Prophylactic tracheotomy and lung cancer resection in patient with low predictive pulmonary function: a randomized clinical trials
Introduction
For a long time, the impact of tracheotomy on the duration of MV has been debated. There are a lot of theoretical advantages that promote the use of tracheotomy in the postoperative period of patients with altered pulmonary function or when it compared with intubation: tracheotomy decreases work of breathing (1,2), eases bronchial secretions clearance (3) and improves patient’s comfort (4). These advantages may facilitate postoperative outcomes. In surgical patients admitted to ICU, a previous study suggested that early tracheotomy performed before postoperative day 7 decreased the duration of MV and the ICU length of stay compared to translaryngeal tracheal intubation (5). Nevertheless, the interest of prophylactic/early tracheotomy remains still questionable even in the field of thoracic surgery where some patients with chronic obstructive pulmonary disease (COPD) have an impaired pulmonary function and marginal respiratory mechanic. However, we know that patients with severe COPD experience significantly more pulmonary complications, prolonged mechanical ventilation (MV) (6,7), and finally had more than a fourthfold 30 days mortality rate (8).
In 2001, date of the beginning of this trial, Heffner identified numerous biases in the studies about this topic (9): retrospective studies, non random assignment, variation in the definition of early vs. late tracheotomy, wide variation of patient included (medical, surgical, trauma) and no clear definition of the weaning protocol. More than 10 years later, no optimal timing has been identified to perform a tracheotomy in medical or surgical patients needing a prolonged ventilation (10). In thoracic surgery, three randomized controlled trials (RCT) have tested the prophylactic minitracheotomy and have showed that it facilitates bronchial secretion removal and sometimes complications related to (11-13). However, none of these studies gave informations about the influence of minitracheotomy on the length of MV and the need of reventilation. One explanation may be that the small inner diameter of the minitracheotomy (4 mm) is less favourable to the respiratory mechanic and the weanability than large diameter (14).
This protocol has been designed to determine if surgical tracheotomy performed in the operating room immediately after lobectomy or pneumonectomy for lung cancer (i.e., prophylactic tracheotomy) in patients with low postoperative pulmonary function could (I) decrease the duration of postoperative MV; and (II) improve the postoperative outcomes. We hypothesized that prophylactic tracheotomy could reduce the length of MV and the number of respiratory complications.
Methods
Design
This prospective, randomized single center controlled trial (NCT01053624) was conducted from October 2001 to October 2014 but was stopped during 3 years in 2006, 2008 and 2011. Included patients were randomized preoperatively into two groups: group T underwent prophylactic tracheotomy with Tracheoflex® (Teleflex, Athlone, Co Westmeath, Ireland) in the operative room immediately after the closure of the thoracotomy and the group C was control patients. The local ethic committee approved the protocole. Patient written informed consent was obtained before randomization.
Patients
Prior surgery, former smokers were encouraged to quite smoking. Two to 3 weeks prior surgery, a chest physiotherapy prescription was ordered and was conducted out of the hospital at physiotherapist‘s discretion. We defined patients with low predictive postoperative function as patients with a predictive postoperative forced expiratory volume in 1 second (ppoFEV1) less than 50%. Preoperative pulmonary assessment included spirometry, diffusing capacity for CO by single breath method (DLco) and arterial blood gases in room air. Maximal oxygen consumption (VO2max) was determined using an incremental exercise test on a cyclo ergometer. Postoperative pulmonary function was calculated with the scintigraphic method (15) for the pneumonectomy patients and with the arythmetic methods for the lobectomy patients if the scintigraphy was lacking (16) using the formula:
Postoperative pulmonary function = preoperative pulmonary function − (1 − number of resected segments/19). We considered that three segments composed the right upper lobe, two the middle lobe, five the right upper lobe, five the left upper lobe and four the left lower lobe.
The inclusion criteria were:
- 30%≤ %ppoFEV1 <50%;
- Age between 18 and 79 years old;
- Preoperative diagnosis of lung cancer or high suspicion of lung cancer;
- Predicted postoperative DLco (ppoDLco) ≥30%;
- Predicted postoperative VO2max (ppoVO2max) ≥10 mL/kg/min;
- Surgical approach by lateral or posterolateral thoracotomy;
- Informed consent obtained by patient.
