
Multidisciplinary sarcoma tumor board: adolescent and young adult soft tissue sarcoma—myxoid liposarcoma and alveolar soft part sarcoma
Soft tissue sarcoma (STS) is a rare cancer, which encompasses over 50 different subtypes of malignancies that develop in the connective tissues such as fat, muscle, and blood vessels. STS can develop anywhere in the body but are mostly found in the extremities, body wall and abdomen. Treatment often includes a combination of surgery, radiation, and systemic therapy. In this multidisciplinary sarcoma tumor board, our institutions discussed two cases of STS that are relevant to the adolescent and young adult (AYA) population—myxoid liposarcoma and alveolar soft part sarcoma. Even within these subtypes, prognostic factors, response to treatment, and survivorship concerns may vary between AYA and older patients.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
We present the following article in accordance with the CARE reporting checklist (available at http://dx.doi.org/10.21037/cco-20-147).
Case 1 (USC)
A 35-year-old woman initially presented with an enlarging, symptomatic right buttock mass and a synchronous left posterior thigh mass. On MRI, the right gluteal mass measured 8 cm × 4 cm × 9 cm and invaded into the gluteus maximus muscle while the left thigh mass measured 13 cm × 10 cm × 22 cm without obvious local tissue invasion (Figure 1). Both showed areas of nodular enhancement following intravenous administration of gadolinium based contrast material. A marrow replacing lesion within the left proximal femur was also identified involving the greater trochanter and femoral neck measuring 2 cm × 2 cm × 4 cm. The etiology of this was unclear and included metastatic disease versus a benign hemangioma or fibrous dysplasia. Percutaneous core needle biopsy of the right buttock mass revealed high grade myxoid liposarcoma with DDIT3 gene rearrangement by fluorescence in situ hybridization (FISH) and presence of round cell transformation. Percutaneous biopsy of the left thigh mass also showed myxoid liposarcoma but no definite round cell component. Staging with CT chest, abdomen, and pelvis (CT CAP) showed two nonspecific anterior chest wall subcutaneous nodules and no other potential sites of metastases.
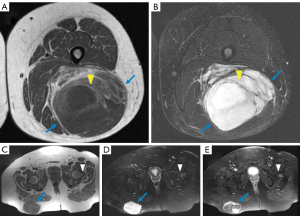
The decision was made to treat with neoadjuvant chemotherapy with 4 cycles of AIM (doxorubicin, ifosfamide and mesna) followed by neoadjuvant radiation to a dose of 50 Gy to the buttock, thigh, and femur. She then underwent surgery for both sites. Final pathology revealed 30% necrosis in the thigh mass and 15% necrosis in the buttock mass consistent with some treatment response, but up to 50% round cell changes. The margins for both specimens were close but negative.
Two months later, she developed lung metastases and a right humeral mass that was biopsy-proven as metastatic myxoid liposarcoma. She was then started on trabectedin. After 9 cycles she had increased liver enzymes that required discontinuation of treatment. She was then switched to eribulin. She underwent resection of the humeral mass with pathology confirming myxoid liposarcoma with less than 5% necrosis. This was followed by adjuvant radiation to a dose of 30 Gy in 10 fractions. She was maintained on eribulin but progressed with a metastasis in the left intertrochanteric femur with a soft tissue component. This was superior to the previously seen lesion, which was stable and favored to be benign. The metastasis was treated with curettage, cryoablation, and placement of a screw followed by adjuvant radiation to 30 Gy in 10 fractions. She resumed eribulin but her liver enzymes rose again so the dose was reduced by 20%. Follow up CT scans of the chest showed partial response to therapy with decrease in size of some of the pulmonary nodules. Therapy had to be held for 4 months due to the development of severe hypothyroidism. It was then resumed with less frequent dosing every 6 weeks. She continues on this dose reduced maintenance regimen at the present time, now 5 years since her original diagnosis. On most recent imaging the pulmonary nodules are stable, the anterior chest wall nodules are no longer seen, and there are no new metastases.
Clinical and differential diagnosis
Myxoid liposarcoma is the second most common subtype of liposarcoma. It accounts for 15–20% of liposarcomas and represents about 5% of all STSs in adults (1). The tumor often arises in the lower extremities and can metastasize when high-grade features are present (characterized histologically by a round cell component) (2). Biopsy plays a crucial role in accurate diagnosis and for myxoid liposarcoma, the DDIT3 gene rearrangement is pathognomonic and can be seen in both low grade and high grade subtypes (3).
