
Germline mutations and prostate cancer: is it time to change treatment algorithms?
Introduction
The most commonly diagnosed non-skin cancer in men is prostate cancer (PCa), affecting approximately 1 in 9 men during their lifetime in Western populations (1). Although early disease can be indolent without threatening life expectancy, advanced and metastatic castration-resistant forms remain incurable. In fact, PCa is the second leading cause of male cancer deaths in USA (1). One of the main therapeutic challenges in PCa is the heterogeneity of the disease in terms of clinical and molecular manifestations. Genetic mutations, specifically in DNA repair genes, are more prevalent than previously recognized and may be associated with aggressive prostate malignancies (2,3). As such, patients could benefit from stratified and personalized treatments derived from somatic or germline genetic testing for specific mutations such as BRCA1/BRCA2 and ATM. The National Comprehensive Cancer Network (NCCN) Prostate Cancer guidelines recently proposed testing for germline DNA repair gene (DRG) mutations in patients with high-risk and metastatic PCa, regardless of family history (4). Despite the many advances in the field, there are currently no clear algorithms or guidelines related to the use of germline genetic testing to guide treatment selection for patients with prostate malignancies. Work is ongoing in three key areas: the use of germline genetic testing to improve screening, establishing treatment algorithms for patients with known pathogenic germline or somatic mutations who are diagnosed with localized disease, and the use of genomic biomarkers to define treatment-selection for patients with advanced prostate cancer.
Genetic mutations in prostate cancer
The heterogeneity of PCa is partly attributed to genetic factors, which make up 57% of the interindividual risk variation (3). Several genetic mutations are implicated in familial PCa, and these include defects in DNA repair genes (DRGs) as listed in Table 1 (5,6). DRGs have an important role in carcinogenesis, since mutations in these genes will induce cells to continue replication without error correction.
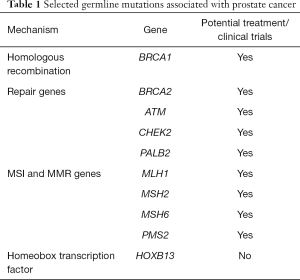
Full table
Germline pathogenic BRCA1/BRCA2 allele mutations have been found in a higher percentage of patients with various cancer types than previously anticipated and are associated with younger age at cancer diagnosis (7). In PCa, 11.8% of men with metastatic PCa harbor heritable (germline) mutations, with BRCA2 being the most common mutated gene (4,5,8,9). The prevalence of these mutations might not be ethnicity-specific, as is the case in breast cancer. Bhaskaran and colleagues sequenced 18 pathogenic mutations in Chinese and American patients with PCa and found no statistically significant difference between the two cohorts (10). Similarly, Wei et al. analyzed 316 Chinese and American men and demonstrated that germline mutations in metastatic as well as localized disease were similar between the two populations (5). Pritchard et al. found germline DRG mutations in a significantly higher percentage of patients with metastatic PCa versus localized disease (11.8% vs. 4.6%, respectively) in a study of 692 men (3). Moreover, the presence of DRG mutations is associated with higher Gleason score ≥8 and higher incidence of distant metastasis at diagnosis (6), in addition to poorer treatment outcomes as reported in several studies (3,6,11,12) and higher likelihood of having grade reclassification during active surveillance (12). These studies support the link between DRG mutations and the overall poorer outcomes in PCa patients.
With the advent of genetic testing in PCa, several essential questions have emerged: who to test, which genetic assay to use, what sample to test on, and which therapy to proceed with based on the results. Testing for these mutations may be performed via commercially available kits that typically use blood or saliva. However, there are no standardized panels for genetic testing, and the examined mutations differ between panels, which makes it challenging to select the appropriate test. In addition, widespread genetic testing for all patients diagnosed with PCa is unlikely to be cost-effective at present and may create some clinical confusion especially in early low-risk disease. Clear guidelines for treatment options after genetic testing based on validated results are lacking. For the purpose of this review, we will divide patients into three distinct categories based on their disease status.
Healthy men (with or without known germline mutations)
PCa is a heritable malignancy and men with first-degree PCa relatives have an approximately two-fold increased risk of PCa (13,14). Particularly, family history of cancers associated with BRCA2 mutations (breast, ovary, pancreas, bladder, melanomas) predisposes mutation-carriers to 2.5 to 8.6-fold higher risk of developing prostate cancer by the age of 65 compared to those who do not have the mutation (7,15,16). Effective screening measures can lead to early detection, treatment, and even cure of PCa. Current screening tools are comprised of digital rectal exam and prostatic serum antigen (PSA). Although PSA screening has decreased the proportion of men presenting with metastatic disease since its introduction in the 1980s, it has also given rise to a large portion of patients who are diagnosed with clinically indolent disease (17). Therefore, widespread PSA screening has been discouraged by many due to concerns regarding over-diagnosis, and many clinicians have advocated limiting screening to high-risk patients, such as those with a positive family history (4). The United States Preventative Task Force have recently renewed guidance for PSA screening in well-informed men age 55–69 as a grade C recommendation (18).