The exclusion criteria were:
- Age less than 18 and more than 79;
- Pregnant woman;
- Preoperative tracheotomy;
- Vocal cord paralysis;
- Phrenic nerve paralysis on the operated side (except for pneumonectomy);
- Neuromuscular disorders;
- Previous pharyngeal or laryngeal surgery;
- Anatomical deformity of the neck making risky a tracheotomy;
- Video assisted thoracoscopic surgery;
- Lung resection less important than planned at the inclusion (ppoFEV1 ≥50%).
Intervention
Lung resection was performed through a lateral or a posterolateral thoracotomy. All patients were given amoxicillin and clavulanic acid as antibiotic prophylaxis. Randomization was done during the intervention when the lung resection and lymphadenectomy were performed. Tracheotomy was performed in the operative room by the thoracic surgeon immediately after the closure of the thoracotomy under the same general anesthesia. The patient was in supine position with the neck extended. A 2 cm transversal skin incision and an elective dissection of the median tissue of the neck were performed with the help of thin retractors. Care was taken to dissect the inferior aspect of the thyroid isthmus and to coagulate the veins in front of the trachea. A transversal incision just below the second tracheal ring was made. We took care to coagulate any bleeding from the tracheal section line. The double lumen bronchial tube was removed and secretions were aspirated. The canula (Tracheoflex®) was inserted and its balloon inflated at the optimal pressure to avoid air leakage or tracheal ischemia. Finally the canula was secured with the neck tapes.
Outcomes measures
The primary outcome measure was the cumulative number of MV days after operation until discharge. We stopped the count of MV days when the criteria of the weaning protocol were reached (17): spontaneous breathing with a level of inspiratory aid ≤10 cm H2O, good level of consciousness, oxygen saturation ≥90% with a fraction of inspired oxygen less than 50% and a positive expiratory pressure ≤5 cm H2O, no need for hight dose of vasoactive or sedative agents. Prolonged MV was defined as the number of MV >2 days. Because the postoperative death could impact positively the duration of MV if early death or negatively if lately death, the duration of prolonged MV has been measured among survivors.
The secondary outcome measures were:
- 60 days mortality rate;
- ICU length of stay;
- Hospital length of stay;
- Incidence of postoperative respiratory complications defined as pneumonia (new chest infiltrate on X-ray film and temperature more than 38.5 °C and purulent sputum), non cardiogenic pulmonary edema, empyema or pleural effusion needing drainage, broncho-pleural fistula, lobar atelectasis needing fiber bronchoscopy, pulmonary embolism proved by computed tomography scan;
- Reventilation, need of non invasive ventilation, need of a tracheotomy because prolonged ventilation more than 7 days;
- Incidence of postoperative cardiac complications defined as arrhythmia needing treatment, cardiac failure needing in trop drug, acute coronary stroke;
- Tracheal complications;
- General complications;
- The report of postoperative complication according a standardized classification (18).
Follow up
Pain relief consisted of a thoracic epidural analgesia (sufentanyl and ropivacaine) or patient’s controlled intravenous narcotic analgesia. All patients received intravenous infusion of paracetamol (4 g per day). At the end of the operation, patients were admitted to the ICU. On the first postoperative days, patients received respiratory physiotherapy twice daily and aspiration of the tracheotomy was performed routinely. Later in the postoperative period, the frequency of tracheal suction and respiratory physiotherapy were dictated by patients’ clinical conditions. Fiber bronchoscopy were indicated (I) in case of persistent sputum retention or lobar atelectasis in spite of additional respiratory physiotherapy and endo tracheal suction (for tracheotomy patients); (II) in case of life threatening complications as pneumonia or bronchopleural fistula. Postoperative complications were prospectively collected and notified on the thoracic and cardiovascular French data base EPITHOR® (19). After operation, the resumption of oral intake was performed according our current practice after major thoracic surgery: no liquid food during 48 h, seated position out of bed during feeding, presence and advice of a nurse during the first meal. The tracheal cannula was removed when the patient was able to seat down out of bed and to cough spontaneously its bronchial sputum. For patients in the control group, the tracheal double lumen tube was removed after at least 30 minutes of spontaneous breathing.
