMA 1994;271:907-13. [Crossref] [PubMed]
- Atkins MB, Lotze MT, Dutcher JP, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol 1999;17:2105-16. [PubMed]
- Atkins MB, Kunkel L, Sznol M, et al. High-dose recombinant interleukin-2 therapy in patients with metastatic melanoma: long-term survival update. Cancer J Sci Am 2000;6 Suppl 1:S11-4. [PubMed]
- Smith FO, Downey SG, Klapper JA, et al. Treatment of metastatic melanoma using interleukin-2 alone or in conjunction with vaccines. Clin Cancer Res 2008;14:5610-8. [Crossref] [PubMed]
- Tang BX, Si L, Chi ZH, et al. A phase II clinical trial of recombinant human interleukin-2 in treatment of advanced melanoma. Tumor 2011;31:1042-5.
- Cui C, Mao L, Chi Z, et al. A phase II, randomized, double-blind, placebo-controlled multicenter trial of endostar in patients with metastatic melanoma. Mol Ther 2013;21:1456-63. [Crossref] [PubMed]
- Ferrucci PF, Minchella I, Mosconi M, et al. Dacarbazine in combination with bevacizumab for the treatment of unresectable/metastatic melanoma: a phase II study. Melanoma Res 2015;25:239-45. [Crossref] [PubMed]
- Spitler LE, Boasbeg PO, Day S, et al. Phase II study of nab-paclitaxel and bevacizumab as first-line therapy for patients with unresectable stage III and IV melanoma. Am J Clin Oncol 2015;38:61-7. [Crossref] [PubMed]
- Kottschade LA, Suman VJ, Perez DG, et al. A randomized phase 2 study of temozolomide and bevacizumab or nab- paclitaxel, carboplatin,and bevacizumab in patients with unresectable stage IV melanoma: a North Central Cancer Treatment Group study, N0775. Cancer 2013;119:586-92. [Crossref] [PubMed]
- Vogl T, Eichle K, Zangos S, et al. Preliminary experience with transarterial chemoembolization (TACE) in liver metastases of uveal malignant melanoma: local tumor control and survival. J Cancer Res Clin Oncol 2007;133:177-84. [Crossref] [PubMed]
- Cui CL, Chi ZH, Yuan XQ, et al. Hepatic intra-arterial bio-chemotherapy for the treatment of melanoma patients with liver metastasis: A phase II clinical study. Chinese Journal of Cancer 2008;27:845-50. [PubMed]
- Sloan AE, Nock CJ, Einstein DB. Diagnosis and treatment of melanoma brain metastasis: a literature review. Cancer Control 2009;16:248-55. [PubMed]
- Xie FT, Liu ZL, Liu QY. Brain metastasis of malignant melanoma: treatment efficacies and prognostic factors. China Medical Engineering 2012;20:14-7.
- Roth TN, Gengler C, Huber GF, et al. Outcome of sinonasal melanoma: clinical experience and review of the literature. Head Neck 2010;32:1385-92. [Crossref] [PubMed]
- Conley JJ, Ackerman AB, editors. Melanoma of the Head and Neck. New York: Georg Thieme Verlag, 1990:154-78.