Genetic testing can potentially guide PCa screening by identifying men who have higher susceptibility more effectively. Currently, there are no clear PCa screening recommendations for carriers of pathogenic germline mutations. Some studies have promising results, for instance, the IMPACT study is an ongoing trial evaluating the role of PSA testing in BRCA1/BRCA2 mutation carriers and preliminary results so far support yearly PSA screening in patients aged 40 to 69 (19). Cheng et al. proposed an algorithm for prostate cancer screening in high-risk patients. They suggest baseline PSA and a digital rectal exam by the physician for men with known pathologic germline mutations or who have first/second-degree relatives with metastatic PCa regardless of genetic testing (20).
Polygenic risk scores (PRS)
PRS are calculated by combining multiple markers for a complex disease, that individually do not have much significance, into a final score that stratifies patients into risk groups. Successful PRS applications were achieved in several diseases including schizophrenia, multiple sclerosis, cardiovascular risk and other complex conditions with a polygenic component (21). Since 2007, Genome-wide association studies (GWAS) have identified germline mutations and more than 100 single nucleotide polymorphisms (SNPs) associated with increased risk of PCa. A single SNP alone has little contribution to PCa, however, the combined effect of multiple SNPs can account for a significant percentage to familial PCa risk (22). This could potentially limit PCa screening to only those at high risk with risk-stratified screening and could improve the benefit-to-harm ratio of screening by reducing false-positives, overdiagnosis and subsequent overtreatment of low-risk disease (23). PRS research in PCa initially had discouraging results, and Dudbridge et al. derived a formula and predicted that with larger sample sizes, researchers could get more successful analysis, and recommended researchers to make more informed decisions in planning their study instead of performing it opportunistically as is the case in most current studies (21). Genetic prediction only become relevant at very large sample sizes including tens of thousands of subjects, which could be possible with national biobanks and international gene consortia (21) A large international consortium, the Prostate Cancer Association Group to Investigate Cancer Associated Alterations in the Genome (PRACTICAL) is currently combining data which includes more than 120,000 patients from many studies on germline variants and is also validating new findings (24).
The Stockholm-1 study assessed the usefulness of PRS in determining prostate biopsy candidates for PCa diagnosis. The investigators created a genetic risk score from 35 validated SNPs, and a non-genetic risk score based on age, PSA, and family history, and applied both models to a cohort of 5,241 patients undergoing prostate biopsy. They found that the genetic model required 22.7% fewer biopsies but at the cost of missing 3% of patients with aggressive PCa (25). In another study, the calculated hazard score from 54 SNPs was a significant predictor of age at diagnosis of aggressive PCa even when family history was excluded from the model. Additionally, the positive predictive value of PSA screening increased with increasing polygenic hazard score (26).
Localized PCa
Genetic testing in select men diagnosed with localized PCa can be clinically relevant for patients and family members. The latest NCCN guidelines recommend germline genetic testing for patients with a positive family history, Ashkenazi Jewish ancestry, and intraductal histology (4). Also, active surveillance without additional interventions is strongly recommended for men with low-risk PCa and life expectancy less than 10 years (4). Similarly, the American College of Medical Genetics and Genomics (ACMG) and the National Society of Genetic Counselors (NSGC) recommend referral of patients to genetic counseling, specifically those with strong history of PCa in first degree relatives (27).
Risk prediction tools for BRCA1 and BRCA2 are used in breast and ovarian cancer patients to identify the individuals who are more likely to carry a mutation. Oliva et al. tested two such mathematical risk calculation models (BRCAPRO and the Manchester Scoring System) in PCa patients based on personal and family history, age, and ethnicity. The two models underperformed and are hence not recommended (28).