Statistical analysis
Due to the lack of information on literature when this study has been planned, the sample size was determined according to capacity to recruit, sample size of studies on the same scientific area and regardless of estimations about effect size. A total of 68 randomized patients were necessary to detect an effect size of 0.8 concerning the primary outcome, for two-sided a type I error of 0.05 and a statistical power of 90%. Finally, we have decided to increase the sample with 10 additional patients (39 in each arm). All analyses were conducted on data from the intention-to-treat population. Baseline characteristics were presented as the mean ± standard deviation or the median (interquartile range) according to statistical distribution for quantitative data and as the number of patients and associated percentages for categorical parameters. Comparisons between randomisation groups were performed using usual statistical tests: chi-squared test or Fisher’s exact test when appropriate for categorical variables and student t-test or Mann-Whitney test when conditions of t-test were not met (assumption of normality studied using Shapiro-Wilk test and homoscedasticity by Fisher-Snedecor test) for quantitative parameters. In multivariate context, regression models (linear for quantitative outcome and logistic for binary dependent variable) were proposed considering covariables fixed according to univariate results and clinical relevance. The interaction between factors were studied and when P<0.05, subgroups analyses were explored. The time-to-event curves were calculated with the Kaplan-Meier method. Statistical analysis was performed using Stata software, version 13 (StataCorp, College Station, TX, USA). The tests were two-sided, with a type I error set at α=0.05.
Results
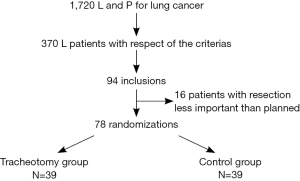
Over a 10-year period, 39 patients were enrolled in each group (Figure 1). The main reasons for non inclusions were increased number of video assisted thoracoscopic surgery for lobectomy patients, refusal of consent, team reluctance about the patient understanding, difficulties in organising inclusion and logistical reasons. No patients were lost to follow up on day 60. Randomized patients’ characteristics were similar for the two groups (Table 1).
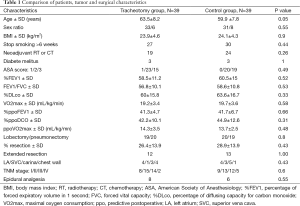
Full table
Primary outcome measure
The duration of MV among survivors (37 patients in each group) was not significantly different between the T group (3.5±6 days, minimum 0, maximum 24) and the C group (4.7±9.3 days, mini 0, maxi 35) (P=0.54).
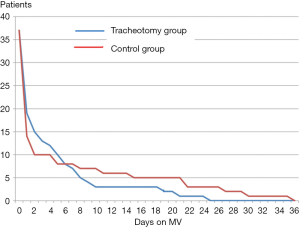
The number and the characteristics of patients with MV >2 days was not significantly different between the T group and C group (Table 2). There was a trend to a decrease of the duration of prolonged MV in tracheotomy patients (9.6±6.9 days) compared to the control patients (17.1±10.9 days) (P=0.07). The difference became significant on postoperative day 4 (P=0.04) (Figure 2, Table 3). Univariate analyses were proposed to study the predictive factors of MV >2 days. Multivariate analysis confirmed that VO2max and pneumonectomy of prolonged MV >2 days were predictive. The results are presented in Table 4.
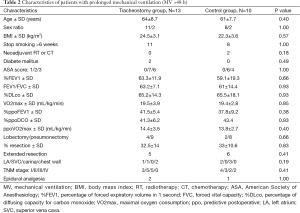
Full table

Full table
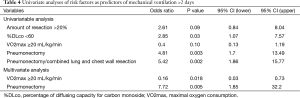
Full table
Secondary outcome measures
The 60 days mortality rate was 5.1% (two deaths in each group). In the tracheotomy group, the two patients had bronchopleural fistulas and died respectively from multiorgan failure on postoperative day 34, and from pulmonary embolism on postoperative day 45. In the control group, one patient died from pneumonia on postoperative day 31 and the other patient died from pneumonia and bowel infarction on postoperative day 5.
