Pitfalls in the pathological diagnosis of lymphoma
Errors due to insufficient data acquisition
Inadequate material including insufficient specimens and sampling errors
Submission of insufficient tissue often occurs during imaging-guided biopsy and leads to difficulty in making an accurate diagnosis. For typical ultrasound or computed tomography (CT)-guided biopsies, we recommend using an 18 gauge (G) cutting needle to ensure enough tissue is acquired for hematoxylin and eosin (HE) staining, immunohistochemistry and molecular testing. Another problem that can arise is when the tissue sampling is not representative of the lesion, which may lead to misdiagnosis. To avoid this problem, pathologists should be in regular communication with clinicians and convey to them the requirement for the specimen. Whenever possible, an excisional biopsy is preferred. Training of junior pathologists in tissue sampling is also important.
Inadequate processing of tissue
Tissue processing involves the fixation, dehydration, embedding, sectioning, staining and mounting of the tissue. Each step is important in yielding adequately stained sections to allow for an accurate diagnosis. Any detail of this process that is neglected may cause potential diagnostic pitfalls. For example, lymph nodes must be fixed promptly and be sliced before fixation, because the fibrous capsule of the lymph node resists the penetration of the fixative. Tissue that is not fixed properly can lead to alterations in morphology and immunohistochemical results. Microscopically, fixed lymph nodes that have not yet been cut often have a peripheral rim of well-preserved structure. The centers of the lymph nodes, however, do not get fixed completely. The center then appears to consist of shrunken lymphocytes, some of which have acentric nuclei and look like plasma cells. Immunohistochemically, CD20 staining can disappear entirely and lead to the misdiagnosis of plasmacytoma instead of diffuse large B cell lymphoma (DLBCL) (Figure 1). Moreover, a misdiagnosis of T cell lymphoma instead of Epsetin-Barr virus (EBV)-positive large B cell lymphoma can also occur (1).
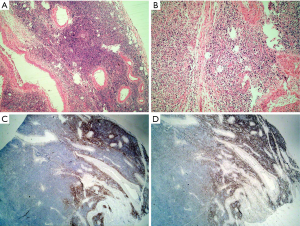
Another example of an important detail in tissue processing that can cause problems is “dry-mounting”. This occurs when the technician dries the slide with a hairdryer without putting it into xylene to make the slide clear. The nuclei of the lymphocytes shrink to darkly stained dots that lack any detail, again making an accurate diagnosis difficult (Figure 2). We encountered this problem for several years until Liu et al. (2) reported on the problem and suggested the proper solution.
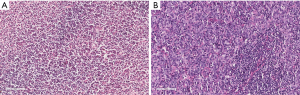
Inadequate clinical information
Since surgical pathology is part of clinical medicine, one cannot make an accurate lymphoma diagnosis without adequate clinical information. The World Health Organization (WHO) emphasizes that diagnosis of such pathologies should integrate clinical, morphological, immunophenotypical, and molecular genetic data. Therefore, regular multidisciplinary discussion plays an important role in the diagnosis of many diseases, especially challenging cases such as lymphomas.
Errors due to personal subjectivity
Although current diagnoses are based on combining results from ancillary techniques, the final diagnosis is still the subjective conclusion of a pathologist. Due to variations in training background and practical experience, pathologists sometimes draw different conclusions from the same objective specimen. These discrepancies can delay the proper treatment for patients, and in extreme circumstances can cause legal problems. To resolve this issue, pathologists can specialize in certain diseases and/or ask experts with experience in a particular field for their opinions.
Errors relating to immunohistochemistry
Insufficient antibody testing
As summarized by Bridget S. Wilkins (3), immunohistochemistry-related errors are shown in Table 1.

Full table
In our experience, the most common error is insufficient range of antibody tests. With an insufficient range of tests, there may be inadequate diagnostic precision. For example, a splenic mantle cell lymphoma was categorized as marginal zone lymphoma and later diagnosed as DLBCL when the patient developed cervical lymph node enlargement. This misdiagnosis likely occurred because CD5 and Cyclin D1 expression were not initially examined (Figure 3).
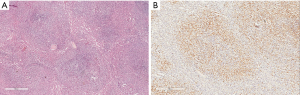
The use of an insufficient panel of immunostains can also cause the misdiagnosis of an ALK positive lymphoma as Hodgkin lymphoma or reactive lymphoid proliferation. Several outside medical centers were consulted for their opinion on a case and did not include ALK in their initial panel of antibodies (Figure 4).
