Risk- and response-adapted strategies for the management of Hodgkin lymphoma
Introduction
Biological insights, new therapies and incremental clinical trial evidence have allowed treatment strategies that result in cure rates for Hodgkin lymphoma (HL) of 80% and higher. Unusually among malignancies, cures in HL can be achieved with both primary therapy, and in many cases with salvage therapy for refractory or relapsed disease, making the assessment of therapeutic benefit more complex, and dependent upon long follow up. A consequence of the high survivorship in a patient population with a peak among young adults is the risk of late adverse effects from therapy. Radiotherapy and chemotherapy can variously induce second malignancies, cardiovascular disease and infertility, with an incidence strongly correlated to the intensity of the initial therapy. In considering the optimal treatment of HL, a balance has to be struck between the need to maintain treatment intensity to maximise cure rates, whilst taking measures to limit excessive early or late toxicity. In doing this, selecting patients with unfavourable disease at presentation or who do not show an early treatment response, for selective intensification of therapy, may provide a means to achieve the correct approach. The widespread use of functional imaging with 18F-fluorodeoxyglucose positron emission tomography (18F-FDG-PET) has enabled investigators to identify non-responders early during treatment. The primary treatment of both early and advanced stage HL has been progressively refined through clinical trials that address the optimal approach with combined or single modality therapy.
Primary therapy
Primary treatment is guided by the clinical stage of disease as described by the Cotswold classification (1) and updated by the Lugano classification to incorporate the use of FDG-PET/computerised tomography (CT) imaging in baseline assessment (2). Patients are divided into early (stage I and favourable stage II) or advanced (high risk stage II, stages III and IV) disease. The standard approach to treatment is with combined chemotherapy and radiotherapy in early disease and predominantly chemotherapy, sometimes followed by consolidation radiotherapy, in advanced stages. The combination of doxorubicin, bleomycin, vinblastine and dacarbazine (ABVD) has become a standard approach in many centers, over three decades after the original randomised control trial demonstrated complete remissions in 75% with a tolerable side effect profile (3) (see Table 1). Contemporary prospective trials have confirmed favourable outcomes with ABVD, reporting complete response rates of 73-89%, 5-year freedom from progression (FFP) of 73-76% and 5-year overall survival (OS) of up to 90% in intermediate and advanced stage HL (4-7). Many attempts to improve upon these results using more complex regimens have proven negative, but one which leads to consistently better tumour control is the escalated BEACOPP regimen (Table 2), developed by the German Hodgkin Study Group (GHSG). It is mostly used as primary therapy in advanced HL after demonstrating greater efficacy over the alternating COPP-ABVD regimen (8,9). After 10 years of follow-up eBEACOPP was associated with a freedom from treatment failure (FFTF) rate of 82%, OS of 86% and second malignancy rate of 6% in patients with advanced stage HL (10). Direct comparative studies have sought to address the question of which regimen is superior, with the conclusion that eBEACOPP produces higher response rates and greater tumour control with prolonged progression-free survival (PFS) but minimal improvement in OS, owing to the efficacy of second line therapy (11-14). A recent systematic review suggested that six cycles of eBEACOPP might give a survival advantage of 7% over ABVD in advanced stage HL (5-year OS; 95% vs. 88%, respectively) (15). This however is at the expense of more acute toxicity, infertility in many cases and a slightly higher risk of secondary acute myeloid leukaemia or myelodysplastic syndromes, although the total number of secondary malignancies has not been shown statistically different to ABVD (12). The main arguments in favour of selecting ABVD over eBEACOPP are lower acute grade 3/4 toxicities, principally myelosuppresion (leukopenia 22% vs. 98%; thrombocytopenia 3% vs. 70%; anaemia 5% vs. 66%), infection (2% vs. 22%), nausea (13% vs. 20%), and alopecia (31% vs. 79%) (16,17). ABVD is associated with fewer hospital admissions with neutropenic sepsis and significantly lower rates of infertility (18,19).
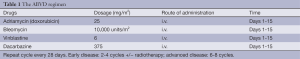
Full table
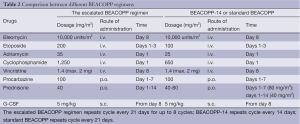
Full table
Prognostic factors at diagnosis
Early stage disease has been stratified into favourable and unfavourable prognostic groups based on age, the presence of B symptoms, number of nodal sites involved, and the maximum size of adenopathy (20,21) (see Table 3). The International Prognostic Score (IPS) for HL was devised as a risk stratification tool for advanced HL that defined adverse prognostic factors at diagnosis based on seven clinical parameters (low albumin, anaemia, male gender, increasing age, advanced stage, leukocytosis, and lymphopenia) (22). This data collection from over 5,000 patients reflected outcomes for those treated mainly in the 1980s, while recently the IPS was re-tested in 740 patients with advanced-stage HL treated in British Columbia over a 30-year period (23). The study found that overall 5-year FFP had improved from 42% to 62%, the IPS retained its prognostic statistical significance, but as the results improved it was not possible to identify a patient group with sufficiently poor prognosis to justify intensified first-line therapy.

Full table
The one group with persistently worse outcomes is the elderly, owing to lower response rates, co-morbidity, lower success rates with salvage therapy and excess deaths from other causes including infections, second malignancies and cardiac disease (24,25).
The limitations of clinical prognostic models have led to the evaluation of molecular and immune-based biomarkers. These include assessment of circulating microRNA (26), infiltrating immune cells such as macrophages (27), the presence of Epstein-Barr virus (28-30), circulating cytokines (31-33), chemokines (34) and surface proteins such as CD30 (35,36), produced by malignant and host immune cells. Gene-expression profiling has identified a transcript signature of tumour associated CD68+ macrophages, whose frequency is directly correlated with treatment failure and survival, outperforming the IPS as a single prognostic biomarker (37). More limited but logistically practical technologies to measure RNA-based gene expression on formalin-fixed paraffin-embedded tissue will soon enable testing to enter the routine diagnostic workup (38). A 23-gene panel has been developed which may identify patients at diagnosis who are at increased risk of treatment failure and death owing to the underlying tumour biology and suppressed anti-tumour immunity (39). These biomarkers may hold promise for the definition of prognosis based upon biology rather than more general clinical findings, but they still require prospective validation before they enter routine use.
