Designing a definitive trial for adjuvant targeted therapy in genotype defined lung cancer: the ALCHEMIST trials
Introduction
Targeted therapies, specifically tyrosine kinase inhibitors (TKIs), have redefined the standard of care for metastatic epidermal growth factor receptor (EGFR)-mutant or anaplastic lymphoma kinase (ALK)-rearranged non-small cell lung cancer (NSCLC) (1-8). The success of these agents exemplifies the current trend toward rationally designed compounds which target specific molecular alterations in cancer. The benefit associated with these agents is similar to the transformative benefit seen with imatinib in gastrointestinal stromal tumor (GIST) or with tamoxifen or trastuzumab for metastatic breast cancers (9-16). But while imatinib, tamoxifen and trastuzumab have long been employed in the adjuvant setting, targeted therapies have not been comprehensively studied in early stage NSCLC (Tables 1,2) (17-20,25-28). About half of all patients who present with potentially curable NSCLC will die from recurrent disease within 5 years (29). By comparison, 5-year survival among 200 patients with high-risk early stage KIT positive GIST treated with imatinib following surgery was 92% (18) and 5-year survival in operable ER positive or HER2 positive breast cancers treated with tamoxifen or trastuzumab is 92% (25) and 91% (26,27) respectively. It is fitting to now aim to transform adjuvant therapy of NSCLC through incorporation of targeted agents just as it has been transformed for these other diseases, but to achieve this a prospective controlled trial in a carefully selected population is needed.

Full table

Full table
Definitive study of adjuvant targeted therapy in NSCLC faces challenges. Such a trial must establish a system to prospectively identify patients with actionable genetic alterations and allocate them appropriately, as targeted therapy does not improve outcomes in unselected populations of early stage NSCLC (Table 2) (21,22). Furthermore, a successful trial must overcome the consistently low participation rate (<5%) in clinical trials among adults with cancer in the United States (30). The Adjuvant Lung Cancer Enrichment Marker Identification and Sequencing Trial (ALCHEMIST) combines the resources of the National Cancer Institute (NCI) with the broad reach of the NCTN to overcome these challenges. The NCTN, built from the previous cooperative group system, will use its academic and community centers to identify eligible patients who will agree to have tumor genotyping performed. The NCI’s Center for Cancer Genetics (CCG) will perform further genomic analysis of all samples. The ALCHEMIST design will allow comprehensive investigation of targeted therapies in resected NSCLC, and will be a platform that can grow with future therapeutic advances (Table 3).

Full table
Design overview
ALCHEMIST is designed to facilitate prospective screening, identification and enrollment of high-risk early stage (IB-IIIA), genotype-selected NSCLC patients in randomized trials of targeted therapy. ALCHEMIST currently consists of three integrated protocols: a screening study (A151216, coordinated by the Alliance for Clinical Trials in Oncology, PI: Geoffrey Oxnard) and two treatment trials (A081105, coordinated by the Alliance for Clinical Trials in Oncology, PI: Ramaswamy Govindan; E4512, coordinated by the ECOG-ACRIN Cancer Research Group, PI: David Gerber). Additional protocols may be included as other compelling therapies, such as immunotherapy agents, emerge.
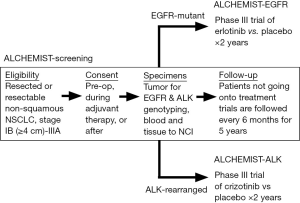
All patients must first consent to ALCHEMIST Screening (A151216) to be considered for ALCHEMIST trials of targeted therapy (Figure 1). Patients can consent prior to surgery or after resection. Eligible patients must have completely resected non-squamous NSCLC. Tumor specimens will be tested centrally for EGFR kinase domain mutations using sequencing, and for ALK rearrangements using FISH; patients must undergo confirmatory central genotyping on study regardless of whether any local genotyping was positive or negative for EGFR or ALK. Remaining tumor tissue will be paired with blood specimens for exploratory genomic analysis by the NCI. Patients whose tumors are found to harbor EGFR mutation or ALK rearrangement will be offered enrollment onto trials of adjuvant targeted therapy. Patients not enrolled onto the adjuvant trials will be followed on ALCHEMIST screening every 6 months for 5 years. In order to advance the understanding of genomic and biological mechanisms of recurrent or resistant disease, tumor samples from any biopsies performed at the time of recurrence will be collected and studied. While it is understood that not all patients will have tissue available at recurrence for genomic analysis, it is expected that a subset of patients will undergo resection or metastectomy as part of their clinical care and therefore will have a larger specimen available for study.