- Ganly I, Patel SG, Singh B, et al. Craniofacial resection for malignant melanoma of the skull base: report of an international collaborative study. Arch Otolaryngol Head Neck Surg 2006;132:73-8. [Crossref] [PubMed]
- Patrick RJ, Fenske NA, Messina JL. Primary mucosal melanoma. J Am Acad Dermatol 2007;56:828-34. [Crossref] [PubMed]
- Benlyazid A, Thariat J, Temam S, et al. Postoperative radiotherapy in head and neck mucosal melanoma: a GETTEC study. Arch Otolaryngol Head Neck Surg 2010;136:1219-25. [Crossref] [PubMed]
- Chan RC, Chan JY, Wei WI. Mucosal melanoma of the head and neck: 32-year experience in a tertiary referral hospital. Laryngoscope 2012;122:2749-53. [Crossref] [PubMed]
- Tacastacas JD, Bray J, Cohen YK, et al. Update on primary mucosal melanoma. J Am Acad Dermatol 2014;71:366-75. [Crossref] [PubMed]
- Greaves WO, Verma S, Patel KP, et al. Frequency and spectrum of BRAF mutations in a retrospective, single-institution study of 1112 cases of melanoma. J Mol Diagn 2013;15:220-6. [Crossref] [PubMed]
- Lourenço SV, Fernandes JD, Hsieh R, et al. Head and neck mucosal melanoma: a review. Am J Dermatopathol 2014;36:578-87. [Crossref] [PubMed]
- Suzuki N, Onda T, Yamamoto N, et al. Mutation of the p16/CDKN2 gene and loss of heterozygosity in malignant mucosal melanoma and adenoid cystic carcinoma of the head and neck. Int J Oncol 2007;31:1061-7. [PubMed]
- Thompson LD, Wieneke JA, Miettinen M. Sinonasal tract and nasopharyngeal melanomas: aclinicopathologic study of 115 cases with a proposed staging system. Am J Surg Pathol 2003;27:594-611. [Crossref] [PubMed]
- Shuman AG, Light E, Olsen SH, et al. Mucosal melanoma of the head and neck: predictors of prognosis. Arch Otolaryngol Head Neck Surg 2011;137:331-7. [Crossref] [PubMed]
- Hanna E, De Monte F, Ibrahim S, et al. Endoscopic resection of sinonasal cancers with and without craniotomy: oncologic results. Arch Otolaryngol Head Neck Surg 2009;135:1219-24. [Crossref] [PubMed]
- de Graeff A, de Leeuw JR, Ros WJ, et al. Pretreatment factors predicting quality of life aftertreatment for head and neck cancer. Head Neck 2000;22:398-407. [Crossref] [PubMed]
- Meleti M, Vescovi P, Mooi WJ, et al. Pigmented lesions of the oral mucosa and perioral tissues: a flow-chart for the diagnosis and some recommendations for the management. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2008;105:606-16. [Crossref] [PubMed]
- Prasad ML, Patel SG, Huvos AG, et al. Primary mucosal melanoma of the head and neck: a proposal for microstaging localized, Stage I (lymph node-negative) tumors. Cancer 2004;100:1657-64. [Crossref] [PubMed]
- Stárek I, Koranda P, Benes P. Sentinel lymph node biopsy: a new perspective in head and neck mucosal melanoma. Melanoma Research 2006;16:423-7. [Crossref] [PubMed]
- Christopherson K, Malyapa RS, Werning JW, et al. Radiation therapy for mucosal melanoma of the head and neck. Am J Clin Oncol 2015;38:87-9. [Crossref] [PubMed]
- Dirix P, Vanstraelen B, Jorissen M, et al. Intensity-modulated radiotherapy for sinonasal cancer:improved outcome compared to conventional radiotherapy. Int J Radiat Oncol Biol Phys 2010;78:998-1004. [Crossref] [PubMed]
- Wu AJ, Gomez J, Zhung JE, et al. Radiotherapy after surgical resection for head and neck mucosal melanoma. Am J Clin Oncol 2010;33:281-5. [PubMed]
- Dauer EH, Lewis JE, Rohlinger AL, et al. Sinonasal melanoma: a clinicopathologic review of 61 cases. Otolaryngol Head Neck Surg 2008;138:347-52. [Crossref] [PubMed]
- Lian B, Si L, Cui C, et al. Phase II randomized trial comparing high-dose IFN-a2b with temozolomide plus cisplatin as systemic adjuvant therapy for resected mucosal melanoma. Clin Cancer Res 2013;19:4488-98. [Crossref] [PubMed]
- Linsley PS, Bradshaw J, Greene J, et al. Intracellular traffiking of CTLA-4 and focal localization towards sites of TCR engagement. Immunity 1996;4:535-43. [Crossref] [PubMed]
- Postow MA, Luke JJ, Bluth MJ, et al. Ipilimumab for patients with advanced mucosal melanoma. Oncologist 2013;18:726-32. [Crossref] [PubMed]
- Del Vecchio M, Di Guardo L, Ascierto PA, et al. Efficacy and safety of ipilimumab 3mg/kg in patients with pretreated, metastatic, mucosal melanoma. Eur J Cancer 2014;50:121-7. [Crossref] [PubMed]
- Taube JM, Anders RA, Young GD, et al. Colocalization of inflmmatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci TransI Med 2012;4:1-22.