Functional imaging with FDG-PET
HL has a FDG avidity rate of 97-100% and when used alongside CT imaging this can lead to a change in stage in 10-30% of patients at baseline (2). Bone marrow biopsy is no longer part of routine staging owing to the high sensitivity (88-100%) and specificity (87-100%) of FDG-PET/CT to detect bone marrow involvement (40-42). The use of FDG-PET imaging to detect residual metabolically active disease is well validated in this context, effectively acting as a surrogate biomarker for chemosensitivity. Superseding the prognostic value of the IPS, and superior to CT, a positive FDG-PET scan after two cycles of ABVD is independently highly predictive of relapse and poorer OS (43-47). Advanced HL has a negative predictive value (NPV) of 97-100% and a positive predictive value (PPV) of 13-27% (48), with one study reporting a NPV of 73% (49). Patients with a PET-2 negative scan have a 2-year PFS of 96% compared to 0-6% for PET-2 positive patients (45). Multivariate analysis demonstrated that the IPS loses its prognostic value when compared with PET-2 since PET-2 positive patients have an equally high risk of treatment failure with both high and low IPS, and conversely, PET-2 negative patients have an equally low risk of treatment failure with both high and low IPS. FDG-PET imaging is interpreted using the Deauville 5-point scoring system (5PS) (50,51). This is well validated and reproducible, however, central review is still preferable in the context of clinical trials. A PET 5PS of 1-2 is considered ‘negative’, 4-5 is considered ‘positive’, whilst a score of 3 is variably interpreted according to the objectives of the trial (52).
The correct timing of FDG-PET scanning is important as test sensitivity can be compromised by the immediate effect of chemotherapy stunning on cellular glucose metabolism (53). In addition, FDG-PET scans should not be performed 7-10 days post chemotherapy during the peak of the inflammatory response to avoid misinterpretation (54,55). The optimal timing of PET-2 scanning is 11-13 days after the second cycle of chemotherapy, prior to the day 14 treatment, within a 28-day cycle (56). Following completion of primary therapy FDG-PET imagining should be performed 3-6 weeks from the final chemotherapy or 12 weeks from completing radiotherapy to avoid a radiotherapy-induced inflammatory response (53,54).
Treatment of early stage favourable Hodgkin lymphoma (HL)
For patients with early stage favourable disease, large randomised trials have helped to define the optimal treatment choices: chemotherapy alone, or combined modality therapy. Radiation therapy alone can elicit cures, however combined modality strategies reduce relapse rates, the need for subsequent salvage therapy and thereby the overall rate of second malignancies (57). Extensive radiation therapy is associated with the development of secondary malignancies 5 to 25 years after primary treatment (58-60), leading investigators to seek reduced exposure, or to eliminate radiation altogether. An historic meta-analysis of 23 randomised trials involving 3,888 patients with early stage HL showed combined chemotherapy and involved field radiotherapy (IFRT) improved disease-free survival compared to radiotherapy alone, halving the 10-year risk of treatment failure (16% vs. 33%; P<0.00001), although the efficacy of salvage chemotherapy after radiotherapy alone meant that there was no clear difference in OS at 10 years (61).
The EORTC H8-F trial demonstrated a 5-year event-free survival (EFS) rate significantly higher after three cycles of MOPP-ABV plus IFRT than after subtotal nodal radiotherapy alone (98% vs. 74%, P<0.001). The 10-year OS estimates were 97% and 92%, respectively (P=0.001) (62). Standard approaches now include ABVD for a variable number of cycles, with or without radiation therapy. A few trials have tested the merit of a chemotherapy-alone strategy. In one, there was no significant difference in relapse rate or OS between those having or omitting extended irradiation after 6 ABVD (63), while in the North American Children’s Oncology Group study, patients with complete remission after chemotherapy were randomized to low-dose (21 Gy) IFRT or no further therapy (64). The trial was stopped when an interim analysis revealed a significant difference in PFS between the arms, and with a median 7.7 years follow-up, there was a significant difference in EFS favouring the radiotherapy group (93% vs. 83%, P<0.004). However, there was no difference in OS, with 10-year estimated survival rates of 96-97%, across all risk groups. A larger trial among adults of chemotherapy alone group resulted in improved OS at 12-year compared to chemotherapy plus extended-field radiotherapy (EFRT) (94% vs. 87%, respectively) (65). However, this was confounded by an increased rate of death from other causes in the combined modality group, and the use of EFRT, which is no longer used, being known to cause greater late toxicity compared with modern radiotherapy techniques. Other randomised trials, meta-analyses and population-based studies in early stage disease have shown superior tumour control with a combined treatment approach compared to chemotherapy alone (66-69).
Contemporary trials have focused on optimising a combined modality strategy. The GHSG HD10 ‘non-inferiority’ trial randomly assigned 1,370 patients with favourable prognosis early stage HL to receive two or four cycles of ABVD with 20 or 30 Gy of IFRT (20). At a median follow-up of 7.5 years, there was no significant difference between four and two cycles of ABVD chemotherapy (5-year OS, 97% vs. 97%; FFTF, 93% vs. 91%) or between 30 Gy and 20 Gy IFRT (5-year OS, 98% vs. 97.5%; FFTF, 93% vs. 93%). However, there were significant differences in major toxicity between four and two cycles of ABVD (grade ≥3 overall toxicity; 52% vs. 33%) and between 30 and 20 Gy IFRT (grade ≥3 overall toxicity; 9% vs. 3%). This trial demonstrated that the duration and intensity of first line treatment could be reduced while maintaining tumour control by using two cycles of ABVD followed by 20 Gy IFRT over a three-month period. In a follow-on study, GHSG HD13, 1,502 patients with favourable prognosis early stage HL received two cycles of ABVD, omitting dacarbazine and/or bleomycin, followed by 30 Gy IFRT (70). FFTF at 5-year was significantly lower in the chemotherapy omitted groups, suggesting that both these drugs make a significant contribution to cure rates.
Treatment of early stage unfavourable Hodgkin lymphoma (HL)
The standard treatment for early stage, unfavourable prognosis HL is combination chemotherapy with ABVD (four to six cycles) followed by IFRT or involved-node radiation (INRT). As with favourable prognosis early HL, a subset of patients with non-bulky disease or those with increased susceptibility to long-term radiotherapy complications may be adequately treated with chemotherapy alone when this is given for additional cycles.
The two key GHSG trials in intermediate stage/early unfavourable prognosis HL are the HD11 and HD14 trials. The HD11 trial compared four cycles of ABVD versus four cycles of BEACOPP, with each arm receiving either 20 or 30 Gy IFRT (71). BEACOPP was associated with increased severe toxicity and showed no additional benefit in disease control over ABVD when given with 30 Gy IFRT. However, 20 Gy IFRT was inferior to 30 Gy when given after four cycles of ABVD. The HD14 trial demonstrated improved tumour control, but not improved survival, with two cycles of eBEACOPP plus two cycles of ABVD with 30 Gy IFRT (2+2 arm) compared to four cycles of ABVD plus 30 Gy IFRT (5-year FFTF 95% vs. 88%, respectively; OS 97% each; P<0.001) (72). Despite the significantly increased acute toxicity with eBEACOPP (grade ≥3 overall toxicity; 87% vs. 51%), the authors suggested a 2+2 strategy was preferable in a broader context as fewer patients progressed (2.5% vs. 8.4%; P<0.001), thereby averting salvage chemotherapy and its toxicity.