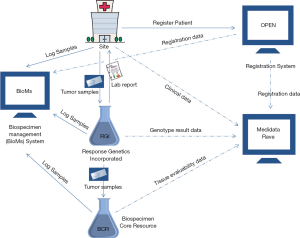
The flow of biospecimens and clinical data on ALCHEMIST are described in Figure 2. All biospecimens are logged and tracked through BioMS, and all genotype and clinical data are collected in Medidata Rave. While there were some existing integrations between the patient registration system (OPEN) and Medidata Rave, infrastructure to interface with laboratory partners (RGI, BCR) and Medidata Rave were built specifically for ALCHEMIST. As noted above and in Figure 2, beyond the predefined molecular alterations to identify potential patients for the EGFR and ALK sub-studies, full genomic characterization of submitted tissue will occur at the NCI-CCG. Regular conference calls and data exchanges between the different entities involved in ALCHEMIST ensure smooth functioning of this complex infrastructure.
Following standard adjuvant therapy, patients with fully resected EGFR-mutant NSCLC will be randomized to erlotinib versus placebo (1:1) for two years under ALCHEMIST-EGFR (A081105). Similarly, patients with fully resected ALK-rearranged NSCLC will be randomized to crizotinib vs. placebo (1:1) for two years under ALCHEMIST-ALK (E4512) following standard adjuvant therapy. As erlotinib and crizotinib are well established standard therapies for advanced NSCLC, it is anticipated that toxicity and compliance will be manageable throughout the NCTN.
The primary endpoint for ALCHEMIST adjuvant therapeutic trials is overall survival (OS). For the EGFR trial (A081105), a total accrual of 450 patients will allow for a 5% rate of study withdrawal and 5% non-confirmation rate of EGFR mutation via central testing, leaving the target accrual of 410 patients to power definitive analysis of the primary endpoint. This sample size will provide at least 85% power to show a hazard ratio (HR) of 0.67 or better in favor of erlotinib over placebo after 183 events, using a one-sided type I error of 5%. There is one planned interim analysis for futility when 50% of the events have been observed, using the O’Brien-Fleming stopping boundary. If the observed HR is greater than or equal to 0.96 (P value is 0.43 or greater), the recommendation will be to stop further accrual (if the trial is still accruing). It is expected that this trial will complete its targeted accrual. For the ALK trial (E4512), a total accrual of 378 patients will allow for a 5% non-confirmation rate of ALK rearrangement via central testing, leaving the target accrual of 360 patients to power definitive analysis of OS. This sample size will provide at least 80% power to detect a HR of 0.67 or better in favor of crizotinib over placebo after 164 events, using a one-sided type I error of 5%. A continuous efficacy-monitoring plan based on the O’Brien-Fleming group sequential boundary for the sequential testing incorporating the Lan and DeMets methodology for the error spending rate function is proposed: 10 planned interim analyses for OS starting at roughly 25% information (42 events under the alternative hypothesis) and one final analysis. At each interim analysis for efficacy, the study will also be monitored for early stopping in favor of the null hypothesis (i.e., futility) using repeated confidence interval methodology similar to that described by Jennison and Turnbull. At each interim analysis, if the nominal (1-2× alpha) confidence interval on the OS HR does not contain the target alternative of 0.67, then the data safety monitoring committee may consider terminating the study early for overall lack of treatment differences.
Rationale
EGFR TKIs are effective therapies, but must be employed in appropriately selected populations. OPTIMAL, a phase III randomized trial of erlotinib versus carboplatin and gemcitabine (1:1) for metastatic, previously untreated EGFR-mutant NSCLC demonstrated a significantly longer progression-free survival (PFS) for patients treated with erlotinib {13.1 [95% confidence interval (CI): 10.58-16.53] vs. 4.6 (4.21-5.42) months; HR: 0.16 (95% CI: 0.10-0.26); P<0.0001} (4). The IPASS trial further showed that clinical criteria alone are not sufficient to predict benefit from EGFR TKI—molecular analysis is needed (6,31). A total of 1,217 East Asian former light or non-smokers with treatment naïve advanced adenocarcinoma were randomized to gefitinib or carboplatin-paclitaxel (6). No advantage in OS was observed between the two arms [18.6 months, HR: 0.91 (95% CI: 0.76-1.10)] (31). While PFS (primary endpoint) was improved in the gefitinib arm [5.7 months, HR: 0.74 (95% CI: 0.65-0.85; P<0.001)], PFS benefit was limited to patients with EGFR mutations, as gefitinib was associated with shorter PFS for patients with wild-type EGFR [HR: 2.85 (95% CI: 2.05-3.98); P<0.001] (31).