Radiologic discussion
Although ultrasound or CT may be done first and may also aid with biopsy, contrast-enhanced MRI remains the imaging modality of choice to best define a myxoid liposarcoma and guide surgical planning (4). The characteristic CT and MRI appearance is related to very high water content. The classic MRI finding is of a well-defined, multilobulated, relatively homogeneous mass with T1 hypointense and marked T2 hyperintense signal, with interspersed small amount of intralesional adipose tissue (septa or nodules), which is seen as T1 hyperintense signaling. It enhances following intravenous administration of contrast material. With increasing round cell component, the mass becomes more heterogeneous and nonspecific, with areas of round cell component having more intermediate T1 and T2 signal (5). In our case, the patient presented with two concurrent masses and it is uncertain whether these represented synchronous primaries or metastatic disease from either one of the primary sites.
With high-grade (round cell) disease, the tumor can metastasize to the lungs, but it also has a distinct propensity to metastasize to atypical non-pulmonary sites such as the bone, soft tissues of the retroperitoneum, contralateral limb, chest wall, and pleura (6). Therefore, patients with high-risk myxoid liposarcoma should undergo whole body imaging to include the chest, abdomen, pelvis and spine as part of their staging evaluation. Studies have suggested a role for whole body MRI (WB-MRI) as this modality successfully detected extra-pulmonary metastatic disease that was occult on synchronous CT scans of the chest, abdomen and pelvis (6). Both bone scintigraphy and 18-F FDG-PET are relatively insensitive for detection of osseous metastasis of myxoid liposarcoma (5). As WB-MRI has demonstrated an ability to identify more sites of metastatic disease compared to other imaging modalities, it has been suggested that WB-MRI should be used at diagnosis and especially when the diagnosis of occult metastasis would change treatment planning. It may also be beneficial at diagnosis of recurrence to rule out other disease sites prior to consideration of metastasectomy (6).
Pathological discussion
Liposarcoma is one of the most common sarcomas found in adults and is characterized by adipocyte differentiation. Myxoid liposarcoma is a distinct variant of liposarcoma, characterized histologically by uniform round to oval shaped primitive non-lipogenic mesenchymal cells and a variable number of small signet-ring lipoblasts in a prominent myxoid stroma with a characteristic branching vascular pattern, sometimes referred to as “chicken wire” in appearance (7).
A unique chromosome translocation, t(12;16)(q13;p11), resulting in a fusion of the DDIT3 gene (also known as CHOP or GADD153) on chromosome 12 and the FUS gene (also referred to as TLS) on chromosome 16, is the key genetic aberration in myxoid liposarcoma (8). More than 90% of myxoid liposarcoma are cytogenetically characterized by this translocation. In rare cases, a variant t(12;22)(q13;q12) has been described in which DDIT3 (CHOP) fuses with EWS, a gene highly related to FUS (8).
Tumors show a spectrum of disease ranging from low-grade to high-grade, poorly-differentiated forms with a prominent round cell component. It is suggested that round cell components above 25% indicate a high-grade neoplasm; however, there have been reports confirming a lower threshold of 5% as the cut off for high-grade tumors (9). Following radiation or chemotherapy, specimens can show a variable degree of necrosis or hyalinization as a response to therapy.
Treatment discussion
For localized, primary disease in myxoid liposarcoma, surgery is the primary treatment modality. Resection with negative margins is ideal to minimize the risk of local recurrence. High-grade myxoid liposarcoma may require wider resections in order to allow for adequate margins; however, when the tumor is adjacent to or invades critical structures such as nerves, blood vessels, or bones, a marginal resection may be necessary to preserve function.
Radiotherapy, in either the preoperative or postoperative setting, is generally performed in patients with high-grade disease, tumor size >5 cm, deep-seated tumors, recurrent disease, or in case of close or positive margins (10). Myxoid liposarcomas are unique in that the tumors are highly radiosensitive, and dramatic responses with pre-operative radiation have been reported (11). One study showed a 59% tumor volume reduction after pre-operative radiation and others have reported 97% local control with combined surgery and radiation (12). Pre-operative radiation therapy may therefore be especially helpful in cases where upfront surgery may be particularly morbid. Radiation therapy in our case was used at initial presentation as neoadjuvant therapy, but was later used after recurrence as consolidation therapy after surgery.
