PET/CT: appropriate application in lymphoma
Introduction
Fluorine 18 fluorodeoxyglucose (18F-FDG) positron emission tomography (PET), and more recently PET-computed tomography (PET/CT) is a very useful and powerful tool in evaluating most lymphoma subtypes. Co-registering functional and morphologic data, this multimodality can accurately diagnose the lymphoma lesion, monitor the treatment response, and predict the prognosis (1). Nowadays, the National Comprehensive Cancer Network (NCCN) guidelines recommend the use of 18F-FDG PET/CT for primary staging, early or final response evaluation, and prognosis in patients with lymphoma. Many risk-adapted therapies based on interim 18F-FDG PET/CT have been currently carried out globally to improve the management and outcome of patients (2,3). However, the optimal application of 18F-FDG PET/CT scans in lymphoma remains problematic because of several variables, such as the standardization of PET/CT interpretation, the meaning of positive PET results, and so on. Thus, the present article reviewed the last decade of publication concerning PET data in lymphoma patients, selected key clinical trials, and discussed the new guidelines to provide an evidence-based approach for the utility of 18F-FDG PET in staging, treatment planning, response assessment, and prognosis evaluation of these patients.
Mechanism of 18F-FDG PET/CT
18F-FDG is a surrogate biomarker for glucose metabolism in vivo and is the most commonly clinical used PET radiotracer (4,5). 18F-FDG is transported into metabolically active cells via glucose transporter proteins, and subsequently phosphorylated in a manner similar to glucose. Phosphorylated 18F-FDG cannot typically dephosphorylate and then trap in the cell (6). Numerous malignant tumors express higher numbers of specific membrane transport proteins, with greater affinity for glucose than normal cells, which permits increased glucose flow into the cancerous cells (7). The positron emitted from 18F bumps into an electron in tumor cells, generates two 511 KeV photons (called the annihilate radiation) emitted in nearly opposite direction that are detected by the PET detector and reconstructed into a metabolic functional imaging of malignant tumor. The low-dose CT imagines co-registered make the attenuation correction of metabolic imaging, and add the anatomic information in vivo in one scanning session. To date, the success of PET/CT in the oncology domain still relies on the use of 18F-FDG, and some recommendation of conduct of 18F-FDG PET/CT are listed in Table 1 (5).

Full table
Histopathologic lymphoma subtypes
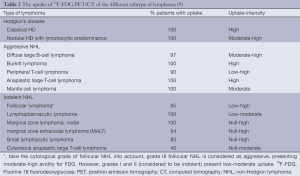
Since lymphomas are a heterogeneous group of neoplastic disease of lymphocyte origin, 18F-FDG avidity of lymphoma lesion correlates better with the histopathologic subtype than with clinical characteristics (8). Most malignant lymphomas, such as HL, diffuse large B cell lymphoma (DLBCL), Burkitt, mantle cell lymphoma (MCL), are shown as generally moderate to high 18F-FDG uptake with a sensitivity of 85-100% (Table 2). While, some indolent NHLs including marginal zone lymphomas (MZL), chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL), and lymphoplasmacytic lymphoma (LPL) have no established role for the clinical usefulness of 18F-FDG PET because of the limited and variable 18F-FDG-avidity (Figure 1). Most of T-cell origin lymphoma is FDG-PET avid, except for the Enteropathy-type T-cell lymphoma (67%) and primary cutaneous anaplastic large cell lymphoma (36%) (Figure 2) (8,10).
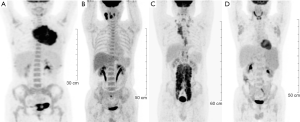
Several reports revealed the transformation rate from indolent lymphomas to aggressive NHL is about 3% per year, up to 15 years after diagnosis, in patients with lymphoma. Despite their recognition and intensification of treatment, the prognosis is poor, with death within less than one year in most patients (11). 18F-FDG PET/CT can reveal the suspicious sites of transformation due to the different avidity between the aggressive lymphomas and the indolent ones. The standardized uptake value (SUV) exceeding ten yields 80% certainty for the identification of aggressive behavior, particularly, in Richter’s transformation for patients with CLL/SLL. These suspicious sites of transformation should be confirmed via the histopathologic biopsy (12).
Staging of lymphomas
18F-FDG PET/CT has demonstrated a better and accurate diagnostic yield, with 97% of sensitivity and 100% of specificity, than contrast-enhanced CT (CECT), especially for normal-sized lymph nodes and extranodal sites (13-17). The finding of the multimodality can upstage the lymphoma (20% to 40% of patients) by detecting more avid-lesions than CECT; while it exhibits no pathological uptake of the morphological lesion identified by CECT, causing downstage of disease (5% to 15% of patients) (14). The current NCCN guidelines recommend initial/baseline PET imaging as an essential test in HL, DLBCL, acquired immune deficiency syndrome (AIDS) related B-cell lymphomas, and as a useful test in selected cases in follicular lymphoma (FL), MZL, MCL, but not recommend it in CLL/SLL because of low-to-moderate 18F-FDG uptakes (18). CECT is still a powerful modality in evaluating no FDG-avid histologies, distinguishing bowels or vessels from lymphadenopathy, and in the setting of compression/thrombosis of mediastinal vessels. Because of the intense physiologic FDG uptake in the brain vortex, detection of intracranial lymphomatous involvement (especially leptomeningeal infiltration) is difficult. Moreover, steroid therapy, which is given to most patients with suspected brain involvement, may interfere with the uptake of FDG and possibly lead to false-negative results (19,20). Magnetic resonance imaging (MRI) is preferred to assess suspected central nervous system (CNS) involvement.
Even though the correction of the stage normally changes in the treatment of lymphoma (5-15% of patients), no evidence revealed that outcome is improved as a result of these data (21-23). However, improving staging accuracy ensures that fewer patients are undertreated or overtreated (24). 18F-FDG PET/CT is particularly important for staging before consideration of radiation therapy.