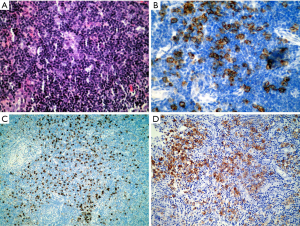
Knowledge of immunohistochemical staining in normal tissue compared to the various types of lymphomas
Familiarity with the immunohistochemical staining pattern of the normal lymph node and the variation observed in lymphoma is critical to the accurate diagnosis of lymphoma. Recognizing the different distribution pattern of CD20 and CD3 positive lymphocytes between normal and lymphoma conditions is essential for the correct diagnosis. In addition, B cell lymphoma-2 (Bcl-2) positivity in the follicular center must be evaluated by the number and distribution pattern of germinal center T cells. This is critical in differentiating follicular lymphoma from reactive proliferation. Furthermore, Bcl-2 positivity cannot be used to distinguish between follicular lymphoma and other small B cell lymphomas, because all of these conditions may express Bcl-2. Similarly, Cyclin D1 is positive in growth center lymphocytes of some small B cell lymphomas/chronic lymphocytic leukemia (CLL) and is also expressed by some plasmacytomas (Figure 5).
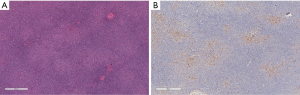
Specificity of antibodies
It is beneficial for pathologists to understand the specificity of antibodies. For example, it is important to know that a monoclonal antibody has specificity for one epitope on one antigen and not for the entire cell expressing that antigen. An antibody may be lineage-associated, for example CD20 and B cells or CD3 and T cells, but is not lineage-specific. Cross-lineage expression of antigens can occasionally occur, such as aberrant expression of CD20 in T and NK cell lymphomas or CD3 expression in non-T cell lymphomas. Additionally, it is rare that B cell lymphomas do not express CD20, but this does happen in some B cell lymphoblastic lymphomas, plasmablastic lymphomas and approximately 25% of rituximab-treated B cell lymphomas. Most monoclonal antibodies that are commonly used have been developed with high sensitivity for use with formalin fixed, paraffin-embedded tissues. However, some antibodies stain with less sensitivity than would be expected, and can vary with fixation, so that true positive cells may be missed. Examples of these antibodies include the CD5, CD10 and Bcl-6 antibodies, among others.
Non-hematolymphoid tumors may express some CD markers, such as CD45, however, this is rarely expressed in carcinomas and sarcomas (4,5). Malignant melanoma can be CD56 and CD117 positive and have an atypical plasmacytoid morphology, which can lead to the misdiagnosis of plasmacytoma. Moreover, immunohistochemistry is oftentimes laboratory specific, which can explain the variation in immunohistochemical staining between different groups. Therefore, it is essential that a pathologist be familiar with the staining results of a particular laboratory.
In addition to immunohistochemistry, flow cytometry is another important tool in diagnostic hematopathology. Flow cytometric immunophenotyping offers the sensitive detection of antigens when antibodies may not be available for formalin fixed paraffin-embedded immunohistochemical immunophenotyping. However, formalin fixed, paraffin-embedded immunohistochemical immunophenotyping is advantageous because it preserves the architecture of the tissue. Additionally, some antibodies are available for immunohistochemistry and not flow cytometry, allowing for the immunohistochemical evaluation of the expression of the proteins in which these antibodies target. Taken together, these techniques should be used as complimentary tools in diagnostic hematopathology.
Errors relating to molecular genetic tests
As more molecular and cytogenetic techniques are undertaken in the department of pathology, they become important supplementary methods to immunohistochemistry. These include clonal analysis of the IGH, IGK, IGL, TCR-B, and TCR-G rearrangements and the fusion genes IGH-BCL2, CCND-IGH, BCL6, MALT1, and MYC, among others. Compared with immunohistochemistry, these techniques are more complex and interpretation of the results requires specialized training. Analyzing these results involves understanding the sensitivity, specificity and limitations of each test. The presence of monoclonality does not necessarily correlate with malignancy, and the results must be interpreted in the context of clinical, morphological, immunohistochemical and other findings. Particularly with small biopsies, there can be pseudo-monoclonality which just reflects the physiological immunoreactions to the highly aggregated super antigen (6) rather than the malignancy. Fortunately, with the development of immunohistochemistry, many molecular changes can now be detected at the level of protein expression, with examples being ALK, MYC and mutation of the epidermal growth factor receptor (EGFR) at exons 19 and 21. It is likely that no more than 5% of lymphoma diagnoses will depend on molecular and cytogenetic tests in the near future.