Chemotherapy is generally reserved for patients with high-risk and advanced disease. In the (non-metastatic) adjuvant setting, high-risk features for which chemotherapy might be considered include high tumor grade, deep-seated tumor, tumor size greater than 5 cm, and positive surgical margins. Comparing the liposarcoma subtypes, there is evidence of differential response and sensitivity to chemotherapy as the myxoid/round cell subtype appears to be especially chemosensitive as measured by RECIST response criteria relative to other liposarcoma subtypes (13). Standard chemotherapy regimens containing doxorubicin and dacarbazine based chemotherapy have been proven to be effective in myxoid liposarcoma (14). In addition, regimens consisting of doublets of doxorubicin/ifosfamide or gemcitabine/docetaxel resulted in response rates of 25% to 35% and survival of 12 to 18 months (15). All of these agents were used or considered in this case with expected side effects necessitating dose adjustments.
Several newer systemic agents have also become important options for the treatment of patients with advanced high grade disease. Trabectedin was approved by the United States Food and Drug Administration (FDA) in 2015 for the treatment of liposarcoma after it was shown to yield superior progression free survival as compared to dacarbazine (16). The most common side effects are nausea, vomiting, fatigue, diarrhea, cytopenias, elevated liver enzymes and hypoalbuminemia. In a phase III multicenter trial comparing trabectedin versus dacarbazine in patients with advanced liposarcoma or leiomyosarcoma after prior therapy with an anthracycline and at least one additional systemic regimen, trabectedin administration resulted in a 45% reduction in the risk of disease progression or death compared with dacarbazine (median PFS for trabectedin vs. dacarbazine, 4.2 vs. 1.5 months; hazard ratio, 0.55; P<0.001). The interim analysis of OS demonstrated a 13% reduction in risk of death in the trabectedin arm compared with dacarbazine (median OS for trabectedin vs. dacarbazine, 12.4 vs. 12.9 months; hazard ratio, 0.87; P=0.37). Eleven percent of patients on each treatment arm had myxoid liposarcoma (16).
Eribulin is a non-taxane microtubule inhibitor. It was first approved for use in metastatic breast cancer and then approved for treatment of liposarcoma by the FDA in 2016. The most common side effects of eribulin are cytopenias, fatigue, nausea, and peripheral neuropathy. Both trabectedin and eribulin have received recent FDA approval for application in the second-line setting for advanced high grade liposarcoma (17). Trabectedin seems to be particularly active in myxoid/round cell liposarcoma and may actually be considered for first-line therapy in selected patients. A phase 3 trial which included 64 (22%) patients with high-grade myxoid liposarcoma, showed equivalent benefit to a neoadjuvant histotype-tailored chemotherapy regimen (trabectedin) compared to the standard chemotherapy regimen of epirubicin and ifosfamide (18).
As in the case of this patient, a combination of surgery, radiation, chemotherapy and novel agents is often required.
AYA considerations
Pediatric liposarcoma has a different spectrum of presentation compared to adult cases. Myxoid liposarcoma is the most common histologic subtype encountered in children and young adults, and the overall prognosis for myxoid tumors is excellent, generally with surgical treatment alone (19). Although younger age portends a better prognosis and outcome, there can be increasing degrees of chronic morbidity associated with longer survival and multiple or combination therapies. As in this case, toxicities associated with chemotherapy can be cumulative and may necessitate continued follow-up and monitoring into survivorship. The sarcoma model of care that involves a multimodality approach best achieved with the expertise concentrated at a comprehensive cancer center has been proposed as an excellent model for overall AYA care (20).
Conclusions
In myxoid liposarcoma, tumors with a round cell component are considered high grade and can metastasize to unusual sites. This unique malignancy can present as synchronous tumors as in the case presented here. Although most often diagnosed in adults, this subtype is common in the AYA population. In these younger patients who are typically better able to tolerate multi-modality therapy, an aggressive combination approach to render these patients disease free may be warranted and can result in long term survival although morbidity must be considered, thoughtful implementation of sequential modalities and systemic therapies can maximize the survival benefit while maintaining a high quality of life. While newer agents show promise in treatment of metastatic disease and add to the systemic options of traditional chemotherapy, additional data is still needed to better assess any long-term effects of protracted courses of these newer agents. This patient had multiple metastatic recurrences that required multiple surgeries, radiation therapy, and extended courses of chemotherapy. Although there is limited data on this protracted approach, this young woman is clinically well five years out from her initial diagnosis and it is hoped that her current maintenance therapy will continue to control the disease with minimal morbidity.