- Topalian SL, Sznol M, McDermott DF, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol 2014;32:1020-30. [Crossref] [PubMed]
- Kim KB, Sosman JA, Fruehauf JP, et al. BEAM: a randomized phase II study evaluating the activity of bevacizumab in combination with carboplatin plus paclitaxel in patients with previously untreated advanced melanoma. J Clin Oncol 2012;30:34-41. [Crossref] [PubMed]
- Grignol VP, Olencki T, Relekar K, et al. A phase 2 trial of bevacizumab and high-dose interferon alpha 2B in metastatic melanoma. J lmmunother 2011;34:509-15.
- von Moos R, Seifert B, Simcock M, et al. First-line temozolomide combined with bevacizumab in metastatic melanoma: a multicentre phase II trial (SAKK 50/07). Ann Oncol 2012;23:531-6. [Crossref] [PubMed]
- Vihinen PP, Hernberg M, Vuoristo MS, et al. A phase II trial of bevacizumab with dacarbazine and daily low-dose interferon-alpha2a as first line treatment in metastatic melanoma. Melanoma Res 2010;20:318-25. [Crossref] [PubMed]
- Yi JH, Yi SY, Lee HR, et al. Dacarbazine-based chemotherapy as fist-line treatment in noncutaneous metastatic melanoma: multicenter, retrospective analysis in Asia. Melanoma Res 2011;21:223-7. [Crossref] [PubMed]
- Harting MS, Kim KB. Biochemotherapy in patients with advanced vulvovaginal mucosal melanoma. Melanoma Res 2004;14:517-20. [Crossref] [PubMed]
- Bartell HL, Bedikian AY, Papadopoulos NE, et al. Biochemotherapy in patients with advanced head and neck mucosal melanoma. Head Neck 2008;30:1592-8. [Crossref] [PubMed]
- Chang W, Lee SJ, Park S, et al. Effect of paclitaxel/carboplatin salvage chemotherapy in noncutaneous versus cutaneous metastatic melanoma. Melanoma Res 2013;23:147-51. [Crossref] [PubMed]
- Lund VJ, Howard DJ, Harding L, et al. Managementoptions and survival in malignant melanoma ofthesinonasal mucosa. Laryngoscope 1999;109:208-11. [Crossref] [PubMed]
- Patel SG, Prasad ML, Escrig M, et al. Primary mucosalmalignant melanoma of the head and neck. Head Neck 2002;24:247-57. [Crossref] [PubMed]
- Singer M, Mutch MG. Anal melanoma. Clin Colon Rectal Surg 2006;19:78-87. [Crossref] [PubMed]
- Chang AE, Karnell LH, Menck HR. The National Cancer Data Base report on cutaneous and noncutaneous melanoma: a summary of 84 836 cases from the past decade. Cancer 1998;83:1664-78. [Crossref] [PubMed]
- Falch C, Stojadinovic A, Hann-von-Weyhern C, et al. Anorectal malignant melanoma: extensive 45-year review and proposal for a novel staging classification. J Am Coll Surg 2013;217:324-35. [Crossref] [PubMed]
- Nam S, Kim CW, Baek SJ, et al. The clinical features and optimal treatment of anorectal malignant melanoma. Ann Surg Treat Res 2014;87:113-7. [Crossref] [PubMed]
- Iddings DM, Fleisig AJ, Chen SL, et al. Practice patterns and outcomes for anorectal melanoma in the USA, reviewing three decades of treatment: is more extensive surgical resection beneficial in all patients. Ann Surg Oncol 2010;17:40-4. [Crossref] [PubMed]
- Nilsson PJ, Ragnarsson-Olding BK. Importance of clear resection margins in anorectal malignant melanoma. Br J Surg 2010;97:98-103. [Crossref] [PubMed]
- Heeney A, Mulsow J, Hyland JM. Treatment and outcomes of anorectal melanoma. Surgeon 2011;9:27-32. [Crossref] [PubMed]
- Matsuda A, Miyashita M, Matsumoto S, et al. Abdominoperineal resection provides better local control but equivalent overall survival to local excision of anorectal malignant melanoma: a systematic review. Ann Surg 2015;261:670-7. [Crossref] [PubMed]
- Bullard KM, Tuttle TM, Rothenberger DA, et al. Surgical therapy for anorectal melanoma. J Am Coll Surg 2003;196:206-11. [Crossref] [PubMed]
- Weinstock MA. Epidemiology and prognosis of anorectal melanoma. Gastroenterology 1993;104:174-8. [PubMed]
- Kim HS, Kim EK, Jun HJ. Noncutaneous malignant melanoma: a prognostic model from a retrospective multicenter study. BMC Cancer 2010;10:167. [Crossref] [PubMed]
- Lian LJ, editor. Lin Qiaozhi study. BMC Cancerlignant melanoma:n. Beijing: Peopledy Medical Publishing House, 2000.