Errors due to complexities in the classification and diagnosis of lymphomas
Errors in the classification of lymphomas are prevalent due to the complexities of the WHO classification system. The variability in an accurate diagnosis differs by each histological subtype of lymphoma. Pongpruttipan et al. (7) found that the chance for misdiagnosis was lowest for DLBCL, nodular sclerosis Hodgkin lymphoma, and subcutaneous penniculitis-like T cell lymphoma (SPTCL). They also indicated that, on average, even among hematopathologists, the frequency of misdiagnosis of lymphoma is approximately 9.6%. Among all of the misdiagnoses of lymphomas, the most imperative to distinguish between is reactive lymphoid disorders from truly neoplastic lesions, because misclassifications can have serious consequences in terms of treatment. Other types of lymphomas that are often misdiagnosed and the key points to properly diagnosing them are described in the following references (8-10). An example of an uncommon case with full morphological examination, immunophenotyping and molecular genetics is presented here to demonstrate the complexities encountered by experienced pathologists (Figure 6). In addition to the immunohistochemical results shown in Figure 6, the Epstein-Barr encoding region (EBER) in situ hybridization indicated that large B cells were present. Biomed-2 multiplex polymerase chain reaction (PCR) demonstrated clonal rearrangement of IGH (FR1-JH, FR3-JH) and TCR (Vβ + Jβ1, Vβ + Jβ2). The diagnoses by different pathologists included: marginal zone lymphoma with EBV positivity, atypical proliferation of lymphoid tissue, and angioimmunoblastic lymphoma with EBV positive large B cell lymphoma transformation. The patient died 6 months after the initial diagnosis.
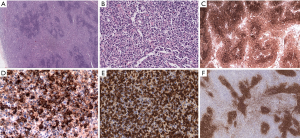
Conclusions
Adequate tissue specimens that are properly prepared and access to detailed background clinical information are essential to final pathological diagnoses. Approximately more than 90% of lymphoma cases can be accurately diagnosed using comprehensive morphological and immunophenotypical examination with the preselected antibody panels for immunostaining. Less than 10% of lymphoma cases are more difficult to diagnose and require molecular genetic testing. In-depth knowledge of the major categories of the WHO lymphoma classification system, awareness of ‘high-risk’ differential diagnoses, as well as collaborative communication and excellent report-writing skills are all important in avoiding pitfalls in the pathological diagnosis of lymphoma.
Acknowledgements
The author would like to thank all the colleagues in the department for their providing the difficult cases and preparation of the slides.
Disclosure: The author declares no conflict of interest.
References
- Li XH. Standardization of pathologic diagnosis of lymphomas. Zhonghua Bing Li Xue Za Zhi 2013;42:217-9. [PubMed]
- Liu Y, Lu SF, Zhou H, et al. Slide drying after staining is the culprit of poor hematoxylin-eosin staining. Zhonghua Bing Li Xue Za Zhi 2013;42:271-2. [PubMed]
- Wilkins BS. Pitfalls in lymphoma pathology: avoiding errors in diagnosis of lymphoid tissues. J Clin Pathol 2011;64:466-76. [PubMed]
- Houreih MA, Eyden BP, Reeve N, et al. Aberrant leukocyte common antigen expression in metastatic small cell lung carcinoma: a rare finding and a potential diagnostic pitfall. Appl Immunohistochem Mol Morphol 2007;15:236-8. [PubMed]
- McDonnell JM, Beschorner WE, Kuhajda FP, et al. Common leukocyte antigen staining of a primitive sarcoma. Cancer 1987;59:1438-41. [PubMed]
- Zhou XY. Application of clonal antigen receptor rearrangement in lymphoma diagnosis. Zhonghua Bing Li Xue Za Zhi 2010;39:347-9. [PubMed]
- Pongpruttipan T, Sitthinamsuwan P, Rungkaew P, et al. Pitfalls in classifying lymphomas. J Med Assoc Thai 2007;90:1129-36. [PubMed]
- Zhao XF. Pitfalls in diagnostic hematopathology: part I. Int J Clin Exp Pathol 2009;2:11-20. [PubMed]
- Zhao XF. Pitfalls in diagnostic hematopathology -- Part II. Int J Clin Exp Pathol 2009;3:39-46. [PubMed]
- Chan JK, Kwong YL. Common misdiagnoses in lymphomas and avoidance strategies. Lancet Oncol 2010;11:579-88. [PubMed]