Case 2 (NTUH)
A 29-year-old man sought medical attention because of worsening lower back pain. MRI of the lumbar spine revealed a suspected L5 metastatic lesion (Figure 2A). CT-guided biopsy of the spine tumor showed epithelioid cell sarcoma, which based on immunohistochemistry staining results, was most consistent with alveolar soft part sarcoma (ASPS). 18-fluoro-deoxy-glucose positron-emission-tomography/computed imaging (FDG PET/CT) revealed avid bilateral lung and multiple bone lesions, including the lumbar spine and left humerus, suggestive of metastatic disease.
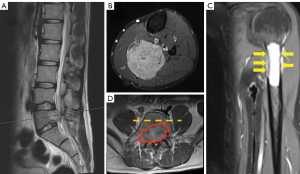
Within one month of initial diagnosis, the patient was referred to our hospital for further treatment. Magnetic resonance imaging (MRI) of the bilateral lower extremities revealed an avidly enhancing mass measuring 8.1 cm on fat-suppressed T1-weighted images after intravenous gadolinium-based contrast administration (Figure 2B). This was considered as the primary location of ASPS that was not captured by the previous FDG PET/CT imaging (to be discussed later). An X-ray and MRI of the left humerus showed impending fracture in the proximal shaft (Figure 2C). There were no neurological impairments by the L5 metastatic tumor, except pain that was controlled with acetaminophen/tramadol.
After discussion by the multi-disciplinary team, the plan was to palliatively stabilize the left humerus through surgery and start systemic treatment for metastatic ASPS. The patient was started on metronomic cyclophosphamide 50 mg/day (reasons to be discussed later) and palliative radiotherapy (3,500 cGy/12 fractions) to the lumbar spine. Two months after metronomic cyclophosphamide treatment, the lumbar spine tumor progressed with cauda equina syndrome and the patient received L5 total laminectomy plus partial corpectomy along with L4 partial laminectomy to relieve the spinal cord compression (Figure 2D).
Because of disease progression, the patient was enrolled into a phase I study with an investigational agent targeting the colony-stimulating factor-1 receptor (CSF-1R). The investigational agent was associated with manageable treatment-associated toxicities and the progression-free survival was 5 months. At the time of progression, the patient had multiple metastatic lung nodules increasing in size but only modest respiratory symptoms such as cough. We then started the patient on epirubicin single agent treatment. Since starting epirubicin the patient has had stable lung metastatic disease for now 18 months since initial diagnosis. Treatment is still ongoing.
Clinical and differential diagnosis
ASPS is a rare histology that comprises about 1% of STS. However, ASPS patients are generally younger with most patients diagnosed between ages of 15 to 35 years of age and a slightly higher female predominance. The most common primary site are the lower extremities but other sites such as the genitourinary tract or head and neck regions have also been reported as primary sites for ASPS. Despite an indolent clinical behavior, up to 40% of more of the patients have metastatic disease at the time of diagnosis (21). The common metastatic sites of ASPS include lung, brain, and bone. Young adults with enlarging mass over lower extremities and buttock or multiple lung metastatic nodules found either asymptomatically during health examinations or with symptoms should be consider ASPS in the differential diagnosis. Because ASPS has a different treatment paradigm as compared to other more common STS, an adequate diagnosis via core biopsy is crucial. The complete staging should also include brain imaging as ASPS is one of the rare STS subtypes that develop brain metastasis (22).
Radiologic discussion
Typical MRI imaging features of ASPS include vascular signal flow voids, large peripheral vessels, and moderate to high enhancement after contrast administration, reflecting the highly vascularized nature of ASPS (23). For staging, because ASPS are highly metastatic, imaging modalities including those for lungs, bone, and brain are essential. As most ASPS are deep-seated, many of the patients are not aware of a primary tumor at the time of the diagnosis of metastatic disease. Although the role of FDG PET/CT imaging for intermediate grade STS such as ASPS is still under debate (24), cases that applied FDG PET/CT to search for the primary location of ASPS have been successful (25). However, when using FDG PET/CT for metastatic patients in search of the primary disease, discussion with the nuclear medicine physician regarding the region of interest (ROI) is critical. In our case, because sarcoma of the extremities is very rare, the FDG PET/CT imaging in search of the primary tumor did not include the lower extremities as part of the ROI, thus missing the primary tumor in the left calf.