The increased use of systemic and multimodality approaches has made Ann Arbor stage less relevant in directing the choice of therapy (24). Thus, the Ann Arbor classification is recommended to modify for anatomic description of disease extent by the recent Lugano Classification. According to the revised staging system (Table 3), the patients with the primary nodal lymphoma can be divided into two categories, the limited stage (Ann Arbor stage I and II, nonbulky) and the advanced stage (stage III or IV). Stage II bulky disease is considered as the limited or advanced stage depended on the histology and several prognostic factors. The extranodal disease is only relevant for the limited stage, not for the advanced-stage, including a single extranodal lesion without nodal involvement and Stage II disease with direct extension to a non-nodal site. Now only patients with HL need be assigned the absent or presence of disease-related symptoms because treatment in HL is still directed by symptoms (24,25).
Bone marrow involvement (BMI)
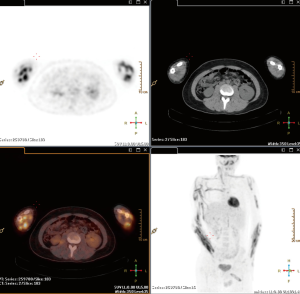
Routine bone marrow biopsy (BMB), usually sampled the posterior iliac crest, may not be representative of the whole disease involvement (26). 18F-FDG PET/CT robustly detects BMI of whole body in lymphoma patients with avid lesions, especially those with a negative iliac crest BMB (Figure 3) (27). The sensitivity and positive predictive value (PPV) in detecting BMI are about 90% and 75%, 100% and 96%, respectively, in HL and DLBCL (28,29). The high sensitivity of 18F-FDG PET/CT scans brought the debate whether the BMB could be obviated in FDG patients already undergone the scan (24). Many recent studies revealed that routine BMB added limited useful clinical information in newly diagnosed patients with HL and DLBCL staged by 18F-FDG PET/CT scans, and the prognosis of these patients with systemic therapy is more related to early interim-PET activity than to the BMB result (29-32). Furthermore, patients with a positive BMB generally have other factors consistent with advanced stage or poor prognosis. Consequently, the Lugano Classification stated that a BMB is no longer required for the routine evaluation of patients with HL and aggressive NHL if an 18F-FDG PET/CT is performed (24,25).
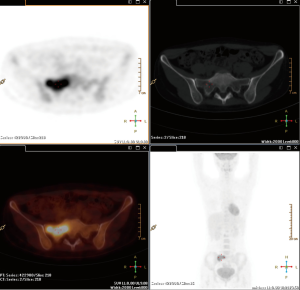
The sensitivity and specificity of 18F-FDG PET/CT for detecting BM involvement in indolent lymphomas are 46% and 93%, respectively (28). Patients with most indolent lymphomas (especially SLL/CLL) may have high percentage of the absence of avidity or the diffuse uptake in the scan, and still need a BMB to detect the involvement of lymphoma.
Bulky lymphoma
The definition of bulky lymphoma is a single nodal mass of 6-10 cm, depended on the histology of lymphoma, or greater than a third of the transthoracic diameter at any level of thoracic vertebrae. The presence of bulky disease is considered a negative prognosis factor in early stage-HL but not in advanced HL. In DLBCL, bulk is predictive of inferior survival in favorable-prognosis disease but not in poor-prognosis disease, probably because the prognosis is determined by multiple adverse factors, not a single one, in the advanced lymphoma (25). However, none of the proposed sizes have been validated in the current therapeutic era; volumetric measurement for tumor bulk and total tumor burden, including methods combining metabolic activity and anatomic size or volume, may be a potential prognostic factor and worth the further investigation.
Response evaluation criteria
The most common anatomic imaging modality used for assessment of treatment response in lymphoma is CECT. The international prognostic index and the FL international prognostic index are currently used clinical prognostic indices for DLBCL and FL, respectively, and the International Prognostic Score is used for HL (33-36). It mainly base on a morphological evaluation with a reduction in tumor size, definitely cannot differentiate tumor from necrosis or fibrotic tissue, and result in false negative results.
18F-FDG PET/CT imaging can provide the metabolic and morphological information of the lesion, and improve the capability of diagnosis and differentiation in the patients with lymphoma. Furth et al. reported in children with HL that 18F-FDG PET/CT diagnosed an early response to therapy with a sensitivity of 100% and a specificity of 68%, and late response to therapy with 100% sensitivity and 78% specificity (37).
However, the definition of ‘negative’ or ‘positive’ of lesion are a matter of ongoing debate. There is variability in interpretation of PET scans among center or even within the same institution. In current clinical practice, visual inspection of 18F-FDG PET or PET/CT images is usually adequate for image interpretation, especial for staging. However, if comparison of 18F-FDG PET/CT imagines was obtained from different center, the final results will be profoundly influenced by many reasons, especially dada analysis method. To standardize the interpretation criteria and make rigorous quality control, the International Harmonization Project (IHP) subcommittee developed consensual criteria of the imaging interpretation for lymphoma, which was set the mediastinal blood pool uptake as a reference background to define FDG PET/CT positivity for a residual mass of 2 cm and greater (38). Although the IHP criteria are useful for the analysis at completion of therapy, it is preferable to have a higher level of uptake (such as liver) as background reference for interim FDG PET/CT because of false-positive rate.
Further refinement of the evaluation criteria led to the Deauville criteria, which have been initially proposed for the assessment of 18F-FDG PET/CT imaging after one or more cycles of therapy (Table 4). The criteria use a five-point scale to determine the FDG uptake in the involved sites relative to that of the mediastinum and the liver (39). 18F-FDG PET scans with scores 1-2 were considered negative; scores 4-5 were considered positive (Figure 4). Normally, score 3 is considered negative, while it’s better to be a threshold for positivity for de-escalation of therapy based on interim PET scan. The criteria have been validated in international multicenter trials for 18F-FDG PET/CT-guide interim response assessments, and the recent NCCN guidelines have set the criteria as standard protocols for the interpretation of the interim and end-of-treatment in HL patients (40,41). Also, these criteria are agreeable for interpreting therapy responses in DLBCL and FL (42-44).