- Tasseron EW, van der Esch EP, Hart AA. A clinicopathological study of 30 melanomas of the vulva. Gynecol Oncol 1992;46:170-5. [Crossref] [PubMed]
- Trimble EL, Lewis JL Jr, Williams LL. Management of vulvar melanoma. Gynecol Oncol 1992;45:254-8. [Crossref] [PubMed]
- Irvin WP Jr, Legallo RL, Stoler MH, et al. Vulvar melanoma: a retrospective analysis and literature review. Gynecol Oncol 2001;83:457-65. [Crossref] [PubMed]
- Wechter ME, Reynolds RK, Haefner HK, et al. Vulvar melanoma: review of diagnosis,staging,and therapy. J Low Genit Tract Dis 2004;8:58-69. [Crossref] [PubMed]
- Micci F, Teixeira MR, Scheistroen M, et al. Cytogenetic characterization of tumors of the vulva and vagina. Genes Chromosomes Cancer 2003;38:137-48. [Crossref] [PubMed]
- Ragnarsson-Olding BK. Primary malignant melanoma of the vulva-an aggressive tumor for modeling the genesis of non-UV light-associated melanomas. Acta Oncol 2004;43:421-35. [Crossref] [PubMed]
- Egan KM, Seddon JM, Glynn RJ, et al. Epidemiologic aspects of uveal melanoma. Surv Ophthalmol 1988;32:239-51. [Crossref] [PubMed]
- Singh AD, Topham A. Incidence of uveal melanoma in the United States: 1973-1997. Ophthalmology 2003;110:956-61. [Crossref] [PubMed]
- Reis L, Eisner MP, Kosary CL, et al, editors. SEER Cancer Statistics Review. 1973-2000. Bethesda, MD: National Cancer Institute, 2003.
- Kivelä T. The epidemiological challenge of the most frequent eye cancer: retinoblastoma, an issue of birth and death. Br J Ophthalmol 2009;93:1129-31. [Crossref] [PubMed]
- Hu DN, Yu GP, McCormick SA, et al. Population-based incidence of uveal melanoma in various races and ethnic groups. Am J Ophthalmol 2005;140:612-7. [Crossref] [PubMed]
- Hurst EA, Harbour JW, Cornelius LA. Ocular melanoma: a review and the relationship to cutaneous melanoma. Arch Dermatol 2003;139:1067-73. [Crossref] [PubMed]
- Rietschel P, Panageas KS, Hanlon C, et al. Variates of survival in metastatic uveal melanoma. J Clin Oncol 2005;23:8076-80. [Crossref] [PubMed]
- Diener-West M, Reynolds SM, Agugliaro DJ, et al. Development of metastatic disease after enrollment in the COMS trials for treatment of choroidal melanoma: Collaborative Ocular Melanoma Study Group Report No. 26. Arch Ophthalmol 2005;123:1639-43. [Crossref] [PubMed]
- Prescher G, Bornfeld N, Hirche H, et al. Prognostic implications of monosomy 3 in uveal melanoma. Lancet 1996;347:1222-5. [Crossref] [PubMed]
- Lake SL, Coupland SE, Taktak AF, et al. Whole-genome microarray detects deletions and loss of heterozygosity of chromosome 3 occurring exclusively in metastasizing uveal melanoma. Invest Ophthalmol Vis Sci 2010;51:4884-91. [Crossref] [PubMed]
- Van Raamsdonk CD, Bezrookove V, Green G, et al. Frequent somatic mutations of GNAQ in uvealmelanoma and blue naevi. Nature 2009;457:599-602. [Crossref] [PubMed]
- Van Raamsdonk CD, Griewank KG, Crosby MB, et al. Mutations in GNA11 in uveal melanoma. N Engl J Med 2010;363:2191-9. [Crossref] [PubMed]
- Xu X, Wei W, Xu X, et al. Oncogenic GNAQ and GNA11 mutations in uveal melanoma in Chinese. PLoS One 2014;9:e109699. [Crossref] [PubMed]
- Brose MS, Volpe P, Feldman M, et al. BRAF and RAS mutations in human lung cancer and melanoma. Cancer Res 2002;62:6997-7000. [PubMed]
- Pollock PM, Meltzer PS. A genome-based strategy uncovers frequent BRAF mutations in melanoma. Cancer Cell 2002;2:5-7. [Crossref] [PubMed]
- Group COMS. Histopathologic characteristics of uveal melanomas in eyes enucleated from the Collaborative Ocular Melanoma Study. COMS report no. 6. Am J Ophthalmol 1998;125:745-66. [Crossref] [PubMed]
- Uveal malignant melanoma: COMS results. In: Singh AD, Damato BE, Perer J, et al, editors. Clinical Ophthalmic Oncology. Philadelphia: Saunders Elsevier, 2007: 268.