The ICU and hospital length of stay were not significantly different between the T group (respectively 12.1±12.2 and 26.1±21 days) and the C group (respectively 9.6±13.6 and 21.6±16 days).
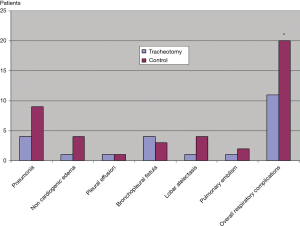
The percentage of patients who developed pneumonia, noncardiogenic edema, lobar atelectasis needing bronchoscopy and pulmonary embolism was respectively 10%, 2.5%, 2.5% and 2.5% in the T group and 23%, 10%, 10% and 5% in the C group. Ten percent of the patients developed a bronchopleural fistula pulmonary in the T group and 7% in the C group. In both groups, 5% of patients developed pleural effusion. Finally, patients who experienced respiratory complications were significantly lower in the T group (28%) than in the C group (51%) (P=0.03). Detailed results on postoperative respiratory complications are exposed in Figure 3.
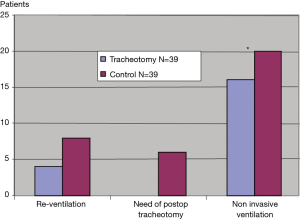
A lower number of patients needed a change of ventilatory support in the T group than in the C group and the difference was significant for the need of non invasive ventilation (P=0.04) (Figure 4). Six patients (15%) needed a tracheotomy in the T group because of a prolonged MV >7 days. Four patients (10%) needed reventilation in the T group compared to 8 patients (20%) in the C group (P=0.2).
Sixteen patients (41%) developed one or more postoperative cardiac complications in each group respectively 10 arhythmias, 6 cardiac failure and 1 acute coronary stroke in the T group and 12 arrhythmias and 5 cardiac failures in the C group.
Tracheotomy related complication were a lack of cutaneous healing needing surgical repair (2 patients) and an accidental dislogment of the canula without consequence (1 patient).
The Figure 5 exposes the rates of postoperative complications according to the standardized classification from Seely and coauthors (18). The difference was not significant between the two groups (P=0.5).
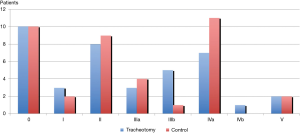
Discussion
The main results of this randomized controlled trials of patients with a low postoperative pulmonary function show that prophylactic tracheotomy provided a more favourable outcome in terms of duration prolonged MV >4 days and respiratory complications.
Among the strategies to reduce postoperative respiratory complications, the place of tracheotomy is not clearly defined even for patients with low pulmonary function. Consequently, no guideline gives criteria for tracheotomy in high risk patients eligible for lung resection. In practice, tracheotomy is usually discussed when a postoperative respiratory complication occurs leading to prolonged MV or reintubation. In fact, tracheotomy provides potential benefits on respiratory mechanics decreasing work of breathing, peak inspiratory pressure, pressure-time product, and intrinsic positive end expiratory pressure in both ventilated (1) and spontaneously breathing patients (2). Recently, a study has confirmed that a large tracheotomy tube size improved the diaphragm effort and weanability indices (14). In the postoperative period, the substantial advantages of the tracheotomy are also to clear easily bronchial secretions (3), to improve patient’s comfort (4), and decrease time of heavy sedation (20). However, despite these advantages, the interest of prophylactic tracheotomy in surgical patient with altered pulmonary function or marginal pulmonary mechanic is not clearly established. Moreover, the optimal timing of tracheotomy in patients with prolonged MV is still debated. In the past decade, there has been a trend to shorten the delay of tracheotomy. Large multicenter studies found that tracheotomy was performed after a median of 11 days at the beginning of the century (21) and more recently that tracheotomy performed on day 7 was associated with potential advantages on weaning, ICU length of stay, and pneumonia, when compared to tracheotomy performed on day 14 (22). There is no longer any doubt that the advantages of minitracheotomy performed with percutaneous dilational technique (less time to perform, less expensive, performed in ICU) has provided the opportunity to test the tracheotomy early postoperatively (4) and even as a prophylactic strategy to reduce postoperative complications (3).