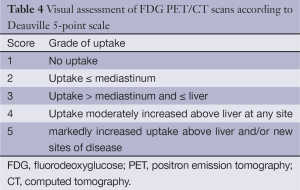
Full table

Semi quantification of FDG uptake using the maximum SUV (SUVmax) at baseline and midtherapy allows evaluation of the lymphoma metabolic changes during induction treatment, so percentage change in the SUVmax (ΔSUVmax) in tumor before and after treatment has been adopted as an interpretation criteria to reduce false-positive of interim PET/CT and to improve prognosis value in NHL. Receiver operator curve in patients with DLBCL identified optimum thresholds for ΔSUVmax as 66% for predicting event-free survival (EFS) in retrospective studies (45-47). Even though many studies accepted 66% as the threshold, the reported cut-off values of ΔSUVmax range from 66% to 91%, suggesting that consistency in scanning protocols, matching conditions for serial scans, and proper calibration and scanner maintenance are mandatory for general application (25,47-49). No validated cut-off value was reported yet.
The metabolic tumor volume (MTV), as a reflection of the disease burden and prognostic factor, is measured on 18F-FDG PET/CT images by the select tumor lesion with uptake above SUVmax 2.5 or using a threshold of liver or mediastinum as a reference organ. Some researches were performed in patients with DLBCL and extranodal natural killer/T cell lymphoma (ENKTCL) (50-52). The total lesion glycolysis (TLG) is also an accurate volume measure to determine disease burden by accounting for the tumor volume and intensity using sophisticated software systems (53,54). These automated volumetric methods may possess better prognosis values, and is one of future directions investigated in patients undergoing interim PET.
End-of-treatment 18F-FDG PET/CT
End-of-treatment FDG PET/CT is used to evaluate the efficacy and monitoring of residual tumor, and to provide the basis for selecting treatment with or without high intensity chemotherapy, radiotherapy or transplantation (55,56). The sensitivity and specificity of 18F-FDG PET/CT for follow-up HL patients were reported to be 94% and 100% and for NHL patients 90% and 88%, respectively. Patient with negative 18F-FDG PET/CT has an excellent prognosis in posttreatment follow-up, in contrast to a positive scan where the risk for disease to relapse increases. Spaepen et al. reported that 56 of 67 NHL patients with negative 18F-FDG PET/CT scan after first-line chemotherapy remained in complete remission (CR) at a median follow-up of 653 days compared to all 26 patients with positive 18F-FDG PET/CT scan who experienced relapse at a median of 73 days (57). In HL patients, several studies demonstrate significantly shorter progression-free survivals (PFS) for PET-positive patients (0-4%) compared with 85-95% for those with a negative scan (1,58-61). However, the therapeutic responses of some indolent lymphomas, such as SLL/CLL, should still be assessed in terms of anatomical relief because of variable or no FDG uptake (38,62).
A complete metabolic response (CMR) represents a good indicator of clinical CR (cCR) leading to less intense therapy or only routine surveillance, whereas persistent abnormal 18F-FDG uptake in residue require a clinical decision whether continuing or switching therapy or considering stem cell transplantation. It is necessary and important to make the histopathological confirmation of positive PET finding before further treatment.
To avoid equivocal interpretation, the scan should ideally performed 6-8 weeks, or at least 3 weeks, after completion of chemotherapy and 8-12 weeks after radiation therapy because of radiation-induced hypermetabolic inflammatory changes (55). Some physiologic uptake often occur after treatments, such as reactive diffuse bone marrow hyperplasia and diffuse splenic uptake (typically 2-4 weeks after therapy); an intense bone marrow hyperplasia after the administration of marrow-stimulating factors; thymic hyperplasia in children and adolescents (2-6 months post-therapy persisting for up to 12-24 months).
Response evaluation during therapy
18F-FDG PET/CT imaging can sensitively detect the metabolic changes of tumor as early as several days after the therapy, and has been recognized as a surrogate marker of chemosensitivity and prognosis (Figure 4) (63-66). In a meta-analysis, interim 18F-FDG PET/CT yielded an overall sensitivity of 81% and a specificity of 97% for advanced-stage HL, and a sensitivity of 78% and a specificity of 87% for DLBCL (67).
Gallamini et al. reported the predictive value of 18F-FDG PET/CT scan in HL patients posttreated with two cycles of doxorubicin, bleomycin, vinblastine and dacarbazine (ABVD). The data showed the 2-year probability of failure-free survivals for PET-2 negative and for PET-2 positive patients were 96% and 6%, respectively (66). The German Hodgkin Study Group (GHSG) evaluated 18F-FDG PET/CT after four cycles of escalated or every-14-day bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone (BEACOPP) chemotherapy in 50 patients with advanced-stage HL and found an NPV of 97%, allowing omission of radiotherapy in 36 patients, none of whom experienced progression. Of the 14 with a positive scan, seven underwent radiotherapy (a PPV of only 14%), and no difference in PFS was found between the PET-negative and PET-positive groups (68). More recently, the best predictive value for interim PET imaging was reported by Zinzani et al. in a retrospective study (n=304) on a subgroup of 147 I-IIA HL patients treated with standard therapy followed by IFRT (69). They found that 97.6% of patients with a negative PET-2 result were in continuous CR (CCR) (median follow-up, 45 months) whereas only 21% of PET-2-positive patients had a CCR (median follow-up, 28 months). The 9-year PFS for PET-2-negative and PET-2-positive patients were 95% and 31% respectively.