Risk adapted radiotherapy—involved-node radiation (INRT)
Trials involving combined modality approaches in early stage HL have largely used IFRT with doses 20 to 40 Gy, given in single fractions of 1.8 to 2.0 Gy (62,73-75). In addition to lowering the radiation dose there has been a focus on reducing the field size to limit toxicity without compromising tumour control. EFRT, which includes adjacent uninvolved sites (e.g., mantle field), has been superseded by IFRT limited to involved regions only (e.g., mediastinal irradiation). A retrospective study of female Hodgkin’s survivors exposed to either EFRT or IFRT reported an overall cumulative incidence at 30 years of breast cancer of 26% in those treated before the age of 21 (76). An increased field size with EFRT compared with similarly dosed IFRT (36-44 Gy) was associated with a 2.7-fold increased breast cancer risk. Cardiovascular complications directly related to field size include accelerated atherosclerosis causing coronary artery disease, cardiac valvular fibrosis and radiation pericarditis (77). Cardiovascular disease represents the most common non-malignant cause of death in long-term survivors (60,78,79). When the heart is within the irradiated field, there is a dose-dependent relationship with doses above 15 Gy, with the incidence of biventricular failure, myocardial infarction, pericardial and valvular disease occurring two- to six-fold higher than in non-irradiated survivors (80). Patients with pre-existing heart disease are at 20% increased risk of cardiac hospitalisation by 10 years when treated with primary mediastinal radiotherapy alongside anthracyline-containing chemotherapy, compared to chemotherapy alone (81). As a consequence the risk of requiring cardiac procedures was up to nine-fold higher than the general population (82). These interventions included coronary artery bypass grafting, angioplasty and valve surgery.
The increasing sophistication of modern radiotherapy conformal planning and cross-sectional imaging has facilitated a move to further reduce field sizes with involved-site radiation (ISRT) and INRT delivering treatment to only detectable nodes (and extranodal lesions) identified at presentation (83). New treatment techniques include image-guided radiotherapy, four-dimensional imaging, deep-inspiration breath-hold (DIBH) and intensity modulated radiotherapy (IMRT) (84). ISRT is more conservative than INRT with the radiation field including only pre- and post-chemotherapy tumour volumes compared to pre- and post-chemotherapy nodal volume (85). Both techniques include a margin of healthy tissue of under 1 cm and use the pre-chemotherapy gross tumour volume (GTV) to determine the clinical target volume (CTV). The key distinction between the two techniques is that INRT integrates imaging with contrast-enhanced CT, fused FDG-PET/CT and MRI with 3-dimensional (3D) planning to further reduce the CTV. In INRT the pre-chemotherapy FDG-PET/CT is fused with the post-chemotherapy planning CT scan enabling the CTV to be contoured whilst sparing uninvolved surrounding structures such as heart and lungs. The patient must undergo baseline imaging in the planned RT position. Comparison of baseline and post-chemotherapy imaging identifies initially involved lymph nodes that had subsequently shrunk or vanished (86). INRT post ABVD has been validated by several studies, showing it to be safe and achieving excellent tumour control in early stage HL with 4-year FFTF of 96% and 5-year PFS of 92% with radiation doses of 30-40 Gy (84,87). The GHSG showed a significant reduction in the planning target volumes (PTV) of 20 patients with early mediastinal HL, with a median PTV of 1,705 cm3 with IFRT and 1,015 cm3 with INRT (88). INRT results in reduced radiation to organs at risk (OAR) with a mean dose to the heart of 17.94 Gy (3D-IFRT) vs. 9.19 Gy (3D-INRT) and 13.76 Gy (IF-IMRT) vs. 7.42 Gy (IN-IMRT). IMRT reduces OAR volume exposed to high doses and is superior for large PTVs involving the anterior mediastinum. Studies with involved-node proton therapy have further reduced the median dose to OAR compared to IN-IMRT and 3D-INRT owing to the unique radiobiology of proton therapy (89). Modern radiotherapy is likely to be shaped by an individualised, highly conformal approach guided by integrated multi-modality imaging.
Response-adapted therapy for early Hodgkin lymphoma (HL)
Controversy over the omission of radiotherapy in early HL has led to the testing of FDG-PET imaging as a means to select those patients who may not require irradiation after chemotherapy. A recent retrospective study assessed the predictive value of FDG-PET scanning after two cycles of ABVD (PET-2) on 2-year failure-free survival (FFS) following treatment with four cycles of ABVD plus IFRT in early HL (90). A total of 15% of patients had a PET-2 positive scan, and after central review the predictive value of PET-2 on PFS was characterised by a very high NPV (95%) and specificity (92%), a low PPV (53%) and sensitivity (65.5%), and an accuracy of 89%. The study found the presence of bulky disease within early stage HL could negatively influence the specificity and PPV of PET-2.
The EORTC H10 study in early HL tested the capacity of FDG-PET scanning after two cycles of ABVD to guide subsequent treatment (91), randomising patients between either standard or PET-directed algorithms. The standard arm for favourable HL was 3 ABVD with 30 Gy INRT, while in the experimental arm a negative PET after two ABVD was followed by two further cycles but no irradiation, and those with a positive PET scan received two cycles of eBEACOPP followed by 30 Gy INRT. For unfavourable disease the standard was 4 ABVD with 30 Gy INRT, while the PET-guided arm used six ABVD for those PET-negative after two cycles, or eBEACOPP with 30 Gy INRT for those remaining PET-positive. A pre-planned interim futility analysis revealed a small but significant increase in early treatment failure in the chemotherapy alone group where radiotherapy was omitted following an early negative PET scan, although the results may have been affected by lack of central review of the scans and the absence of local blinding of the reporting. Nonetheless both trial arms had excellent outcomes with 1-year PFS of 95% and 97%, respectively.
The UK RAPID trial used FDG-PET response-directed therapy following three cycles of ABVD in early HL to randomised PET-negative patients between IFRT and no further radiotherapy (NFT) (92). Analysis after a median 5 years follow up showed the PFS at 3 years to be 94.6% in the irradiated group and 90.8% in the non-irradiated, with no difference in overall survival: 97.1% in the irradiated and 99% in the non-irradiated group. The investigators concluded that excellent outcomes are achievable without radiotherapy in selected cases, albeit with a slight increase in the risk of recurrence.
The GHSG HD16 trial is currently recruiting 1,100 favourable early stage HL patients and randomising those who are PET negative after two cycles of ABVD to receive either 20 Gy IFRT or no further treatment (NCT00736320).
Treatment of advanced stage Hodgkin lymphoma (HL)
Opinion is divided as to whether 68 cycles of ABVD or six cycles of eBEACOPP should be the standard of care for advanced HL. The use of interim FDG-PET scanning to identify early non-responders provides a response-adapted approach whereby those with PET-positive disease after two cycles of ABVD can be risk stratified and go on to receive the more intensive eBEACOPP. Conversely, investigators have examined the role of early FDG-PET scanning as a means to de-escalate treatment to avoid unnecessary toxicity in early responders. In contrast to early stage HL, maximising treatment efficacy takes precedence over limiting toxicity.