In our study we chose to use a tracheotomy tube with a 8 mm inner diameter rather than minitracheotomy (inner diameter of 4 mm) to have the maximal benefits in terms of airway resistance, respiratory mechanic, bronchial toilet and, in case of prolonged ventilation, to increase patient comfort with secure airway. The tracheotomy was performed immediately after the lung resection by the surgeon in the operating room, so it was advantageous in term of cost, duration of procedure and disponibility (23).
Inclusion of patients of heterogenous group has pointed out to probably altered the results on the role of tracheotomy (5,9). In our study, the majority of our patients had moderate or severe COPD and all of them underwent major lung resection for cancer by thoracotomy. For a long time, we know that patients with severe COPD are six times more likely to have major postoperative pulmonary complications after thoracic surgery than patients without COPD (6). More recently, in a retrospective study including 244 patients, Sekine et al. have shown that patients with COPD experienced more pneumonia and prolonged MV, and finally had more than a fourthfold 30 days mortality rate (8). Today, we know that noninvasive ventilation (NIV) is effective in decreasing the need for tracheal intubation in patients with mild or moderate COPD (24). Nevertheless, a prospective study has identified some limits of NIV in thoracic surgery: increased respiratory rate, number of bronchoscopy performed, and time spent under NIV were identified as risk factors associated with NIV failure (25). Patient with NIV failure developed more pneumonias, had a higher mortality rate than patients with NIV success and finally 42% of them needed a tracheotomy. In our opinion, these results show that a range of high risk patients with poor pulmonary function, marginal pulmonary mechanic, and risky for sputum retention who can expect benefits from prophylactic tracheotomy.
Various definitions of high risk patient have been proposed in previous RCT and sometime without measurable criteria (12). Others RCT included all patients eligible for lung resection (11,13). Because of the use of NIV in our practice, we selected patients with the most altered postoperative pulmonary function. We chose ppoFEV1 to define the operative risk of our patients because it is the association of two rigorous and easily measurable parameters world-widely used before lung resection: FEV1 and the amount of resected lung. Indeed, according to international preoperative assessment guidelines (26,27), FEV1 is often recommended before lung resection and, for a long time, has been one of the most reliable test to assess the operative risk (28). Moreover, FEV1 could be combined with the expected loss in pulmonary function to calculate the ppoFEV1, another risk factor for lung resection (7,29). In a prospective observational study, Nakahara et al have shown that ppoFEV1 was inversely related to the need of postoperative bronchoscopy, tracheostomy and the postoperative death (30). In a retrospective review of clinical records of patients with FEV1 and/or forced vital capacity ≤50%, Magdaleinat et al. reports a 8.5% mortality rate and 70% morbidity rate with a rate of pneumonia, tracheotomy and prolonged MV of 25%, 24%, and 20% respectively (7). A loss in pulmonary function >15% was associated with a significant higher rate of pulmonary complications. In a previous prospective study, we found that (I) ppoFEV1 was the best predictor of postoperative hypoxemia and postoperative pulmonary complications in lobectomy patients; and (II) patients who experienced pulmonary complications had a lower ppoFEV1 that patients who did not (29). Finally, postoperative respiratory complications are the main causes of major morbidity and mortality following lung resection (18), reaching the rate of 35% after pneumonectomy in our French practice (31), and this issue is largely impacted by risk factors such as low FEV1, malignant disease and major resection (19).