However, in an analysis of early-stage HL, Hutchings et al. reported a PPV for interim 18F-FDG PET after 2-3 cycles of ABVD chemotherapy of only 30% whereas the NPV was maintained at 95% (3). In this group, the 2-year PFS in the interim PET-negative and PET-positive patients were 97-100% and 70-80%, respectively. Barnes et al. showed that interim PET did not predict outcome in ninety-six patients with nonbulky limited-stage HL patients, with PFS in positive and negative patients 87% vs. 91%, respectively, whereas the end-of-treatment PET result was predictive of outcome, with a PFS of 94% for PET-negative vs. 54% for PET-positive patients (70). More results revealed that the role of FDG-PET in the prediction of ultimate outcome is clearer in advanced-stage than early stage HL (1,71,72).
The interim PET/CT scan can predict the outcome of patients of NHL undergoing the systematic chemotherapy, but the prognosis value is somewhat less convincing than that of HL (73). Initial retrospective studies in DLBCL suggested a difference in outcome for patients with positive and negative findings on interim PET (64,74), while more recent studies have demonstrated good NPV but more variable PPV. PFS for the PET-positive groups has ranged from 18% to 74% (1,25). The difference in PET results may be related to evaluating criteria, variable duration of follow-up, patient populations, and different types of treatment adapted, different number of chemotherapy cycles, and timing of scanning during the chemotherapy cycle, and so on. The lower PPV is partial related to the higher risk of infections among patients treated with higher dose-density and intensity strategies and among typically older patients. In addition the use of rituximab may produce a high incidence of false positive results by inducing an inflammatory response by activation of antibody dependent cellular cytotoxicity and complement dependent cytotoxicity. Moskowitz et al. treated 98 patients with DLBCL using a dose-dense R-CHOP-like regimen, with 18F-FDG PET/CT after four cycles. The biopsy data of FDG-positive residue proved 87% of patients were false positive. PFS of interim PET-positive/biopsy-negative patients was identical to that in patients with a negative interim PET scan (73).
The use of quantitation to improve on visual assessment has been explored in DLBCL. Lin et al. assessed the prognostic value of early 18F-FDG PET/CT in 92 patients with DLBCL with the ΔSUVmax criteria, and yielded an accuracy of 76.1% to predict event free survival (EFS). The 2-year estimate for EFS was 21% in patients with SUVmax reduction ≤65.7% compared with 79% in those with reduction >65.7% (45). Now, the ΔSUVmax criteria are being prospectively applied in several multicenter studies exploring response-adapted treatment with immunochemotherapy. Changes in the MTV and TLG are additional exploratory quantitation measures. However, some preliminary reports have suggested changes in the MTV and TLG are not predictive in DLBCL.
Since several criteria exist in the response evaluation system, it will definitely affect the prognosis value of 18F-FDG PET/CT in patients with NHL. Many researchers suggested that the prospective studies should been performed in homogenous populations receiving the same treatment to determine the optimal one (75). Itti et al. and Casasnovas et al. compared the Deauville criteria and ΔSUVmax criteria for assessing the prognostic value of interim PET/CT in DLBCL, and results showed that ΔSUVmax leads to slightly better performance and interobserver reproducibility than the Deauville criteria (42,47). While, Manohar et al. insisted that 5-point scale is preferentially over other criteria because of simplicity in interpretation and reproducibility when assessed end-of-treatment 18F-FDG PET/CT in 69 patients with aggressive NHL (61). More investigation need to be explored in the future’s clinical trials. Anyway, standardization of PET methods is mandatory for the use of quantitative approaches and desirable for routine clinical practice.
PET-directed treatment
To date, there is no direct evidence that altering therapy on the basis of interim PET findings improves patient outcome. More the 80-90% of early-stage lymphoma patients are cured with standard therapy. However, a substantial number of early-stage lymphoma patients are subject to some amount of overtreatment, and this is an argument for using early PET/CT to identify good-risk, early-stage patients eligible for less-intensive treatment. Multiple PET-directed randomized studies were initiated to determine the outcome of therapy de-escalation in patients who achieve an early CR as well as escalation in non-responding patients. But only few have reported interim results. The Hay et al. investigated the prognosis of patients, with nonbulky stage IA-IIA HL, treated with combined modality therapy (CMT) using ABVD plus radiotherapy, or ABVD alone. The results showed that 8-year PFS was 87% and overall survival 96% in patients who achieved CR/unconfirmed complete response (CRu) after two cycles of ABVD. In contrast, among those without CR/CRu after two cycles of ABVD, 8-year PFS was 88% and overall survival 95% (76). In the HD15 trial of the GHSG, a subset of 69 HL patients (stages IIB, III, IV) with a large mediastinal mass or extranodal disease were treated with either 8 or 6 cycles of escalated BEACOPP or with 8 cycles of BEACOPP14. Those with a residual mass of at least 2.5 cm underwent an 18F-FDG PET/CT scan. Patients with a negative study were not further treated, whereas those with a positive scan received involved field radiation. The frequency of consolidative radiotherapy was only 11% compared with 70% in prior studies antedating the use of PET scans, with no difference in overall survival (1,77).
Kasamon et al. used a risk-adapted approach in 59 newly diagnosed patients with various histologies of aggressive NHL. 18F-FDG PET/CT was performed after two to three cycles of therapy. Of those 33 patients, 28 underwent ASCT and experienced a 2-year EFS of 75% (67% for all PET-positive patients), which was better than the anticipated 20% that was based on historical controls (78). Mostly recently, a phase II clinical trial was conducted by using interim PET as a guide to risk-adapted therapy in high-risk patients with newly diagnosed DLBCL (79). Patients achieving negative FDG-PET continued receiving three additional cycles of R-MegaCHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone), whereas PET-positive patients received two courses of R-IFE (rituximab, ifosfamide, etoposide) followed by BEAM (BCNU, etoposide, cytarabine, melphalan) and autologous stem-cell transplantation. Patients in CR after interim PET had significantly better 3-year PFS than those with partial response (81% vs. 57%) but not a statistically significant longer OS. Three-year PFS (81% vs. 33%) and OS (95% vs. 33%) were significantly better for negative than for positive interim PET patients.
In summary, on the basis of the available data, midtreatment clinical trials addressed important clinical questions and should not performed as standard practice yet.