Several randomised trials are currently evaluating PET-directed therapy in advanced HL. Studies designed to assess whether early PET negative scans can de-escalate standard therapy include the RATHL study comparing ABVD with AVD (bleomycin omitted) (93,94), the GHSG HD18 trial, investigating 6 versus 4 cycles of BEACOPP, the Italian lymphoma study group HD0801 trial, investigating 30 Gy RT versus no RT after six cycles of ABVD, and the Group pour l'Etude des Lymphomes de l'Adulte (GELA) AHL and Israeli RHC trials both comparing BEACOPP versus ABVD. The studies all have the primary endpoint of PFS of between 2 to 5 years. The GHSG HD18 trial uses a four-point scale for interim FDG-PET interpretation, similar to the Deauville 5PS; however since the principal objective was to test de-escalation, researchers chose a more conservative cut-off for a positive PET scan in an attempt to reduce false-negative results (95). This has resulted in a higher PET-2 positive rate of 47% after two cycles of eBEACOPP (56), and stands in contrast to a rate of 15-20% with ABVD in three of the other PET-directed trials (94,96,97).
Studies evaluating whether early PET positive scans can be used to escalate standard therapy include the RATHL study (93), escalating ABVD to either six cycles of BEACOPP-14 or four cycles of eBEACOPP, the US Intergroup S0816 trial (97), escalating ABVD to six cycles of eBEACOPP, the Italian lymphoma study group HD0607 trial (96), escalating ABVD to four cycles of eBEACOPP and four baseline BEACOPP, the GHSG HD18 trial (95), investigating the addition of Rituximab to eBEACOPP, and the Israeli RHC trials both comparing escalating eBEACOPP or ABVD to eBEACOPP with or without INRT.
Some trials start with eBEACOPP and de-escalate to ABVD with a negative PET-2, whilst other trials start with ABVD and escalate to eBEACOPP with a positive PET-2. These are clearly alternative strategies, with the critical difference being that escalated therapy will affect a much smaller patient subset, of approximately 15-20% with a positive interim PET scan. Preliminary results from the Italian HD0607 trial have shown a 15% positive PET-2 rate following two cycles of ABVD, with 41/222 patients escalated to four cycles of eBEACOPP plus four cycles of stdBEACOPP, with 73% achieving complete remission (96). The 1-year PFS in the PET-2 positive group was 80.5%, demonstrating the merits of an early switch in therapy. The majority (64%) of positive PET-2 scans had residual FDG uptake from a single bulky mediastinal site (98). A retrospective trial that analysed an identical strategy of escalated therapy reported a 2-year FFS of 91% overall, and 62% in the escalated PET-2 positive group (99), which was clearly superior to historical prospective trials that reported a 2-year FFS of 80% overall (100,101), and just 12% in the PET-2 positive group maintained on ABVD (45).
Results from the UK RATHL trial have demonstrated a PET-2 negative rate of 84%, with a PFS event rate of 9% in the first year among this group (93). Patients had less deterioration in lung function if they were randomised to stop bleomycin (mean change in diffusion capacity; 4.3% vs. 12.8%) (94). Longer term treatment failure rates and survival data are awaited, to determine whether this was linked to any difference in efficacy. In the PET-2 positive arm, patients escalated with eBEACOPP or BEACOPP-14 achieved CR in 76%, at completion FDG-PET scanning (PET-3). The response rates did not differ between the two intensified BEACOPP regimens. The rate of treatment failure was 22% in the PET-2 positive arm. These initial results suggest that early FDG-PET scanning may be a useful tool for treatment modulation, both for escalating and de-escalating therapy.
Failure of primary therapy
Patients with primary refractory or relapsed disease may still be cured with salvage regimens and high dose consolidation therapy, with those who received more intensive chemotherapy (alternating MOPP/ABVD or BEACOPP) being harder to salvage successfully than those who received ABVD (102,103). Predictably, performance status, age above 50 years and the ability to tolerate salvage chemotherapy and ASCT directly impacts on survival (104,105). Primary refractory disease carries a poorer prognosis than relapsed disease after primary therapy (106). In relapsed disease, short time to relapse (within one year of completing induction therapy), advanced stage at relapse, and anaemia are poor prognostic factors (107). The use of FDG-PET scanning prior to ASCT is strongly predictive of subsequent relapse, with one study demonstrating an EFS of >80% if PET-negative pre-ASCT, compared to 29% if PET-positive (108).
Conclusions
Recent studies have explored a risk-adapted strategy to HL, using early FDG-PET scanning to guide therapy by acting as a marker of chemosensitivity. PET-2 scanning is highly predictive of outcome in patients with advanced HL treated with ABVD (45), especially when reading is standardised using the 5PS, rather than the use of standardised uptake values (SUV). The scale provides reproducible results with a high level of concordance seen across studies (51,109). The cut-off for a positive and negative PET scan can be adapted according to the clinical trial design, in order to avoid over-treatment or under-treatment. A PET tailored approach appears promising, but until the current studies report their primary endpoints, changing treatment based solely on interim PET scanning cannot be mandated as a standard of care (52). As a result, the optimal management of ABVD-treated patients with advanced stage HL who have a positive PET-2 remains uncertain: current recommendations are that treatment should not be altered unless there is clear evidence of disease progression or unacceptable toxicity (48). In early stage HL, if a pre-treatment plan has been made to give primary therapy with ABVD followed by radiotherapy, then there is no clear role for early PET assessment (52). However, end of treatment FDG-PET/CT is recommended for all patients to inform on the need for radiotherapy, further biopsies and follow-up schedules.
The current standard of care for early stage HL is two cycles of ABVD and 20 Gy radiotherapy for favourable prognosis, and four cycles of ABVD and 30 Gy for unfavourable prognosis (52). If radiotherapy is to be omitted on the basis of a negative post-chemotherapy PET scan then patients should receive a minimum of three cycles of ABVD and should be aware of the slight increase in the risk of recurrence. However, radiotherapy is recommended for early stage patients with bulky disease. Combining two cycles of eBEACOPP with two cycles of ABVD and 30 Gy is a treatment option in early unfavourable HL, as per the HD14 trial protocol (72).
The standard of care for advanced HL in patients aged 16 to 60 is either 6-8 cycles of ABVD or six cycles of eBEACOPP, with consideration given to the differences in efficacy and toxicity between these two regimens. Consolidation radiotherapy is recommended for ABVD-treated patients to sites of original bulky disease or where there is residual tissue is >1.5 cm on CT, although the early results of the PET-negative arms in RATHL are promising and may in due course provide evidence that radiotherapy can be avoided for this group. PET-negative patients who received eBEACOPP do not require consolidation radiotherapy to residual tissue (52).
The treatment of HL continues to evolve, and the studies now in progress or in follow up promise to further refine the approach, maintaining the high expectation of cure but also lowering the risk of long-term morbidity.
Acknowledgements
Disclosure: PW Johnson has received honoraria for participation in advisory boards and educational symposia from Millenium-Takeda and Bristol Myers Squibb.