No prospective, placebo controlled trial has examined adjuvant treatment with EGFR TKI in an appropriately selected population. The BR19 trial randomized patients with completely resected NSCLC to receive gefitinib or placebo for 2 years (21). The trial closed early due to lack of benefit, enrolling 503 of 1,242 planned patients (21). There was no significant difference in OS (HR: 1.24; 95% CI: 0.94-1.64; P=0.14) or DFS (HR: 1.22; 95% CI: 0.93-1.61; P=0.15) between the two arms (Table 2) (21). However, patients were not selected based on EGFR mutation status, and only 15 patients were found to have EGFR activating mutations (21). The RADIANT trial selected for EGFR expression or amplification, not EGFR mutation, in patients with resected NSCLC and showed no improvement in OS for erlotinib vs. placebo (Table 2) (22,32). Median DFS was improved with erlotinib vs. placebo in 161 patients with EGFR mutations (46.4 vs. 28.5 months; HR: 0.61, 95% CI: 0.38-0.98, P=0.039), however, this result was not statistically significant due to the hierarchical testing procedure employed by the trial (22,32). Retrospective data from MSKCC suggests improved DFS (HR: 0.43, 95% CI: 0.26-0.72, P=0.001) and OS with adjuvant EGFR TKI in resected EGFR-mutant NSCLC, but OS figures are not significant (HR: 0.50, 95% CI: 0.23-1.08, P=0.076) (Table 2) (23).
Motivated by this retrospective data, the SELECT trial enrolled 100 EGFR-mutant NSCLC patients to prospectively test the efficacy and feasibility of adjuvant erlotinib in molecularly-selected patients (24,33). Median DFS and OS have not yet been reached, but the 2-year DFS observed by the trial was 90% (n=89) (Table 2) (24). Importantly, many patients underwent dose reduction, which allowed improved compliance for the duration of adjuvant therapy. Building off the feasibility demonstrated in the SELECT trial, ALCHEMIST-Screening (A151216) in conjunction with ALCHEMIST-EGFR (A081105) will identify patients with resected EGFR-mutant lung cancer for enrollment onto a randomized, placebo-controlled trial to power definitive analyses regarding efficacy of EGFR TKI in resected EGFR-mutant NSCLC.
Phase III trials have also confirmed the efficacy of targeted TKI therapy in advanced ALK-rearranged NSCLC, but no prospective studies have examined adjuvant TKI in early stage ALK+ patients (7,8). A randomized study of second-line crizotinib vs. pemetrexed or docetaxel for ALK+ locally advanced or metastatic NSCLC demonstrated a median PFS of 7.7 months for crizotinib vs. 3.0 months for chemotherapy (HR: 0.49, 95% CI: 0.37-0.64, P<0.001) (7). These findings, combined with recent data that point to poorer prognosis for early stage ALK+ patients (34), make crizotinib an attractive option for incorporation into curative therapy for these patients. ALCHEMIST-ALK (E4512) represents a definitive trial to evaluate adjuvant ALK TKI in this subpopulation of NSCLC.
Endpoints and design rationale
The primary objective of ALCHEMIST-Screening is to facilitate accrual to ALCHEMIST trials of targeted therapy. ALCHEMIST-Screening aims to genotype up to 8,000 high-risk early stage NSCLC patients in order to fully accrue ALCHEMIST-EGFR and ALCHEMIST-ALK, based on estimated 15% prevalence of EGFR kinase domain mutations and 5% prevalence of ALK rearrangements (7,35,36). The desired rate of accrual to the therapeutic randomized trials is 16 patients per month: ~10 patients for the ALCHEMIST-EGFR trial and ~6 per month for ALCHEMIST-ALK trial, in order to reach completion within four years.
OS was chosen as the primary endpoint for ALCHEMIST trials of targeted therapy because it represents the most significant endpoint for patients with curable disease. In this setting, OS is the best measure for absolute benefit of a new treatment relative to standard of care. OS as an endpoint can be diluted by the effect of subsequent therapies, but if the benefit afforded by targeted therapy in the adjuvant setting does not surpass the benefit of targeted therapy at recurrence for control arm patients, then the value of adjuvant targeted therapy is less clear compared to treatment at time of recurrence.