Surveillance 18F-FDG PET/CT scan
The utility of 18F-FDG PET/CT in the follow-up is not recommended because the published data failed to prove its clinical value (80,81). More than 60% of relapses of patients with HL and aggressive NHL were diagnosed clinically, especially in aggressive NHL in cases with extranodal involvement. Jerusalem et al. showed doubtful cost-benefit profitability of follow-up with 18F-FDG PET/CT since the appearance of an important number of false positive required the performance of complementary tests to determine possible relapse (82). Meanwhile, the false-positive result of 18F-FDG PET/CT scan may cause possible and unnecessary radiation exposure and patient anxiety. In clinic, CECT and laboratory examinations, such as serum lactate dehydrogenase, are recommended to follow up the relapse of lymphoma. The frequency of examinations depends on the histologies. For example, patients with HL or DLBCL should visit hospital every 3 months during the first 2 years, to every 6 months for the next 3 years, and then annually thereafter to monitor for late relapse and treatment-related adverse effect; while patients with FL should be observed every 3 to 6 months (24). When patients are suspicious of the relapse of lymphoma, 18F-FDG PET/CT scan should be performed at the first instance.
PET/CT in stem cell transplantation
Compare to standard chemotherapy, treatment with high doses of chemotherapy followed by stem cell transplantation offer long-term disease free survival in more than 50% of transplanted patients (83). 18F-FDG PET/CT has demonstrated to be an effective tool to differentiate between responders and non-responders to this treatment, with a sensitivity of 69% and a specificity of 81% (84). In an addition, PET-positive prior to transplantation was associated with a 3-fold greater risk of progression and a 4-fold greater risk of death than patients presenting a negative result in the functional study (85,86). Qiao et al. noted that post-transplantation PET/CT scan might also be considered as a prognostic factor in terms of survival comparing to pre-transplantation scan (87).
Conclusions
18F-FDG PET/CT scan has become an important part of the management of lymphoma, and been recommended for staging, detection of recurrence, evaluation of response to treatment, and prediction of response in patients with lymphoma due to the high sensitivity and specificity rates. Even though an interim PET scan possesses the predictive value, its role in directing treatment remains investigational. Any alteration during the treatment should be confirmed by the biopsy.
Acknowledgements
Disclosure: The author declares no conflict of interest.
References
- Kostakoglu L, Cheson BD. State-of-the-Art Research on “Lymphomas: Role of Molecular Imaging for Staging, Prognostic Evaluation, and Treatment Response”. Front Oncol 2013;3:212. [PubMed]
- Roland V, Bodet-Milin C, Moreau A, et al. Impact of high-dose chemotherapy followed by auto-SCT for positive interim [18F] FDG-PET diffuse large B-cell lymphoma patients. Bone Marrow Transplant 2011;46:393-9. [PubMed]
- Hutchings M, Loft A, Hansen M, et al. FDG-PET after two cycles of chemotherapy predicts treatment failure and progression-free survival in Hodgkin lymphoma. Blood 2006;107:52-9. [PubMed]
- Gallagher BM, Fowler JS, Gutterson NI, et al. Metabolic trapping as a principle of oradiopharmaceutical design: some factors resposible for the biodistribution of [18F] 2-deoxy-2-fluoro-D-glucose. J Nucl Med 1978;19:1154-61. [PubMed]
- Larson SM, Schwartz LH. 18F-FDG PET as a candidate for “qualified biomarker”: functional assessment of treatment response in oncology. J Nucl Med 2006;47:901-3. [PubMed]
- Ishiwata K, Tomura M, Ido T, et al. In vivo assessment of 6-deoxy-6-[18F]fluoro-D-galactose as a PET tracer for studying galactose metabolism. Int J Rad Appl Instrum B 1989;16:775-81. [PubMed]
- Ishiwata K, Yamaguchi K, Kameyama M, et al. 2-Deoxy-2-[18F]fluoro-D-galactose as an in vivo tracer for imaging galactose metabolism in tumors with positron emission tomography. Int J Rad Appl Instrum B 1989;16:247-54. [PubMed]
- Weiler-Sagie M, Bushelev O, Epelbaum R, et al. (18)F-FDG avidity in lymphoma readdressed: a study of 766 patients. J Nucl Med 2010;51:25-30. [PubMed]
- Alvarez Páez AM, Nogueiras Alonso JM, Serena Puig A. 18F-FDG-PET/CT in lymphoma: two decades of experience. Rev Esp Med Nucl Imagen Mol 2012;31:340-9. [PubMed]
- Schöder H, Noy A, Gönen M, et al. Intensity of 18fluorodeoxyglucose uptake in positron emission tomography distinguishes between indolent and aggressive non-Hodgkin’s lymphoma. J Clin Oncol 2005;23:4643-51. [PubMed]
- Al-Tourah AJ, Gill KK, Chhanabhai M, et al. Population-based analysis of incidence and outcome of transformed non-Hodgkin’s lymphoma. J Clin Oncol 2008;26:5165-9. [PubMed]
- Noy A, Schöder H, Gönen M, et al. The majority of transformed lymphomas have high standardized uptake values (SUVs) on positron emission tomography (PET) scanning similar to diffuse large B-cell lymphoma (DLBCL). Ann Oncol 2009;20:508-12. [PubMed]
- Schaefer NG, Hany TF, Taverna C, et al. Non-Hodgkin lymphoma and Hodgkin disease: coregistered FDG PET and CT at staging and restaging--do we need contrast-enhanced CT? Radiology 2004;232:823-9. [PubMed]