References
- Lister TA, Crowther D, Sutcliffe SB, et al. Report of a committee convened to discuss the evaluation and staging of patients with Hodgkin’s disease: Cotswolds meeting. J Clin Oncol 1989;7:1630-6. [PubMed]
- Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol 2014;32:3059-68. [PubMed]
- Bonadonna G, Zucali R, Monfardini S, et al. Combination chemotherapy of Hodgkin’s disease with adriamycin, bleomycin, vinblastine, and imidazole carboxamide versus MOPP. Cancer 1975;36:252-9. [PubMed]
- Gobbi PG, Levis A, Chisesi T, et al. ABVD versus modified stanford V versus MOPPEBVCAD with optional and limited radiotherapy in intermediate- and advanced-stage Hodgkin’s lymphoma: final results of a multicenter randomized trial by the Intergruppo Italiano Linfomi. J Clin Oncol 2005;23:9198-207. [PubMed]
- Gordon LI, Hong F, Fisher RI, et al. Randomized phase III trial of ABVD versus Stanford V with or without radiation therapy in locally extensive and advanced-stage Hodgkin lymphoma: an intergroup study coordinated by the Eastern Cooperative Oncology Group (E2496). J Clin Oncol 2013;31:684-91. [PubMed]
- Hoskin PJ, Lowry L, Horwich A, et al. Randomized comparison of the stanford V regimen and ABVD in the treatment of advanced Hodgkin’s Lymphoma: United Kingdom National Cancer Research Institute Lymphoma Group Study ISRCTN 64141244. J Clin Oncol 2009;27:5390-6. [PubMed]
- Johnson PW, Radford JA, Cullen MH, et al. Comparison of ABVD and alternating or hybrid multidrug regimens for the treatment of advanced Hodgkin’s lymphoma: results of the United Kingdom Lymphoma Group LY09 Trial (ISRCTN97144519). J Clin Oncol 2005;23:9208-18. [PubMed]
- Tesch H, Diehl V, Lathan B, et al. Moderate dose escalation for advanced stage Hodgkin’s disease using the bleomycin, etoposide, adriamycin, cyclophosphamide, vincristine, procarbazine, and prednisone scheme and adjuvant radiotherapy: a study of the German Hodgkin’s Lymphoma Study Group. Blood 1998;92:4560-7. [PubMed]
- Diehl V, Franklin J, Pfreundschuh M, et al. Standard and increased-dose BEACOPP chemotherapy compared with COPP-ABVD for advanced Hodgkin’s disease. N Engl J Med 2003;348:2386-95. [PubMed]
- Engert A, Diehl V, Franklin J, et al. Escalated-dose BEACOPP in the treatment of patients with advanced-stage Hodgkin’s lymphoma: 10 years of follow-up of the GHSG HD9 study. J Clin Oncol 2009;27:4548-54. [PubMed]
- Mounier N, Brice P, Bologna S, et al. ABVD (8 cycles) versus BEACOPP (4 escalated cycles ≥ 4 baseline): final results in stage III-IV low-risk Hodgkin lymphoma (IPS 0-2) of the LYSA H34 randomized trial. Ann Oncol 2014;25:1622-8. [PubMed]
- Bauer K, Skoetz N, Monsef I, et al. Comparison of chemotherapy including escalated BEACOPP versus chemotherapy including ABVD for patients with early unfavourable or advanced stage Hodgkin lymphoma. Cochrane Database Syst Rev 2011;CD007941. [PubMed]
- Viviani S, Zinzani PL, Rambaldi A, et al. ABVD versus BEACOPP for Hodgkin’s lymphoma when high-dose salvage is planned. N Engl J Med 2011;365:203-12. [PubMed]
- Carde P, Karrasch M, Fortpied C. ABVD (8cycles) versus BEACOPP (4 escalated cycles => 4 baseline) in stage III-IV high-risk Hodgkin Lymphoma (HL): First results of EORTC 20012 Intergroup randomized phase III clinical trial. J Clin Oncol 2012;30:abstr 8002.
- Skoetz N, Trelle S, Rancea M, et al. Effect of initial treatment strategy on survival of patients with advanced-stage Hodgkin’s lymphoma: a systematic review and network meta-analysis. Lancet Oncol 2013;14:943-52. [PubMed]
- Federico M, Luminari S, Iannitto E, et al. ABVD compared with BEACOPP compared with CEC for the initial treatment of patients with advanced Hodgkin’s lymphoma: results from the HD2000 Gruppo Italiano per lo Studio dei Linfomi Trial. J Clin Oncol 2009;27:805-11. [PubMed]
- Engel C, Loeffler M, Schmitz S, et al. Acute hematologic toxicity and practicability of dose-intensified BEACOPP chemotherapy for advanced stage Hodgkin’s disease. German Hodgkin’s Lymphoma Study Group (GHSG). Ann Oncol 2000;11:1105-14. [PubMed]
- Hodgson DC, Pintilie M, Gitterman L, et al. Fertility among female hodgkin lymphoma survivors attempting pregnancy following ABVD chemotherapy. Hematol Oncol 2007;25:11-5. [PubMed]
- Behringer K, Mueller H, Goergen H, et al. Gonadal function and fertility in survivors after Hodgkin lymphoma treatment within the German Hodgkin Study Group HD13 to HD15 trials. J Clin Oncol 2013;31:231-9. [PubMed]
- Engert A, Plütschow A, Eich HT, et al. Reduced treatment intensity in patients with early-stage Hodgkin’s lymphoma. N Engl J Med 2010;363:640-52. [PubMed]
- Cosset JM, Henry-Amar M, Meerwaldt JH, et al. The EORTC trials for limited stage Hodgkin’s disease. The EORTC Lymphoma Cooperative Group. Eur J Cancer 1992;28A:1847-50. [PubMed]
- Hasenclever D, Diehl V. A prognostic score for advanced Hodgkin’s disease. International Prognostic Factors Project on Advanced Hodgkin’s Disease. N Engl J Med 1998;339:1506-14. [PubMed]
- Moccia AA, Donaldson J, Chhanabhai M, et al. International Prognostic Score in advanced-stage Hodgkin’s lymphoma: altered utility in the modern era. J Clin Oncol 2012;30:3383-8. [PubMed]
- Mauch PM, Kalish LA, Marcus KC, et al. Long-term survival in Hodgkin’s disease relative impact of mortality, second tumors, infection, and cardiovascular disease. Cancer J Sci Am 1995;1:33-42. [PubMed]
- Roach M 3rd, Brophy N, Cox R, et al. Prognostic factors for patients relapsing after radiotherapy for early-stage Hodgkin’s disease. J Clin Oncol 1990;8:623-9. [PubMed]
- Jones K, Nourse JP, Keane C, et al. Plasma microRNA are disease response biomarkers in classical Hodgkin lymphoma. Clin Cancer Res 2014;20:253-64. [PubMed]
- Kamper P, Bendix K, Hamilton-Dutoit S, et al. Tumor-infiltrating macrophages correlate with adverse prognosis and Epstein-Barr virus status in classical Hodgkin’s lymphoma. Haematologica 2011;96:269-76. [PubMed]