- Group COMS. COMS Manual of Procedures: accession no. PBS 179 693. Springfield, VA: National Technical Information Service, 1995.
- Malignant melanoma of the uvea. In: Edge DB, Byrd DR, Compton CC, et al, editors. AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer, 2010:547-59.
- Shields CL, Kaliki S, Furuta M, et al. American Joint Committee on Cancer Classification of Uveal Melanoma (Anatomic Stage) Predicts Prognosis in 7731 Patients: The 2013 Zimmerman Lecture. Ophthalmology 2015;122:1180-6. [Crossref] [PubMed]
- AJCC Ophthalmic Oncology Task Force. International Validation of the American Joint Committee on Cancer’s 7th Edition Classification of Uveal Melanoma. JAMA Ophthalmol 2015;133:376-83.
- Onken MD, Worley LA, Ehlers JP, et al. Gene expression profiling in uveal melanomareveals two molecular classes and predicts metastatic death. Cancer Res 2004;64:7205-9. [Crossref] [PubMed]
- Tschentscher F, Husing J, Holter T, et al. Tumor classification based on gene expression profiling shows that uveal melanomas with and without monosomy 3 represent two distinct entities. Cancer Res 2003;63:2578-84. [PubMed]
- Onken MD, Worley LA, Char DH, et al. Collaborative Ocular Oncology Group report number: prospective validation of a multi-gene prognostic assay in uveal melanoma. Ophthalmology 2012;119:1596-603. [Crossref] [PubMed]
- Diener-West M, Earle JD, Fine SL, et al. Collaborative Ocular Melanoma Study Group. The COMS randomized trial of iodine 125 brachytherapy for choroidal melanoma, III: initial mortality findings. COMS Report No. 18. Arch Ophthalmol 2001;119:969-82. [Crossref] [PubMed]
- Patel M, Smyth E, Chapman PB, et al. Therapeutic implications of the emerging molecular biology of uveal melanoma. Clin Cancer Res 2011;17:2087-100. [Crossref] [PubMed]
- Harbour JW, Chao DL. A molecular revolution in uveal melanoma: implications for patient care and targeted therapy. Ophthalmology 2014;121:1281-8. [Crossref] [PubMed]
- Carvajal RD, Sosman JA, Quevedo JF, et al. Effect of selumetinibvs chemotherapy on progression-free survival in uveal melanoma: a randomized clinical trial. JAMA 2014;311:2397-405. [Crossref] [PubMed]
- Carvajal RD, Schwartz GK, Mann H, et al. Study design and rationale for a randomised, placebo-controlled, double-blind study to assess the efficacy ofselumetinib (AZD6244; ARRY-142886) in combination with dacarbazine in patients with metastatic uveal melanoma (SUMIT). BMC Cancer 2015;15:467. [Crossref] [PubMed]
- Zimmer L, Vaubel J, Mohr P, et al. Phase II DeCOG-study of ipilimumab in pretreated and treatment-naive patients with metastatic uveal melanoma. PLoS One 2015;10:e0118564. [Crossref] [PubMed]