- Moog F, Bangerter M, Diederichs CG, et al. Extranodal malignant lymphoma: detection with FDG PET versus CT. Radiology 1998;206:475-81. [PubMed]
- Ömür Ö, Baran Y, Oral A, et al. Fluorine-18 fluorodeoxyglucose PET-CT for extranodal staging of non-Hodgkin and Hodgkin lymphoma. Diagn Interv Radiol 2014;20:185-92. [PubMed]
- Isasi CR, Lu P, Blaufox MD. A metaanalysis of 18F-2-deoxy-2-fluoro-D-glucose positron emission tomography in the staging and restaging of patients with lymphoma. Cancer 2005;104:1066-74. [PubMed]
- Bednaruk-Młyński E, Pieńkowska J, Skórzak A, et al. Comparison of positron emission tomography/computed tomography with classical contrast-enhanced computed tomography in the initial staging of Hodgkin lymphoma. Leuk Lymphoma 2015;56:377-82. [PubMed]
- Fueger BJ, Yeom K, Czernin J, et al. Comparison of CT, PET, and PET/CT for staging of patients with indolent non-Hodgkin’s lymphoma. Mol Imaging Biol 2009;11:269-74. [PubMed]
- Even-Sapir E, Lievshitz G, Perry C, et al. Fluorine-18 fluorodeoxyglucose PET/CT patterns of extranodal involvement in patients with Non-Hodgkin lymphoma and Hodgkin's disease. Radiol Clin North Am 2007;45:697-709. [PubMed]
- Paes FM, Kalkanis DG, Sideras PA, et al. FDG PET/CT of extranodal involvement in non-Hodgkin lymphoma and Hodgkin disease. Radiographics 2010;30:269-91. [PubMed]
- Raanani P, Shasha Y, Perry C, et al. Is CT scan still necessary for staging in Hodgkin and non-Hodgkin lymphoma patients in the PET/CT era? Ann Oncol 2006;17:117-22. [PubMed]
- Menzel C, Döbert N, Mitrou P, et al. Positron emission tomography for the staging of Hodgkin’s lymphoma--increasing the body of evidence in favor of the method. Acta Oncol 2002;41:430-6. [PubMed]
- Sasaki M, Kuwabara Y, Koga H, et al. Clinical impact of whole body FDG-PET on the staging and therapeutic decision making for malignant lymphoma. Ann Nucl Med 2002;16:337-45. [PubMed]
- Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for Initial Evaluation, Staging, and Response Assessment of Hodgkin and Non-Hodgkin Lymphoma: The Lugano Classification. J Clin Oncol 2014;32:3059-68. [PubMed]
- Barrington SF, Mikhaeel NG, Kostakoglu L, et al. Role of Imaging in the Staging and Response Assessment of Lymphoma: Consensus of the International Conference on Malignant Lymphomas Imaging Working Group. J Clin Oncol 2014;32:3048-58. [PubMed]
- Wang J, Weiss LM, Chang KL, et al. Diagnostic utility of bilateral bone marrow examination: significance of morphologic and ancillary technique study in malignancy. Cancer 2002;94:1522-31. [PubMed]
- Schaefer NG, Strobel K, Taverna C, et al. Bone involvement in patients with lymphoma: the role of FDG-PET/CT. Eur J Nucl Med Mol Imaging 2007;34:60-7. [PubMed]
- Wu LM, Chen FY, Jiang XX, et al. 18F-FDG PET, combined FDG-PET/CT and MRI for evaluation of bone marrow infiltration in staging of lymphoma: a systematic review and meta-analysis. Eur J Radiol 2012;81:303-11. [PubMed]
- El-Galaly TC, d’Amore F, Mylam KJ, et al. Routine bone marrow biopsy has little or no therapeutic consequence for positron emission tomography/computed tomography-staged treatment-naive patients with Hodgkin lymphoma. J Clin Oncol 2012;30:4508-14. [PubMed]
- Adams HJ, Kwee TC, de Keizer B, et al. Systematic review and meta-analysis on the diagnostic performance of FDG-PET/CT in detecting bone marrow involvement in newly diagnosed Hodgkin lymphoma: is bone marrow biopsy still necessary? Ann Oncol 2014;25:921-7. [PubMed]
- Weiler-Sagie M, Kagna O, Dann EJ, et al. Characterizing bone marrow involvement in Hodgkin’s lymphoma by FDG-PET/CT. Eur J Nucl Med Mol Imaging 2014;41:1133-40. [PubMed]
- Khan AB, Barrington SF, Mikhaeel NG, et al. PET-CT staging of DLBCL accurately identifies and provides new insight into the clinical significance of bone marrow involvement. Blood 2013;122:61-7. [PubMed]
- Shipp M. Prognostic factors in non-Hodgkin's lymphoma. Curr Opin Oncol 1992;4:856-62. [PubMed]
- Shipp MA. Prognostic factors in aggressive non-Hodgkin’s lymphoma: who has “high-risk” disease? Blood 1994;83:1165-73. [PubMed]
- Solal-Céligny P, Roy P, Colombat P, et al. Follicular lymphoma international prognostic index. Blood 104:1258-65. [PubMed]
- Hasenclever D, Diehl V. A prognostic score for advanced Hodgkin’s disease. International Prognostic Factors Project on Advanced Hodgkin’s Disease. N Engl J Med 1998;339:1506-14. [PubMed]
- Furth C, Steffen IG, Erdrich AS, et al. Explorative analyses on the value of interim PET for prediction of response in pediatric and adolescent non-Hodgkin lymphoma patients. EJNMMI Res 2013;3:71. [PubMed]
- Juweid ME, Stroobants S, Hoekstra OS, et al. Use of positron emission tomography for response assessment of lymphoma: consensus of the Imaging Subcommittee of International Harmonization Project in Lymphoma. J Clin Oncol 2007;25:571-8. [PubMed]
- Meignan M, Gallamini A, Meignan M, et al. Report on the First International Workshop on Interim-PET-Scan in Lymphoma. Leuk Lymphoma 2009;50:1257-60. [PubMed]
- Barrington SF, Qian W, Somer EJ, et al. Concordance between four European centres of PET reporting criteria designed for use in multicentre trials in Hodgkin lymphoma. Eur J Nucl Med Mol Imaging 2010;37:1824-33. [PubMed]