- Chetaille B, Bertucci F, Finetti P, et al. Molecular profiling of classical Hodgkin lymphoma tissues uncovers variations in the tumor microenvironment and correlations with EBV infection and outcome. Blood 2009;113:2765-3775. [PubMed]
- Diepstra A, van Imhoff GW, Schaapveld M, et al. Latent Epstein-Barr virus infection of tumor cells in classical Hodgkin’s lymphoma predicts adverse outcome in older adult patients. J Clin Oncol 2009;27:3815-21. [PubMed]
- Jarrett RF, Stark GL, White J, et al. Impact of tumor Epstein-Barr virus status on presenting features and outcome in age-defined subgroups of patients with classic Hodgkin lymphoma: a population-based study. Blood 2005;106:2444-51. [PubMed]
- Casasnovas RO, Mounier N, Brice P, et al. Plasma cytokine and soluble receptor signature predicts outcome of patients with classical Hodgkin’s lymphoma: a study from the Groupe d’Etude des Lymphomes de l’Adulte. J Clin Oncol 2007;25:1732-40. [PubMed]
- Sarris AH, Kliche KO, Pethambaram P, et al. Interleukin-10 levels are often elevated in serum of adults with Hodgkin’s disease and are associated with inferior failure-free survival. Ann Oncol 1999;10:433-40. [PubMed]
- Vassilakopoulos TP, Nadali G, Angelopoulou MK, et al. Serum interleukin-10 levels are an independent prognostic factor for patients with Hodgkin’s lymphoma. Haematologica 2001;86:274-81. [PubMed]
- Sauer M, Plütschow A, Jachimowicz RD, et al. Baseline serum TARC levels predict therapy outcome in patients with Hodgkin lymphoma. Am J Hematol 2013;88:113-5. [PubMed]
- Visco C, Nadali G, Vassilakopoulos TP, et al. Very high levels of soluble CD30 recognize the patients with classical Hodgkin’s lymphoma retaining a very poor prognosis. Eur J Haematol 2006;77:387-94. [PubMed]
- Jones K, Vari F, Keane C, et al. Serum CD163 and TARC as disease response biomarkers in classical Hodgkin lymphoma. Clin Cancer Res 2013;19:731-42. [PubMed]
- Steidl C, Lee T, Shah SP, et al. Tumor-associated macrophages and survival in classic Hodgkin’s lymphoma. N Engl J Med 2010;362:875-85. [PubMed]
- Geiss GK, Bumgarner RE, Birditt B, et al. Direct multiplexed measurement of gene expression with color-coded probe pairs. Nat Biotechnol 2008;26:317-25. [PubMed]
- Scott DW, Chan FC, Hong F, et al. Gene expression-based model using formalin-fixed paraffin-embedded biopsies predicts overall survival in advanced-stage classical Hodgkin lymphoma. J Clin Oncol 2013;31:692-700. [PubMed]
- El-Galaly TC, d’Amore F, Mylam KJ, et al. Routine bone marrow biopsy has little or no therapeutic consequence for positron emission tomography/computed tomography-staged treatment-naive patients with Hodgkin lymphoma. J Clin Oncol 2012;30:4508-14. [PubMed]
- Moulin-Romsee G, Hindié E, Cuenca X, et al. (18)F-FDG PET/CT bone/bone marrow findings in Hodgkin’s lymphoma may circumvent the use of bone marrow trephine biopsy at diagnosis staging. Eur J Nucl Med Mol Imaging 2010;37:1095-105. [PubMed]
- Adams HJ, Kwee TC, de Keizer B, et al. Systematic review and meta-analysis on the diagnostic performance of FDG-PET/CT in detecting bone marrow involvement in newly diagnosed Hodgkin lymphoma: is bone marrow biopsy still necessary? Ann Oncol 2014;25:921-7. [PubMed]
- Hutchings M, Loft A, Hansen M, et al. FDG-PET after two cycles of chemotherapy predicts treatment failure and progression-free survival in Hodgkin lymphoma. Blood 2006;107:52-9. [PubMed]
- Hutchings M, Mikhaeel NG, Fields PA, et al. Prognostic value of interim FDG-PET after two or three cycles of chemotherapy in Hodgkin lymphoma. Ann Oncol 2005;16:1160-8. [PubMed]
- Gallamini A, Hutchings M, Rigacci L, et al. Early interim 2-[18F]fluoro-2-deoxy-D-glucose positron emission tomography is prognostically superior to international prognostic score in advanced-stage Hodgkin’s lymphoma: a report from a joint Italian-Danish study. J Clin Oncol 2007;25:3746-52. [PubMed]
- Gallamini A, Rigacci L, Merli F, et al. The predictive value of positron emission tomography scanning performed after two courses of standard therapy on treatment outcome in advanced stage Hodgkin’s disease. Haematologica 2006;91:475-81. [PubMed]
- Zinzani PL, Tani M, Fanti S, et al. Early positron emission tomography (PET) restaging: a predictive final response in Hodgkin’s disease patients. Ann Oncol 2006;17:1296-300. [PubMed]
- Barrington SF, Mikhaeel NG, Kostakoglu L, et al. Role of imaging in the staging and response assessment of lymphoma: consensus of the International Conference on Malignant Lymphomas Imaging Working Group. J Clin Oncol 2014;32:3048-58. [PubMed]
- Gallamini A, Barrington SF, Biggi A, et al. The predictive role of interim positron emission tomography for Hodgkin lymphoma treatment outcome is confirmed using the interpretation criteria of the Deauville five-point scale. Haematologica 2014;99:1107-13. [PubMed]
- Meignan M, Gallamini A, Meignan M, et al. Report on the First International Workshop on Interim-PET-Scan in Lymphoma. Leuk Lymphoma 2009;50:1257-60. [PubMed]
- Barrington SF, Qian W, Somer EJ, et al. Concordance between four European centres of PET reporting criteria designed for use in multicentre trials in Hodgkin lymphoma. Eur J Nucl Med Mol Imaging 2010;37:1824-33. [PubMed]
- Follows GA, Ardeshna KM, Barrington SF, et al. Guidelines for the first line management of classical Hodgkin lymphoma. Br J Haematol 2014;166:34-49. [PubMed]
- Engles JM, Quarless SA, Mambo E, et al. Stunning and its effect on 3H-FDG uptake and key gene expression in breast cancer cells undergoing chemotherapy. J Nucl Med 2006;47:603-8. [PubMed]
- Juweid ME, Stroobants S, Hoekstra OS, et al. Use of positron emission tomography for response assessment of lymphoma: consensus of the Imaging Subcommittee of International Harmonization Project in Lymphoma. J Clin Oncol 2007;25:571-8. [PubMed]
- Spaepen K, Stroobants S, Dupont P, et al. [(18)F]FDG PET monitoring of tumour response to chemotherapy: does [(18)F]FDG uptake correlate with the viable tumour cell fraction? Eur J Nucl Med Mol Imaging 2003;30:682-8. [PubMed]