Pathological discussion
ASPS are generally poorly circumscribed, friable, and frequently surrounded by numerous tortuous and large caliber vessels. Microscopically, uniform rounded and often polygonal cells are separated by dense fibrous trabeculae into nests and sometimes show loss of cellular cohesion, resulting in a pseudo-alveolar pattern (Figure 3A). The findings of positive diastase-resistant periodic acid-Schiff (PAS) staining combined with a cytoplasmic crystalloid structure helps support the diagnosis (26). In terms of immunohistochemistry findings, ASPS are typically positive for Cathepsin K and can be focally positive for desmin, while being largely negative for cytokeratin, EMA, HMB-45, melan-A, and synaptophysin. A positive nuclear staini pattern for TFE3 serves as useful diagnostic marker as it suggests the characteristic translocation involving the very coding gene; with the caveat that TFE3 is also expressed in other TFE3-rearranged tumors (27) (Figure 3B).
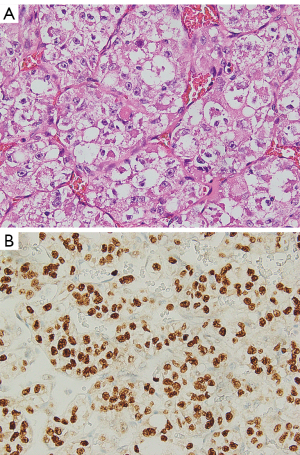
ASPS is characterized by a specific translocation between chromosome 17 and chromosome X [der(17)t(x;17)(p11.2q25)], yielding the fusion-product ASPL-TFE3 gene. This chimeric transcription factor is pathognomonic of ASPS and is associated with downstream c-MET activation. The molecular confirmatory tests include fluorescent in situ hybridization (FISH) to detect the TFE3 rearrangement and RT-PCR to detect the ASPL-TFE3 fusion.
The pathologic diagnosis of ASPS is not usually challenging, especially with a typical clinical picture. If the pathologic sample was taken from a metastatic site, however, then a variety of differential diagnoses might be taken into consideration, including renal cell carcinoma, hepatocellular carcinoma, perivascular epithelioid cell tumor (PEComa), paraganglioma, and granular cell tumor. A careful histological examination and adequate panel of immunostaining should easily discriminate among these diagnoses. Furthermore, the extrarenal locations and lack of expression of HMB45 or melan-A, respectively, can help with the distinction from the small subsets of renal cell carcinoma or PEComa that harbor TFE3 rearrangement while displaying somewhat ASPS-like morphology.
Treatment discussion
For localized ASPS patients, surgical treatment remains the gold standard. After surgical treatment, because of the indolent nature of ASPS, adjuvant chemotherapy and radiotherapy are not routinely recommended.
For metastatic disease, the evidence suggests that anti-angiogenic agents or immune checkpoint inhibitors of programmed cell death 1 receptor or its ligand (PD-1/PD-L1) are important options (28). Sunitinib, sorafenib, pazopanib, anlotinib, and cediranib all have retrospective or prospective studies that demonstrated the benefit of multi-targeted anti-angiogenic tyrosine kinase inhibitors. Worthy of note is the CASPS study, in which progressive metastatic ASPS patients were randomized to either cediranib or placebo (29). After 24 weeks or at progression, patients in the placebo arm could opt for cediranib treatment. At 24 weeks, patients treated with cediranib had an average tumor decrease of 8.3% as compared to patients receiving placebo who had an average increase of 13.4%. However, perhaps due to the indolent and varied natural history of ASPS and the cross over design of this study, differences in the secondary endpoints of median PFS and OS were not detected (29). Anlotinib is another anti-angiogenic multi-targeted inhibitor that has shown high potential of activity. In a single-arm prospective clinical trial testing the efficacy of anlotinib in metastatic STS, 13 patients with ASPS had a 12-week PFS-rate, median PFS, and OS of 77%, 21 months, and not-reached, respectively (30).
ICI’s are gaining a role in the treatment of metastatic ASPS. In the Alliance study A094401, ASPS patients were one of the few histologies that responded to the combination of nivolumab and ipilimumab (31). Single agent atezolizumab, a PD-L1 antibody, has shown an objective response rate of 42% in metastatic ASPS patients, although the time to response was longer than most other cancer types (32). The combination of an anti-angiogenic agent and an ICI antibody has also shown activity in ASPS patients. The combination of axitinib and pembrolizumab had an objective response rate of 54.5% (11/16) in ASPS patients (33). Based on these findings, anti-angiogenic agents and ICI either singly or in combination should be considered as early treatments for advanced ASPS patients.