For the first time to our knowledge, a RCT shows a potential benefit of prophylactic tracheotomy on the duration of MV in selected patients eligible for lung resection. Among patients who experienced more than 2 days of MV, there was a trend to a shorter duration of MV in the tracheotomy patients compared to control patients. The difference began to be significant from the fourth postoperative day. The benefit observed in the tracheotomy group was not ascribable to greater severity of illness in the control group. The patients in both groups had similar characteristics at study inclusion, particularly %DLco and VO2max known as important parameters of preoperative evaluation (26,27). The increased airway resistance (sputum retention) and the decreased thoracopulmonary compliance linked to the lung resection and chest wound injury are two major causes of the increased work of breathing and altered breathing pattern after thoracotomy (32). In this situation, our results emphasize the advantages of tracheotomy performed as a prophylactic method giving to our patients immediate and favourable conditions to be weaned from MV. Our multivariate analysis indicates that pneumonectomy or patients with low VO2max should get a better benefit from this strategy.
Main pulmonary complications leading to respiratory failure after lung resection are sputum retention, atelectasis and pneumonia. In our study, the rate of atelectasis was fouthfold less important in tracheotomy patients than in control. Our results are in agreement with the preventing role of tracheotomy in sputum retention or atelectasis demonstrated in previous studies (11,12,33). With an incidence of 11% to 25%, pneumonia is a major concern representing the main cause of respiratory failure in COPD patients (7,8,29). In a RCT, Bonde and coworkers found a lower but not significant rate of pneumonia in tracheotomy patients (28%) compared to control patients (38%). The RCT by Issa and coworkers found a significant lower rate of pneumonia in tracheotomy patients (13%) compared to control patients (60%) but this latter study had only 15 patients in each group and an unusual increased rate of pneumonia in control patients.
In our study, the rate of pneumonia was 10% in tracheotomy group and 23% in the control group (P=0.2) and this complication tends to occur within the first 5 postoperative days (respectively 75% and 66% in each group) as previously reported (34). Taken together, these results argue for the preventing influence of tracheotomy on the occurrence of pneumonia.
Non cardiogenic pulmonary edema occurs with an incidence of 15% after lung resection (35) and the 13% rate of our study was quiet similar. The causes of this severe complication included excessive negative pulmonary pressure which can results from airway obstruction after extubation (36). Moreover, we know that the upper airway resistance contributes to 25% to 40% of the airflow resistance (37). Interestingly, we observed that non cardiogenic edema occurs in 1 patient of the tracheotomy group and 4 patients of the control group. In our opinion, one cannot exclude the preventing role of tracheotomy for this complication.
In our population of high risk patients, the 60-day mortality rate was 5.1%. It is an acceptable rate compared to the 30-day mortality rate of 3.8% in the overall population of patients eligible for lung cancer resection (19) and the 5% to 6% after pneumonectomy (31,38). With two deaths in each group and a mortality rate of 5.1%, our results are quiet similar to the RCT by Bonde and coauthors who found 5.8% hospital mortality rate and three deaths in each group (12). Our study confirms that prophylactic tracheotomy in lung cancer surgery does not worse but does not provide any benefit in terms of hospital mortality as in the general population of ICU patients (10) or in cardiac surgery patients where early tracheotomy was tested (4). Like others studies, we did not demonstrated benefits of tracheotomy in terms of ICU and hospital length of stay (4,11,13,22).
The rate of tracheotomy complication was 3.8% in our series. Two were minor complications (lack of cutaneous healing). The accidental decannulation occurred in ICU and the cannula was immediately replaced without life-threatening consequences nor death related to. It is a relatively low rate in accordance with the literature (39) and the surgical technique (40) but we evaluated only peri-operative complications and not late complications.