The type I error rate (1-sided 0.05) for the two adjuvant trials is higher than the standard rate (2-sided 0.05) to accommodate the low prevalence of EGFR kinase mutations and ALK rearrangements in NSCLC and make the studies feasible. OS estimates for placebo arms for the purpose of sample size calculations were based on historical data of standard of care outcomes from unselected early stage NSCLC. It is important to note that, while there is less historical data for the standard of care outcome in early stage EGFR-mutant or ALK-rearranged patients, there are indications that EGFR-mutant NSCLC may predict a better prognosis, while ALK-rearranged NSCLC may be associated with a poorer prognosis (23,34,36,37).
The rate of agreement between the local and central testing results for EGFR and ALK will be monitored within the ALCHEMIST screening trial. Specifically, each locally deemed EGFR-mutant or wild-type patient will also be classified by central assessment. Similarly, each patient deemed locally as ALK-rearranged or not by FISH will be classified by the central assessment. For each locally used assay, agreement will be defined as the proportion of patients deemed mutant (or wild-type) by local and central assessment divided by the number of evaluable patients, where an evaluable patient is one who has a local assessment result and has submitted tissue for central assessment. An agreement rate of 90% or higher between the local assay and the central assessment will be deemed acceptable.
Conclusions
If the ALCHEMIST trials of targeted therapy reach their primary objectives, genotype-directed TKIs will become an important addition to standard curative therapy for early stage NSCLC patients with appropriate molecular alterations. The results of these trials may encourage further study of highly active targeted agents as part of curative therapy for NSCLC. For example, a trial studying adjuvant PD-1 inhibition is in development as part of the ALCHEMIST effort. Tissue collection and analysis through ALCHEMIST-Screening may also reveal new molecular targets, and may in turn pave the way for novel therapies to be studied in the adjuvant setting for appropriate populations.
Acknowledgements
Funding: Research described in this publication is supported by the National Cancer Institute of the National Institutes of Health under Award Numbers U10CA180821 and U10CA180882 (to the Alliance for Clinical Trials in Oncology).
Footnote
Conflicts of Interest: GR Oxnard has received consulting fees from Astra Zeneca and Boehringer Ingelheim and honoraria from Chugai.
References
- Paez JG, Jänne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 2004;304:1497-500. [PubMed]
- Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 2010;362:2380-8. [PubMed]
- Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med 2010;363:1693-703. [PubMed]
- Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2011;12:735-42. [PubMed]
- Gridelli C, De Marinis F, Di Maio M, et al. Gefitinib as first-line treatment for patients with advanced non-small-cell lung cancer with activating Epidermal Growth Factor Receptor mutation: implications for clinical practice and open issues. Lung Cancer 2011;72:3-8. [PubMed]
- Fukuoka M, Wu YL, Thongprasert S, et al. Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS). J Clin Oncol 2011;29:2866-74. [PubMed]
- Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med 2013;368:2385-94. [PubMed]
- Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med 2014;371:2167-77. [PubMed]
- von Mehren M, Blanke C, Joensuu H, et al. High incidence of durable responses induced by imatinib mesylate in patients with unresectable and metastatic gastrointestinal stromal tumor Proc Am Soc Clin Oncol 2002;21:403a. (abstract 1608).
- Heinrich MC, Corless CL, Demetri GD, et al. Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J Clin Oncol 2003;21:4342-9. [PubMed]
- Debiec-Rychter M, Sciot R, Le Cesne A, et al. KIT mutations and dose selection for imatinib in patients with advanced gastrointestinal stromal tumours. Eur J Cancer 2006;42:1093-103. [PubMed]
- Demetri GD, Benjamin RS, Blanke CD, et al. NCCN Task Force report: management of patients with gastrointestinal stromal tumor (GIST)--update of the NCCN clinical practice guidelines. J Natl Compr Canc Netw 2007;5 Suppl 2:S1-29; quiz S30.