Although systemic treatment is the main focus in the treatment of metastatic ASPS, because of the slow-growing nature of ASPS, local treatment of metastatic disease is not unreasonable. For patients with solitary brain metastases, surgery with or without post-surgical radiotherapy (either stereotactic surgery or whole-brain radiotherapy) should be considered (34). Bone metastatic sites that are associated with high-risk of fracture should consider surgical fixation. However, the lung metastatic nodules of ASPS are generally multifocal and bilateral in nature, thus surgical metastasectomy of the lung nodules are not advised routinely.
For our patient, because of the claims regulation of National Health Insurance in Taiwan, neither anti-angiogenic agents nor ICIs are reimbursed specifically for ASPS patients. Pazopanib was the only reimbursed anti-angiogenic agent for advanced STS patients (including ASPS) but only in the second or later-line setting when other first-line systemic chemotherapies have failed (based on the PALETTE study) (35). In addition, STS patients with bone metastases were excluded from pazopanib reimbursement as well. The patient and his family could not afford any of the anti-angiogenic agents or ICI’s. Thus, with the treatment options limited, we could only start with systemic chemotherapy. Metronomic cyclophosphamide has been used to treat other advanced STS with moderate efficacy but low toxicities, although ASPS cases treated with metronomic cyclophosphamide have not been reported (36). Nonetheless, metronomic cyclophosphamide has been shown to inhibit the angiogenesis process in preclinical models, which provides more evidence that metronomic cyclophosphamide may be considered for the treatment of advanced ASPS patients (37). There is currently no standard treatment for ASPS patients who failed anti-angiogenic agents and/or immunotherapy. Although ASPS are generally considered refractory to chemotherapy, reports have suggested that a small minority of ASPS patients may still respond to cytotoxic chemotherapy (38). For this patient, because no other treatment options were available, the patient agreed to start anthracycline treatment after failure of two-lines of systemic treatment.
AYA considerations
As previously mentioned, most ASPS patients present in the AYA age range and therefore are able to tolerate multiple therapies. While some studies have suggested that younger age at diagnosis is associated with better prognosis in STS, the more favorable anatomical locations that are more amenable to resection—such as extremity, head and neck, or orbit—may also explain these better outcomes in ASPS. AYA patients with a metastatic epithelioid tumor should include ASPS in the differential diagnosis and a detailed physical examination with imaging that includes the extremities and trunk should be performed. Despite the indolent clinical course of ASPS, metastatic ASPS patients eventually will succumb to the disease with 5-year survival rate at 20%. Although the promise of newer immune checkpoint inhibitors exists, communication and attention to psychosocial factors that uniquely affect the young adult population should be addressed.
ASPS is a rare STS that commonly presents in AYA patients but the outcome remains unsatisfactory. For localized tumors, surgery is the only curative method. For metastatic disease, anti-angiogenic therapy and immunotherapy show promise and should be considered as first-line treatment. However, based on different medical systems in different countries, the treatment may need to be discussed and tailored individually, such as in our case.
Acknowledgments
We would like to acknowledge our colleagues Wei-Hsin Lin, MD, Jen-Chieh Lee, MD, PhD, Koping Chang, MD, Che-Yu Hsu, MD, and Tom Wei-Wu Chen, MD from the Departments of Orthopedic Surgery, Pathology, and Oncology, National Taiwan University Hospital, Taipei, for participation in this international Multidisciplinary Tumor Board discussion and provision and write-up of the ASPS case.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist (available at http://dx.doi.org/10.21037/cco-20-147).