- Gallamini A, Kostakoglu L. Interim FDG-PET in Hodgkin lymphoma: a compass for a safe navigation in clinical trials? Blood 2012;120:4913-20. [PubMed]
- Franklin J, Pluetschow A, Paus M, et al. Second malignancy risk associated with treatment of Hodgkin’s lymphoma: meta-analysis of the randomised trials. Ann Oncol 2006;17:1749-60. [PubMed]
- Boivin JF, Hutchison GB, Zauber AG, et al. Incidence of second cancers in patients treated for Hodgkin’s disease. J Natl Cancer Inst 1995;87:732-41. [PubMed]
- van Leeuwen FE, Klokman WJ, Veer MB, et al. Long-term risk of second malignancy in survivors of Hodgkin’s disease treated during adolescence or young adulthood. J Clin Oncol 2000;18:487-97. [PubMed]
- Ng AK, Bernardo MP, Weller E, et al. Long-term survival and competing causes of death in patients with early-stage Hodgkin’s disease treated at age 50 or younger. J Clin Oncol 2002;20:2101-8. [PubMed]
- Specht L, Gray RG, Clarke MJ, et al. Influence of more extensive radiotherapy and adjuvant chemotherapy on long-term outcome of early-stage Hodgkin’s disease: a meta-analysis of 23 randomized trials involving 3,888 patients. International Hodgkin’s Disease Collaborative Group. J Clin Oncol 1998;16:830-43. [PubMed]
- Fermé C, Eghbali H, Meerwaldt JH, et al. Chemotherapy plus involved-field radiation in early-stage Hodgkin’s disease. N Engl J Med 2007;357:1916-27. [PubMed]
- Straus DJ, Portlock CS, Qin J, et al. Results of a prospective randomized clinical trial of doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) followed by radiation therapy (RT) versus ABVD alone for stages I, II, and IIIA nonbulky Hodgkin disease. Blood 2004;104:3483-9. [PubMed]
- Nachman JB, Sposto R, Herzog P, et al. Randomized comparison of low-dose involved-field radiotherapy and no radiotherapy for children with Hodgkin’s disease who achieve a complete response to chemotherapy. J Clin Oncol 2002;20:3765-71. [PubMed]
- Meyer RM, Gospodarowicz MK, Connors JM, et al. ABVD alone versus radiation-based therapy in limited-stage Hodgkin’s lymphoma. N Engl J Med 2012;366:399-408. [PubMed]
- Pavlovsky S, Maschio M, Santarelli MT, et al. Randomized trial of chemotherapy versus chemotherapy plus radiotherapy for stage I-II Hodgkin’s disease. J Natl Cancer Inst 1988;80:1466-73. [PubMed]
- Meyer RM, Gospodarowicz MK, Connors JM, et al. Randomized comparison of ABVD chemotherapy with a strategy that includes radiation therapy in patients with limited-stage Hodgkin’s lymphoma: National Cancer Institute of Canada Clinical Trials Group and the Eastern Cooperative Oncology Group. J Clin Oncol 2005;23:4634-42. [PubMed]
- Herbst C, Rehan FA, Brillant C, et al. Combined modality treatment improves tumor control and overall survival in patients with early stage Hodgkin’s lymphoma: a systematic review. Haematologica 2010;95:494-500. [PubMed]
- Koshy M, Rich SE, Mahmood U, et al. Declining use of radiotherapy in stage I and II Hodgkin’s disease and its effect on survival and secondary malignancies. Int J Radiat Oncol Biol Phys 2012;82:619-25. [PubMed]
- Behringer K, Goergen H, Hitz F, et al. Omission of dacarbazine or bleomycin, or both, from the ABVD regimen in treatment of early-stage favourable Hodgkin’s lymphoma (GHSG HD13): an open-label, randomised, non-inferiority trial. Lancet 2014. [Epub ahead of print]. [PubMed]
- Eich HT, Diehl V, Görgen H, et al. Intensified chemotherapy and dose-reduced involved-field radiotherapy in patients with early unfavorable Hodgkin’s lymphoma: final analysis of the German Hodgkin Study Group HD11 trial. J Clin Oncol 2010;28:4199-206. [PubMed]
- von Tresckow B, Plütschow A, Fuchs M, et al. Dose-intensification in early unfavorable Hodgkin’s lymphoma: final analysis of the German Hodgkin Study Group HD14 trial. J Clin Oncol 2012;30:907-13. [PubMed]
- Noordijk EM, Carde P, Dupouy N, et al. Combined-modality therapy for clinical stage I or II Hodgkin’s lymphoma: long-term results of the European Organisation for Research and Treatment of Cancer H7 randomized controlled trials. J Clin Oncol 2006;24:3128-35. [PubMed]
- Engert A, Schiller P, Josting A, et al. Involved-field radiotherapy is equally effective and less toxic compared with extended-field radiotherapy after four cycles of chemotherapy in patients with early-stage unfavorable Hodgkin’s lymphoma: results of the HD8 trial of the German Hodgkin’s Lymphoma Study Group. J Clin Oncol 2003;21:3601-8. [PubMed]
- Arakelyan N, Jais JP, Delwail V, et al. Reduced versus full doses of irradiation after 3 cycles of combined doxorubicin, bleomycin, vinblastine, and dacarbazine in early stage Hodgkin lymphomas: results of a randomized trial. Cancer 2010;116:4054-62. [PubMed]
- De Bruin ML, Sparidans J, van’t Veer MB, et al. Breast cancer risk in female survivors of Hodgkin’s lymphoma: lower risk after smaller radiation volumes. J Clin Oncol 2009;27:4239-46. [PubMed]
- Hancock SL, Tucker MA, Hoppe RT. Factors affecting late mortality from heart disease after treatment of Hodgkin’s disease. JAMA 1993;270:1949-55. [PubMed]
- Ng AK. Review of the cardiac long-term effects of therapy for Hodgkin lymphoma. Br J Haematol 2011;154:23-31. [PubMed]
- Aleman BM, van den Belt-Dusebout AW, et al. Long-term cause-specific mortality of patients treated for Hodgkin’s disease. J Clin Oncol 2003;21:3431-9. [PubMed]
- Mulrooney DA, Yeazel MW, Kawashima T, et al. Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: retrospective analysis of the Childhood Cancer Survivor Study cohort. BMJ 2009;339:b4606. [PubMed]
- Myrehaug S, Pintilie M, Yun L, et al. A population-based study of cardiac morbidity among Hodgkin lymphoma patients with preexisting heart disease. Blood 2010;116:2237-40. [PubMed]
- Galper SL, Yu JB, Mauch PM, et al. Clinically significant cardiac disease in patients with Hodgkin lymphoma treated with mediastinal irradiation. Blood 2011;117:412-8. [PubMed]
- Girinsky T, van der Maazen R, et al. Involved-node radiotherapy (INRT) in patients with early Hodgkin lymphoma: concepts and guidelines. Radiother Oncol 2006;79:270-7. [PubMed]