In conclusion, prophylactic tracheotomy in patients with ppo FEV1 <50% who underwent thoracotomy for lung cancer resection provided benefits in terms of duration of prolonged MV and respiratory complications but was not associated with a decreased mortality rate, ICU length of stay, hospital length of stay and non-respiratory complications. Patients with pneumonectomy or with VO2max <20 mL/kg/min should get a better benefit from this strategy.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Diehl JL, El Atrous S, Touchard D, et al. Changes in the work of breathing induced by tracheotomy in ventilator-dependent patients. Am J Respir Crit Care Med 1999;159:383-8. [PubMed]
- Moscovici da Cruz V, Demarzo SE, et al. Effects of tracheotomy on respiratory mechanics in spontaneously breathing patients. Eur Respir J 2002;20:112-7. [PubMed]
- Abdelaziz M, Naidu B, Agostini P. Is prophylactic minitracheostomy beneficial in high-risk patients undergoing thoracotomy and lung resection? Interact Cardiovasc Thorac Surg 2011;12:615-8. [PubMed]
- Trouillet JL, Luyt CE, Guiguet M, et al. Early percutaneous tracheotomy versus prolonged intubation of mechanically ventilated patients after cardiac surgery: a randomized trial. Ann Intern Med 2011;154:373-83. [PubMed]
- Rodriguez JL, Steinberg SM, Luchetti FA, et al. Early tracheostomy for primary airway management in the surgical critical care setting. Surgery 1990;108:655-9. [PubMed]
- Kroenke K, Lawrence VA, Theroux JF, et al. Postoperative complications after thoracic and major abdominal surgery in patients with and without obstructive lung disease. Chest 1993;104:1445-51. [PubMed]
- Magdeleinat P, Seguin A, Alifano M, et al. Early and long-term results of lung resection for non-small-cell lung cancer in patients with severe ventilatory impairment. Eur J Cardiothorac Surg 2005;27:1099-105. [PubMed]
- Sekine Y, Behnia M, Fujisawa T. Impact of COPD on pulmonary complications and on long-term survival of patients undergoing surgery for NSCLC. Lung Cancer 2002;37:95-101. [PubMed]
- Heffner JE. The role of tracheotomy in weaning. Chest 2001;120:477S-81S. [PubMed]
- Gomes Silva BN, Andriolo RB, Saconato H, et al. Early versus late tracheostomy for critically ill patients. Available online: http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD007271.pub2/abstract
- Randell TT, Tierala EK, Lepäntalo MJ, et al. Prophylactic minitracheostomy after thoracotomy: a prospective, random control, clinical trial. Eur J Surg 1991;157:501-4. [PubMed]
- Bonde P, Papachristos I, McCraith A, et al. Sputum retention after lung operation: prospective, randomized trial shows superiority of prophylactic minitracheostomy in high-risk patients. Ann Thorac Surg 2002;74:196-202; discussion 202-3. [PubMed]
- Issa MM, Healy DM, Maghur HA, et al. Prophylactic minitracheotomy in lung resections. A randomized controlled study. J Thorac Cardiovasc Surg 1991;101:895-900. [PubMed]
- Valentini I, Tonveronachi E, Gregoretti C, et al. Different tracheotomy tube diameters influence diaphragmatic effort and indices of weanability in difficult to wean patients. Respir Care 2012;57:2012-8. [PubMed]
- Ali MK, Mountain CF, Ewer MS, et al. Predicting loss of pulmonary function after pulmonary resection for bronchogenic carcinoma. Chest 1980;77:337-42. [PubMed]
- Juhl B, Frost N. A comparison between measured and calculated changes in the lung function after operation for pulmonary cancer. Acta Anaesthesiol Scand Suppl 1975;57:39-45. [PubMed]
- Sevrage de la ventilation mécanique — XXIe Conférence de consensus en réanimation et médecine d’urgence. Reanimation 2001;10:697-705.