- Le Roux PY, Gastinne T, Le Gouill S, et al. Prognostic value of interim FDG PET/CT in Hodgkin's lymphoma patients treated with interim response-adapted strategy: comparison of International Harmonization Project (IHP), Gallamini and London criteria. Eur J Nucl Med Mol Imaging 2011;38:1064-71. [PubMed]
- Itti E, Meignan M, Berriolo-Riedinger A, et al. An international confirmatory study of the prognostic value of early PET/CT in diffuse large B-cell lymphoma: comparison between Deauville criteria and ΔSUVmax. Eur J Nucl Med Mol Imaging 2013;40:1312-20. [PubMed]
- Dupuis J, Berriolo-Riedinger A, Julian A, et al. Impact of [(18)F]fluorodeoxyglucose positron emission tomography response evaluation in patients with high-tumor burden follicular lymphoma treated with immunochemotherapy: a prospective study from the Groupe d’Etudes des Lymphomes de l’Adulte and GOELAMS. J Clin Oncol 2012;30:4317-22. [PubMed]
- Tychyj-Pinel C, Ricard F, Fulham M, et al. PET/CT assessment in follicular lymphoma using standardized criteria: central review in the PRIMA study. Eur J Nucl Med Mol Imaging 2014;41:408-15. [PubMed]
- Lin C, Itti E, Haioun C, et al. Early 18F-FDG PET for prediction of prognosis in patients with diffuse large B-cell lymphoma: SUV-based assessment versus visual analysis. J Nucl Med 2007;48:1626-32. [PubMed]
- Itti E, Lin C, Dupuis J, et al. Prognostic value of interim 18F-FDG PET in patients with diffuse large B-Cell lymphoma: SUV-based assessment at 4 cycles of chemotherapy. J Nucl Med 2009;50:527-33. [PubMed]
- Casasnovas RO, Meignan M, Berriolo-Riedinger A, et al. SUVmax reduction improves early prognosis value of interim positron emission tomography scans in diffuse large B-cell lymphoma. Blood 2011;118:37-43. [PubMed]
- Nols N, Mounier N, Bouazza S, et al. Quantitative and qualitative analysis of metabolic response at interim positron emission tomography scan combined with International Prognostic Index is highly predictive of outcome in diffuse large B-cell lymphoma. Leuk Lymphoma 2014;55:773-80. [PubMed]
- Yang DH, Ahn JS, Byun BH, et al. Interim PET/CT-based prognostic model for the treatment of diffuse large B cell lymphoma in the post-rituximab era. Ann Hematol 2013;92:471-9. [PubMed]
- Song MK, Chung JS, Shin HJ, et al. Clinical significance of metabolic tumor volume by PET/CT in stages II and III of diffuse large B cell lymphoma without extranodal site involvement. Ann Hematol 2012;91:697-703. [PubMed]
- Song MK, Chung JS, Shin HJ, et al. Clinical value of metabolic tumor volume by PET/CT in extranodal natural killer/T cell lymphoma. Leuk Res 2013;37:58-63. [PubMed]
- Song MK, Chung JS, Shin HJ, et al. Prognostic value of metabolic tumor volume on PET / CT in primary gastrointestinal diffuse large B cell lymphoma. Cancer Sci 2012;103:477-82. [PubMed]
- Werner-Wasik M, Nelson AD, Choi W, et al. What is the best way to contour lung tumors on PET scans? Multiobserver validation of a gradient-based method using a NSCLC digital PET phantom. Int J Radiat Oncol Biol Phys 2012;82:1164-71. [PubMed]
- Hatt M, Visvikis D, Albarghach NM, et al. Prognostic value of 18F-FDG PET image-based parameters in oesophageal cancer and impact of tumour delineation methodology. Eur J Nucl Med Mol Imaging 2011;38:1191-202. [PubMed]
- Zanoni L, Cerci JJ, Fanti S. Use of PET/CT to evaluate response to therapy in lymphoma. Q J Nucl Med Mol Imaging 2011;55:633-47. [PubMed]
- Okada M, Sato N, Ishii K, et al. FDG PET/CT versus CT, MR imaging, and 67Ga scintigraphy in the posttherapy evaluation of malignant lymphoma. Radiographics 2010;30:939-57. [PubMed]
- Spaepen K, Stroobants S, Dupont P, et al. Prognostic value of positron emission tomography (PET) with fluorine-18 fluorodeoxyglucose ([18F]FDG) after first-line chemotherapy in non-Hodgkin’s lymphoma: is [18F]FDG-PET a valid alternative to conventional diagnostic methods? J Clin Oncol 2001;19:414-9. [PubMed]
- Cremerius U, Fabry U, Neuerburg J, et al. Positron emission tomography with 18F-FDG to detect residual disease after therapy for malignant lymphoma. Nucl Med Commun 1998;19:1055-63. [PubMed]
- Zinzani PL, Magagnoli M, Chierichetti F, et al. The role of positron emission tomography (PET) in the management of lymphoma patients. Ann Oncol 1999;10:1181-4. [PubMed]
- Wiedmann E, Baican B, Hertel A, et al. Positron emission tomography (PET) for staging and evaluation of response to treatment in patients with Hodgkin’s disease. Leuk Lymphoma 1999;34:545-51. [PubMed]
- Manohar K, Mittal BR, Raja S, et al. Comparison of various criteria in interpreting end of therapy F-18 labeled fluorodeoxyglucose positron emission tomography/computed tomography in patients with aggressive non-Hodgkin lymphoma. Leuk Lymphoma 2013;54:714-9. [PubMed]
- Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol 2007;25:579-86. [PubMed]
- Zinzani PL, Gandolfi L, Broccoli A, et al. Midtreatment 18F-fluorodeoxyglucose positron-emission tomography in aggressive non-Hodgkin lymphoma. Cancer 2011;117:1010-8. [PubMed]
- Kostakoglu L, Goldsmith SJ, Leonard JP, et al. FDG-PET after 1 cycle of therapy predicts outcome in diffuse large cell lymphoma and classic Hodgkin disease. Cancer 2006;107:2678-87. [PubMed]