- Paumier A, Ghalibafian M, Beaudre A, et al. Involved-node radiotherapy and modern radiation treatment techniques in patients with Hodgkin lymphoma. Int J Radiat Oncol Biol Phys 2011;80:199-205. [PubMed]
- Specht L, Yahalom J, Illidge T, et al. Modern radiation therapy for Hodgkin lymphoma: field and dose guidelines from the international lymphoma radiation oncology group (ILROG). Int J Radiat Oncol Biol Phys 2014;89:854-62. [PubMed]
- Girinsky T, Specht L, Ghalibafian M, et al. The conundrum of Hodgkin lymphoma nodes: to be or not to be included in the involved node radiation fields. The EORTC-GELA lymphoma group guidelines. Radiother Oncol 2008;88:202-10. [PubMed]
- Maraldo MV, Aznar MC, Vogelius IR, et al. Involved node radiation therapy: an effective alternative in early-stage hodgkin lymphoma. Int J Radiat Oncol Biol Phys 2013;85:1057-65. [PubMed]
- Koeck J, Abo-Madyan Y, Lohr F, et al. Radiotherapy for early mediastinal Hodgkin lymphoma according to the German Hodgkin Study Group (GHSG): the roles of intensity-modulated radiotherapy and involved-node radiotherapy. Int J Radiat Oncol Biol Phys 2012;83:268-76. [PubMed]
- Hoppe BS, Flampouri S, Su Z, et al. Consolidative involved-node proton therapy for Stage IA-IIIB mediastinal Hodgkin lymphoma: preliminary dosimetric outcomes from a Phase II study. Int J Radiat Oncol Biol Phys 2012;83:260-7. [PubMed]
- Rigacci L, Puccini B, Zinzani PL, et al. The prognostic value of positron emission tomography performed after two courses (interim-pet) of standard therapy on treatment outcome in early stage hodgkin lymphoma. A multicentric study by the fondazione italiana linfomi (FIL). Am J Hematol 2015. [Epub ahead of print]. [PubMed]
- Raemaekers JM, André MP, Federico M, et al. Omitting radiotherapy in early positron emission tomography-negative stage I/II Hodgkin lymphoma is associated with an increased risk of early relapse: Clinical results of the preplanned interim analysis of the randomized EORTC/LYSA/FIL H10 trial. J Clin Oncol 2014;32:1188-94. [PubMed]
- Radford J, Barrington S, Counsell N. Involved field radiotherapy versus no further treatment in patients with clinical stage IA and IIA Hodgkin lymphoma and a negative PET scan after 3 cycles of ABVD: Results of the UK NCRI RAPID trial. Blood 2012;120:abstr 547.
- Johnson P, Federico M, Fossa A, et al. Responses And Chemotherapy Dose Adjustment Determined By PET-CT Imaging: First Results From The International Response Adapted Therapy In Advanced Hodgkin Lymphoma (RATHL) Study. Hematol Oncol 2013;31:138.
- Johnson PW, Federico M, Fossa A, et al. Response rates and toxicity of response-adapted therapy in advanced Hodgkin lymphoma: Initial results from the International RATHL Study. Haematologica 2013;98:2. [PubMed]
- Gallamini A, O’Doherty M. Report of satellite workshop on interim-PET in Hodgkin lymphoma: 8th International Symposium on Hodgkin Lymphoma, Cologne, 23 October 2010. Leuk Lymphoma 2011;52:583-6. [PubMed]
- Gallamini A, Rossi A, Patti C, et al. Early Treatment Intensification in Advanced-Stage High-Risk Hodgkin Lymphoma (HL) Patients, with a Positive FDG-PET Scan After Two ABVD Courses - First Interim Analysis of the GITIL/FIL HD0607 Clinical Trial. 57th ASH Annual Meeting & Exposition. Orlando, FL, USA, 2012;120:550.
- Press OW, LeBlanc M, Rimsza LM, et al. A Phase II Trial Of Response-Adapted Therapy Of Stages III–Iv Hodgkin Lymphoma Using Early Interim FDG-PET Imaging: US Intergroup S0816. Hematol Oncol 2013;31:124.
- Biggi A, Chauvie S, Gallamini A. Concordance in interim PET reporting in the prospective HD 0607 clinical trial in advanced-stage Hodgkin lymphoma. J Nucl Med 2012;53:1378.
- Gallamini A, Patti C, Viviani S, et al. Early chemotherapy intensification with BEACOPP in advanced-stage Hodgkin lymphoma patients with a interim-PET positive after two ABVD courses. Br J Haematol 2011;152:551-60. [PubMed]
- Canellos GP, Niedzwiecki D. Long-term follow-up of Hodgkin’s disease trial. N Engl J Med 2002;346:1417-8. [PubMed]
- Canellos GP, Anderson JR, Propert KJ, et al. Chemotherapy of advanced Hodgkin’s disease with MOPP, ABVD, or MOPP alternating with ABVD. N Engl J Med 1992;327:1478-84. [PubMed]
- Josting A, Müller H, Borchmann P, et al. Dose intensity of chemotherapy in patients with relapsed Hodgkin’s lymphoma. J Clin Oncol 2010;28:5074-80. [PubMed]
- Bonfante V, Santoro A, Viviani S, et al. Outcome of patients with Hodgkin’s disease failing after primary MOPP-ABVD. J Clin Oncol 1997;15:528-34. [PubMed]
- Josting A, Rueffer U, Franklin J, et al. Prognostic factors and treatment outcome in primary progressive Hodgkin lymphoma: a report from the German Hodgkin Lymphoma Study Group. Blood 2000;96:1280-6. [PubMed]
- Fermé C, Mounier N, Diviné M, et al. Intensive salvage therapy with high-dose chemotherapy for patients with advanced Hodgkin’s disease in relapse or failure after initial chemotherapy: results of the Groupe d’Etudes des Lymphomes de l’Adulte H89 Trial. J Clin Oncol 2002;20:467-75. [PubMed]
- Longo DL, Duffey PL, Young RC, et al. Conventional-dose salvage combination chemotherapy in patients relapsing with Hodgkin’s disease after combination chemotherapy: the low probability for cure. J Clin Oncol 1992;10:210-8. [PubMed]
- Josting A, Franklin J, May M, et al. New prognostic score based on treatment outcome of patients with relapsed Hodgkin’s lymphoma registered in the database of the German Hodgkin’s lymphoma study group. J Clin Oncol 2002;20:221-30. [PubMed]
- Moskowitz CH, Matasar MJ, Zelenetz AD, et al. Normalization of pre-ASCT, FDG-PET imaging with second-line, non-cross-resistant, chemotherapy programs improves event-free survival in patients with Hodgkin lymphoma. Blood 2012;119:1665-70. [PubMed]
- Biggi A, Barrington S, Hutchings M. Analysis of the Deauville criteria for the assessment of interim PET in advanced stage Hodgkin Lymphoma patients enrolled in the IVS study: II. Reliability of score and concordance among reviewers. J Nucl Med 2012;53:506. [PubMed]