- Seely AJ, Ivanovic J, Threader J, et al. Systematic classification of morbidity and mortality after thoracic surgery. Ann Thorac Surg 2010;90:936-42; discussion 942. [PubMed]
- Bernard A, Rivera C, Pages PB, et al. Risk model of in-hospital mortality after pulmonary resection for cancer: a national database of the French Society of Thoracic and Cardiovascular Surgery (Epithor). J Thorac Cardiovasc Surg 2011;141:449-58. [PubMed]
- Nieszkowska A, Combes A, Luyt CE, et al. Impact of tracheotomy on sedative administration, sedation level, and comfort of mechanically ventilated intensive care unit patients. Crit Care Med 2005;33:2527-33. [PubMed]
- Esteban A, Anzueto A, Alía I, et al. How is mechanical ventilation employed in the intensive care unit? An international utilization review. Am J Respir Crit Care Med 2000;161:1450-8. [PubMed]
- Terragni PP, Antonelli M, Fumagalli R, et al. Early vs late tracheotomy for prevention of pneumonia in mechanically ventilated adult ICU patients: a randomized controlled trial. JAMA 2010;303:1483-9. [PubMed]
- Weissbrod PA, Merati AL. Is percutaneous dilational tracheotomy equivalent to traditional open surgical tracheotomy with regard to perioperative and postoperative complications? Laryngoscope 2012;122:1423-4. [PubMed]
- Auriant I, Jallot A, Hervé P, et al. Noninvasive ventilation reduces mortality in acute respiratory failure following lung resection. Am J Respir Crit Care Med 2001;164:1231-5. [PubMed]
- Riviere S, Monconduit J, Zarka V, et al. Failure of noninvasive ventilation after lung surgery: a comprehensive analysis of incidence and possible risk factors. Eur J Cardiothorac Surg 2011;39:769-76. [PubMed]
- Colice GL, Shafazand S, Griffin JP, et al. Physiologic evaluation of the patient with lung cancer being considered for resectional surgery: ACCP evidenced-based clinical practice guidelines (2nd edition). Chest 2007;132:161S-77S.
- Brunelli A, Charloux A, Bolliger CT, et al. ERS/ESTS clinical guidelines on fitness for radical therapy in lung cancer patients (surgery and chemo-radiotherapy). Eur Respir J 2009;34:17-41. [PubMed]
- Markos J, Mullan BP, Hillman DR, et al. Preoperative assessment as a predictor of mortality and morbidity after lung resection. Am Rev Respir Dis 1989;139:902-10. [PubMed]
- Filaire M, Bedu M, Naamee A, et al. Prediction of hypoxemia and mechanical ventilation after lung resection for cancer. Ann Thorac Surg 1999;67:1460-5. [PubMed]
- Nakahara K, Ohno K, Hashimoto J, et al. Prediction of postoperative respiratory failure in patients undergoing lung resection for lung cancer. Ann Thorac Surg 1988;46:549-52. [PubMed]
- Thomas PA, Berbis J, Baste JM, et al. Pneumonectomy for lung cancer: contemporary national early morbidity and mortality outcomes. J Thorac Cardiovasc Surg 2015;149:73-82. [PubMed]
- Peters RM, Wellons HA Jr, Htwe TM. Total compliance and work of breathing after thoracotomy. J Thorac Cardiovasc Surg 1969;57:348-55. [PubMed]
- Au J, Walker WS, Inglis D, et al. Percutaneous cricothyroidostomy (minitracheostomy) for bronchial toilet: results of therapeutic and prophylactic use. Ann Thorac Surg 1989;48:850-2. [PubMed]
- Montravers P, Veber B, Auboyer C, et al. Diagnostic and therapeutic management of nosocomial pneumonia in surgical patients: results of the Eole study. Crit Care Med 2002;30:368-75. [PubMed]
- Parquin F, Marchal M, Mehiri S, et al. Post-pneumonectomy pulmonary edema: analysis and risk factors. Eur J Cardiothorac Surg 1996;10:929-32; discussion 933. [PubMed]
- McConkey PP. Postobstructive pulmonary oedema--a case series and review. Anaesth Intensive Care 2000;28:72-6. [PubMed]
- Levitzky MG, editor. Pulmonary physiology. New York: McGraw-Hill Medical, 2007.
- Shapiro M, Swanson SJ, Wright CD, et al. Predictors of major morbidity and mortality after pneumonectomy utilizing the Society for Thoracic Surgeons General Thoracic Surgery Database. Ann Thorac Surg 2010;90:927-34; discussion 934-5. [PubMed]
- Goldenberg D, Ari EG, Golz A, et al. Tracheotomy complications: a retrospective study of 1130 cases. Otolaryngol Head Neck Surg 2000;123:495-500. [PubMed]
- Koitschev A, Simon C, Blumenstock G, et al. Surgical technique affects the risk for tracheostoma-related complications in post-ICU patients. Acta Otolaryngol 2006;126:1303-8. [PubMed]