- Hutchings M, Mikhaeel NG, Fields PA, et al. Prognostic value of interim FDG-PET after two or three cycles of chemotherapy in Hodgkin lymphoma. Ann Oncol 2005;16:1160-8. [PubMed]
- Gallamini A, Rigacci L, Merli F, et al. The predictive value of positron emission tomography scanning performed after two courses of standard therapy on treatment outcome in advanced stage Hodgkin’s disease. Haematologica 2006;91:475-81. [PubMed]
- Terasawa T, Lau J, Bardet S, et al. Fluorine-18-fluorodeoxyglucose positron emission tomography for interim response assessment of advanced-stage Hodgkin’s lymphoma and diffuse large B-cell lymphoma: a systematic review. J Clin Oncol 2009;27:1906-14. [PubMed]
- Markova J, Kobe C, Skopalova M, et al. FDG-PET for assessment of early treatment response after four cycles of chemotherapy in patients with advanced-stage Hodgkin’s lymphoma has a high negative predictive value. Ann Oncol 2009;20:1270-4. [PubMed]
- Zinzani PL, Rigacci L, Stefoni V, et al. Early interim 18F-FDG PET in Hodgkin’s lymphoma: evaluation on 304 patients. Eur J Nucl Med Mol Imaging 2012;39:4-12. [PubMed]
- Barnes JA, LaCasce AS, Zukotynski K, et al. End-of-treatment but not interim PET scan predicts outcome in nonbulky limited-stage Hodgkin’s lymphoma. Ann Oncol 2011;22:910-5. [PubMed]
- Sher DJ, Mauch PM, Van Den Abbeele A, et al. Prognostic significance of mid- and post-ABVD PET imaging in Hodgkin’s lymphoma: the importance of involved-field radiotherapy. Ann Oncol 2009;20:1848-53. [PubMed]
- Filippi AR, Botticella A, Bellò M. Interim positron emission tomography and clinical outcome in patients with early stage Hodgkin lymphoma treated with combined modality therapy. Leuk Lymphoma 2013;54:1183-7. [PubMed]
- Moskowitz CH, Schöder H, Teruya-Feldstein J, et al. Risk-adapted dose-dense immunochemotherapy determined by interim FDG-PET in Advanced-stage diffuse large B-Cell lymphoma. J Clin Oncol 2010;28:1896-903. [PubMed]
- Spaepen K, Stroobants S, Dupont P, et al. Early restaging positron emission tomography with (18)F-fluorodeoxyglucose predicts outcome in patients with aggressive non-Hodgkin’s lymphoma. Ann Oncol 2002;13:1356-63. [PubMed]
- Tateishi U. PET/CT in malignant lymphoma: basic information, clinical application, and proposal. Int J Hematol 2013;98:398-405. [PubMed]
- Hay AE, Klimm B, Chen BE, et al. An individual patient-data comparison of combined modality therapy and ABVD alone for patients with limited-stage Hodgkin lymphoma. Ann Oncol 2013;24:3065-9. [PubMed]
- Markova J, Kahraman D, Kobe C, et al. Role of [18F]-fluoro-2-deoxy-D-glucose positron emission tomography in early and late therapy assessment of patients with advanced Hodgkin lymphoma treated with bleomycin, etoposide, adriamycin, cyclophosphamide, vincristine, procarbazine and prednisone. Leuk Lymphoma 2012;53:64-70. [PubMed]
- Kasamon YL, Wahl RL, Ziessman HA, et al. Phase II study of risk-adapted therapy of newly diagnosed, aggressive non-Hodgkin lymphoma based on midtreatment FDG-PET scanning. Biol Blood Marrow Transplant 2009;15:242-8. [PubMed]
- Pardal E, Coronado M, Martín A, et al. Intensification treatment based on early FDG-PET in patients with high-risk diffuse large B-cell lymphoma: a phase II GELTAMO trial. Br J Haematol 2014;167:327-36. [PubMed]
- Liedtke M, Hamlin PA, Moskowitz CH, et al. Surveillance imaging during remission identifies a group of patients with more favorable aggressive NHL at time of relapse: a retrospective analysis of a uniformly-treated patient population. Ann Oncol 2006;17:909-13. [PubMed]
- Zinzani PL, Stefoni V, Tani M, et al. Role of [18F]fluorodeoxyglucose positron emission tomography scan in the follow-up of lymphoma. J Clin Oncol 2009;27:1781-7. [PubMed]
- Jerusalem G, Beguin Y, Fassotte MF, et al. Early detection of relapse by whole-body positron emission tomography in the follow-up of patients with Hodgkin’s disease. Ann Oncol 2003;14:123-30. [PubMed]
- Brice P, Bouabdallah R, Moreau P, et al. Prognostic factors for survival after high-dose therapy and autologous stem cell transplantation for patients with relapsing Hodgkin’s disease: analysis of 280 patients from the French registry. Société Française de Greffe de Moëlle. Bone Marrow Transplant 1997;20:21-6. [PubMed]
- Terasawa T, Dahabreh IJ, Nihashi T. Fluorine-18-fluorodeoxyglucose positron emission tomography in response assessment before high-dose chemotherapy for lymphoma: a systematic review and meta-analysis. Oncologist 2010;15:750-9. [PubMed]
- Svoboda J, Andreadis C, Elstrom R, et al. Prognostic value of FDG-PET scan imaging in lymphoma patients undergoing autologous stem cell transplantation. Bone Marrow Transplant 2006;38:211-6. [PubMed]
- Poulou LS, Thanos L, Ziakas PD. Unifying the predictive value of pretransplant FDG PET in patients with lymphoma: a review and meta-analysis of published trials. Eur J Nucl Med Mol Imaging 2010;37:156-62. [PubMed]
- Qiao W, Zhao J, Wang C, et al. Predictive value of (18)F-FDG hybrid PET/CT for the clinical outcome in patients with non-Hodgkin’s lymphoma prior to and after autologous stem cell transplantation. Hematology 2010;15:21-7. [PubMed]