Peer Review File: Available at http://dx.doi.org/10.21037/cco-20-147
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/cco-20-147). WWT serves as an unpaid editorial board member of Chinese Clinical Oncology from Oct 2018 to Sep 2020. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jo VY, Fletcher CD. WHO classification of soft tissue tumours: an update based on the 2013 (4th) edition. Pathology 2014;46:95-104. [Crossref] [PubMed]
- Kilpatrick SE, Doyon J, Choong PFM, et al. Nascimento AG. The clinicopathologic spectrum of myxoid and round cell liposarcoma. Cancer 1996;77:1450-8. [Crossref] [PubMed]
- Antonescu CR, Elahi A, Humphrey M, et al. Specificity of TLS-CHOP rearrangement for classic myxoid/round cell liposarcoma: absence in predominantly myxoid well-differentiated liposarcomas. J Mol Diagn 2000;2:132-8. [Crossref] [PubMed]
- O'Regan KN, Jagannathan J, Krajewski K, et al. Imaging of liposarcoma: classification, patterns of tumor recurrence, and response to treatment. AJR Am J Roentgenol 2011;197:W37-43. [Crossref] [PubMed]
- Sheah K, Ouellette HA, Torriani M, et al. Metastatic myxoid liposarcomas: imaging and histopathologic findings. Skeletal Radiol 2008;37:251-8. [Crossref] [PubMed]
- Stevenson JD, Watson JJ, Cool P, et al. Whole-body magnetic resonance imaging in myxoid liposarcoma: A useful adjunct for the detection of extra-pulmonary metastatic disease. Eur J Surg Oncol 2016;42:574-80. [Crossref] [PubMed]
- Lemeur M, Mattei JC, Souteyrand P, et al. Prognostic factors for the recurrence of myxoid liposarcoma: 20 cases with up to 8 years follow-up. Orthop Traumatol Surg Res 2015;101:103-7. [Crossref] [PubMed]
- ten Heuvel SE, Hoekstra HJ, van Ginkel RJ, et al. Clinicopathologic prognostic factors in myxoid liposarcoma: a retrospective study of 49 patients with long-term follow-up. Ann Surg Oncol 2007;14:222-9. [Crossref] [PubMed]
- Smith TA, Easley KA, Goldblum JR. Myxoid/round cell liposarcoma of the extremities. A clinicopathologic study of 29 cases with particular attention to extent of round cell liposarcoma. Am J Surg Pathol 1996;20:171-80. [Crossref] [PubMed]
- Chapman TR, Jour G, Hoch BL, et al. Myxoid liposarcomas demonstrate a profound response to neoadjuvant radiation therapy: an mri-based volumetric analysis and pathological correlation. Int J Radiat Oncol Biol Phys 2014;90:S756-7. [Crossref]
- Wilke CT, Wilson J, Ogilvie C, et al. Radiologic and pathologic response after neoadjuvant radiation therapy for myxoid liposarcoma of the extremities. Int J Radiat Oncol Biol Phys 2014;90:S765. [Crossref]
- Chung PW, Deheshi BM, Ferguson PC, et al. Radiosensitivity translates into excellent local control in extremity myxoid liposarcoma: a comparison with other soft tissue sarcomas. Cancer 2009;115:3254-61. [Crossref] [PubMed]
- Jones RL, Fisher C, Al-Muderis O, et al. Differential sensitivity of liposarcoma subtypes to chemotherapy. Eur J Cancer 2005;41:2853-60. [Crossref] [PubMed]
- Patel SR, Burgess MA, Plager C, et al. Myxoid liposarcoma. Experience with chemotherapy. Cancer 1994;74:1265-9. [Crossref] [PubMed]
- Katz D, Boonsirikamchai P, Choi H, et al. Efficacy of first-line doxorubicin and ifosfamide in myxoid liposarcoma. Clin Sarcoma Res 2012;2:2. [Crossref] [PubMed]
- Demetri GD, von Mehren M, Jones RL, et al. Efficacy and Safety of Trabectedin or Dacarbazine for Metastatic Liposarcoma or Leiomyosarcoma After Failure of Conventional Chemotherapy: Results of a Phase III Randomized Multicenter Clinical Trial. J Clin Oncol 2016;34:786-93. [Crossref] [PubMed]
- Schöffski P, Chawla S, Maki RG, et al. Eribulin versus dacarbazine in previously treated patients with advanced liposarcoma or leiomyosarcoma: a randomised, open-label, multicentre, phase 3 trial. Lancet 2016;387:1629-37. [Crossref] [PubMed]
- Gronchi A, Ferrari S, Quagliuolo V, et al. Histotype-tailored neoadjuvant chemotherapy versus standard chemotherapy in patients with high-risk soft-tissue sarcomas (ISG-STS 1001): an international, open-label, randomised, controlled, phase 3, multicentre trial. Lancet Oncol 2017;18:812-822. [Crossref] [PubMed]