- Sawka CA, Pritchard KI, Shelley W, et al. A randomized crossover trial of tamoxifen versus ovarian ablation for metastatic breast cancer in premenopausal women: a report of the National Cancer Institute of Canada Clinical Trials Group (NCIC CTG) trial MA.1. Breast Cancer Res Treat 1997;44:211-5. [PubMed]
- Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001;344:783-92. [PubMed]
- Andersson M, Lidbrink E, Bjerre K, et al. Phase III randomized study comparing docetaxel plus trastuzumab with vinorelbine plus trastuzumab as first-line therapy of metastatic or locally advanced human epidermal growth factor receptor 2-positive breast cancer: the HERNATA study. J Clin Oncol 2011;29:264-71. [PubMed]
- Valero V, Forbes J, Pegram MD, et al. Multicenter phase III randomized trial comparing docetaxel and trastuzumab with docetaxel, carboplatin, and trastuzumab as first-line chemotherapy for patients with HER2-gene-amplified metastatic breast cancer (BCIRG 007 study): two highly active therapeutic regimens. J Clin Oncol 2011;29:149-56. [PubMed]
- Dematteo RP, Ballman KV, Antonescu CR, et al. Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: a randomised, double-blind, placebo-controlled trial. Lancet 2009;373:1097-104. [PubMed]
- Joensuu H, Eriksson M, Sundby Hall K, et al. One vs three years of adjuvant imatinib for operable gastrointestinal stromal tumor: a randomized trial. JAMA 2012;307:1265-72. [PubMed]
- International Breast Cancer Study Group. Tamoxifen after adjuvant chemotherapy for premenopausal women with lymph node-positive breast cancer: International Breast Cancer Study Group Trial 13-93. J Clin Oncol 2006;24:1332-41. [PubMed]
- Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med 2005;353:1659-72. [PubMed]
- Goss GD, O'Callaghan C, Lorimer I, et al. Gefitinib versus placebo in completely resected non-small-cell lung cancer: results of the NCIC CTG BR19 study. J Clin Oncol 2013;31:3320-6. [PubMed]
- Kelly K, Altorki NK, Eberhardt WE, et al. A randomized, double-blind phase 3 trial of adjuvant erlotinib (E) versus placebo (P) following complete tumor resection with or without adjuvant chemotherapy in patients (pts) with stage IB-IIIA EGFR positive (IHC/FISH) non-small cell lung cancer (NSCLC): RADIANT results. J Clin Oncol 2014;32:abstr 7501.
- D'Angelo SP, Janjigian YY, Ahye N, et al. Distinct clinical course of EGFR-mutant resected lung cancers: results of testing of 1118 surgical specimens and effects of adjuvant gefitinib and erlotinib. J Thorac Oncol 2012;7:1815-22. [PubMed]
- Pennell NA, Neal JW, Chaft JE, et al. SELECT: A multicenter phase II trial of adjuvant erlotinib in resected early-stage EGFR mutation-positive NSCLC. J Clin Oncol 2014;32:abstr 7514.
- Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005;365:1687-717. [PubMed]
- Smith I, Procter M, Gelber RD, et al. 2-year follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer: a randomised controlled trial. Lancet 2007;369:29-36. [PubMed]
- Goldhirsch A, Gelber RD, Piccart-Gebhart MJ, et al. 2 years versus 1 year of adjuvant trastuzumab for HER2-positive breast cancer (HERA): an open-label, randomised controlled trial. Lancet 2013;382:1021-8. [PubMed]
- Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med 2005;353:1673-84. [PubMed]
- American Cancer Society. Cancer Facts & Figures 2015. Atlanta: American Cancer Society, 2015.
- Institute of Medicine (US) Forum on Drug Discovery, Development, and Translation. Transforming Clinical Research in the United States: Challenges and Opportunities: Workshop Summary. Washington (DC): National Academies Press (US), 2010.
- Soria JC, Mok TS, Cappuzzo F, et al. EGFR-mutated oncogene-addicted non-small cell lung cancer: current trends and future prospects. Cancer Treat Rev 2012;38:416-30. [PubMed]
- Shepherd FA, Altorki NK, Eberhardt WEE, et al. Adjuvant erlotinib (E) versus placebo (P) in non-small cell lung cancer (NSCLC) patients (pts) with tumors carrying EGFR-sensitizing mutations from the RADIANT trial. J Clin Oncol 2014;32:abstr 7513.
- Neal JW, Pennell NA, Govindan R, et al. The SELECT study: a multicenter phase II trial of adjuvant erlotinib in resected epidermal growth factor receptor (EGFR) mutation-positive non-small cell lung cancer (NSCLC). J Clin Oncol 2012;30:abstr 7010.
- Yang P, Kulig K, Boland JM, et al. Worse disease-free survival in never-smokers with ALK+ lung adenocarcinoma. J Thorac Oncol 2012;7:90-7. [PubMed]
- Garassino MC, Marsoni S, Floriani I. Testing epidermal growth factor receptor mutations in patients with non-small-cell lung cancer to choose chemotherapy: the other side of the coin. J Clin Oncol 2011;29:3835-7; author reply 3837-9. [PubMed]
- Rosell R, Moran T, Queralt C, et al. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med 2009;361:958-67. [PubMed]
- Marks JL, Broderick S, Zhou Q, et al. Prognostic and therapeutic implications of EGFR and KRAS mutations in resected lung adenocarcinoma. J Thorac Oncol 2008;3:111-6. [PubMed]