- Huh WW, Yuen C, Munsell M, et al. Liposarcoma in children and young adults: a multi-institutional experience. Pediatr Blood Cancer 2011;57:1142-6. [Crossref] [PubMed]
- Reed DR, Naghavi A, Binitie O. Sarcoma as a Model for Adolescent and Young Adult Care. J Oncol Pract 2019;15:239-47. [Crossref] [PubMed]
- Portera CA Jr, Ho V, Patel SR, et al. Alveolar soft part sarcoma: clinical course and patterns of metastasis in 70 patients treated at a single institution. Cancer 2001;91:585-91. [Crossref] [PubMed]
- Chou YS, Liu CY, Chen WM, et al. Brain, the last fortress of sarcoma: similar dismal outcome but discrepancy of timing of brain metastasis in bone and soft tissue sarcoma. J Surg Oncol 2011;104:765-70. [Crossref] [PubMed]
- McCarville MB, Muzzafar S, Kao SC, et al. Imaging features of alveolar soft-part sarcoma: a report from Children's Oncology Group Study ARST0332. AJR Am J Roentgenol 2014;203:1345-52. [Crossref] [PubMed]
- Macpherson RE, Pratap S, Tyrrell H, et al. Retrospective audit of 957 consecutive 18F-FDG PET-CT scans compared to CT and MRI in 493 patients with different histological subtypes of bone and soft tissue sarcoma. Clin Sarcoma Res 2018;8:9. [Crossref] [PubMed]
- Montgomery JR, Conrad GR, Sinha P, et al. FDG PET of alveolar soft part sarcoma. Clin Nucl Med 2010;35:827-9. [Crossref] [PubMed]
- Zarrin-Khameh N, Kaye KS. Alveolar soft part sarcoma. Arch Pathol Lab Med 2007;131:488-91. [PubMed]
- Tsuji K, Ishikawa Y, Imamura T. Technique for differentiating alveolar soft part sarcoma from other tumors in paraffin-embedded tissue: comparison of immunohistochemistry for TFE3 and CD147 and of reverse transcription polymerase chain reaction for ASPSCR1-TFE3 fusion transcript. Hum Pathol 2012;43:356-63. [PubMed]
- Paoluzzi L, Maki RG. Diagnosis, prognosis, and treatment of alveolar soft-part sarcoma: a review. JAMA Oncol 2019;5:254-60. [Crossref] [PubMed]
- Judson I, Morden JP, Kilburn L, et al. Cediranib in patients with alveolar soft-part sarcoma (CASPS): a double-blind, placebo-controlled, randomised, phase 2 trial. Lancet Oncol 2019;20:1023-34. [Crossref] [PubMed]
- Chi Y, Fang Z, Hong X, et al. Safety and efficacy of anlotinib, a multikinase angiogenesis inhibitor, in patients with refractory metastatic soft-tissue sarcoma. Clin Cancer Res 2018;24:5233-8. [Crossref] [PubMed]
- D'Angelo SP, Mahoney MR, Van Tine BA, et al. Nivolumab with or without ipilimumab treatment for metastatic sarcoma (Alliance A091401): two open-label, non-comparative, randomised, phase 2 trials. Lancet Oncol 2018;19:416-26. [Crossref] [PubMed]
- Coyne G, Sharon E, Moore N, et al. Phase II study of atezolizumab in patients with alveolar soft part sarcoma. CTOS Annual Meeting 2018:Paper 021.
- Wilky BA, Trucco MM, Subhawong TK, et al. Axitinib plus pembrolizumab in patients with advanced sarcomas including alveolar soft-part sarcoma: a single-centre, single-arm, phase 2 trial. Lancet Oncol 2019;20:837-48. [Crossref] [PubMed]
- Tao X, Hou Z, Wu Z, et al. Brain metastatic alveolar soft-part sarcoma: Clinicopathological profiles, management and outcomes. Oncol Lett 2017;14:5779-84. [PubMed]
- van der Graaf WT, Blay JY, Chawla SP, et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2012;379:1879-86. [Crossref] [PubMed]
- Mir O, Domont J, Cioffi A, et al. Feasibility of metronomic oral cyclophosphamide plus prednisolone in elderly patients with inoperable or metastatic soft tissue sarcoma. Eur J Cancer 2011;47:515-9. [Crossref] [PubMed]
- Hanahan D, Bergers G, Bergsland E. Less is more, regularly: metronomic dosing of cytotoxic drugs can target tumor angiogenesis in mice. J Clin Invest 2000;105:1045-7. [Crossref] [PubMed]
- Reichardt P, Lindner T, Pink D, et al. Chemotherapy in alveolar soft part sarcomas. What do we know? Eur J Cancer 2003;39:1511-6. [Crossref] [PubMed]