Advanced PCa
Despite the efficacy of androgen deprivation therapy in metastatic PCa, patients often progress to castration-resistant disease which is incurable and fatal. Moreover, advanced PCa patients with germline pathogenic mutations may have more aggressive disease and poorer outcomes, as discussed above. As such, the NCCN guidelines recommend all high-risk and advanced PCa patients to undergo germline genetic testing. Germline DRG mutations are emerging as predictive biomarkers for response to novel targeted therapies. Defects in homologous recombination repair genes suggest potential susceptibility to poly ADP ribose polymerase inhibitors (PARPi) and platinum-based chemotherapy; whereas microsatellite instability and mismatch repair gene mutations could predict response to immune checkpoint inhibitors and make patients eligible for pertinent clinical trials. It is important to note that somatic mutations may arise in the tumor tissue due to genetic instability with disease progression and therapy, so repeating genetic testing on tumor DNA in these patients might be favorable (20). Genetic testing for somatic mutations is outside the scope of this review.
PARP inhibitors
DNA damage repair pathways prevent replication collapse in cells exposed to endogenous and exogenous DNA damage. The nuclear enzyme poly ADP ribose polymerase (PARP) plays a key role in this pathway. More specifically, PARP complex binds to DNA with single-strand breaks and initiates repair by using the contralateral DNA strand, and if the repair is unsuccessful, programmed cell death ensues. PARP inhibitors (PARPi) trap the PARP complex blocking repair and eventually leading to a double-strand break and cell death. PARPi have emerged as attractive therapies and are FDA-approved for ovarian and breast cancers since they ensue cellular toxicity in cells deficient in BRCA1/BRCA2. Usually, bi-allelic loss is required for sensitization. In recent years, several PARPi have been under study in patients with prostate malignancies.
Available PARPi such as olaparib, niraparib, and rucaparib are taken in a tablet form by mouth, and they are generally well tolerated. The most common side effects of olaparib include hematologic toxicity (anemia), fatigue, nausea and vomiting. Side effect profile of niraparib is similar to olaparib, with hematologic toxicities such as anemia and thrombocytopenia being the most common.
Mateo et al. conducted two phase II clinical trials on the use of the PARPi olaparib in metastatic CRPC (mCRPC) patients who had progression of disease after at least one regimen of chemotherapy. In TOPARP-A, which was reported in 2015, fifty patients with mCRPC received olaparib as monotherapy, with 33% having a statistically significant response. The investigators conducted biomarker studies for both somatic and germline DNA on fresh tissue biopsy samples and 33% of patients had DRG mutations, 88% of which had a response to olaparib. Radiographic progression-free survival was 9.8 months in patients with DRG mutations versus 2.7 months in patients without (11). In TOPARP-B, patients with mCRPC who had progression of disease after at least one chemotherapy regimen underwent DNA sequencing on tumor biopsies, and patients were eligible to participate if DRG mutations were detected. The results indicate that olaparib has the greatest effect against heavily pretreated mCRPC with DRG mutations, the highest response being in tumors with BRCA1/2 aberrations (29).
PCa is a BRCA-associated cancer and studies have shown the clinical benefit of PARPi in BRCA-associated cancers, with similar response in both somatic and germline BRCA1/BRCA2 mutations (7). Regarding ATM mutations, Marshall et al. reported that mCRPC patients harboring ATM mutations had inferior outcomes to olaparib compared to those with BRCA1/BRCA2 mutations (30). Similarly, in TRITON2, the investigators found that ATM mutation carriers did not respond to rucaparib. This is in contrast to the TOPARP-A trial in which four out of six patients with ATM mutation initially responded to olaparib, although only two of these patients maintained a PSA response at week 12 (11).
The majority of current PARPi trials focus on mCRPC (Table 2), and it is not yet clear if patients with earlier stages of disease may benefit from PARPi treatment. The phase III PROfound trial which was recently published with positive results for olaparib in men with mCRPC with BRCA1/2 or ATM gene mutations. Trial data showed a statistically-significant and clinically-meaningful improvement in the primary endpoint of radiographic progression-free survival with olaparib compared to enzalutamide or abiraterone (31). This led olaparib to be granted FDA approval for mCRPC with germline and somatic homologous recombination repair gene mutations (32). Similarly, rucaparib was recently granted FDA approval for BRCA-associated mCRPC (33).
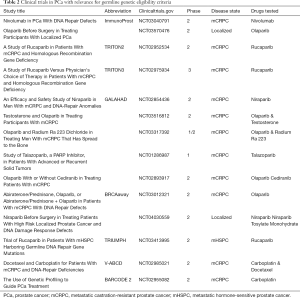
Full table
Another area of growing research is the combination of PARPi and androgen receptor (AR) blockade. PARP has been associated with a protumor effect by promoting AR transcription in AR-positive prostate cells (34). Also, AR blockade upregulates PARP, and high PARP activity is observed in men treated with androgen deprivation therapy. This has led to studies investigating the role of the combination in PCa, with one phase II trial favoring the combination. The median progression-free survival was 13.8 months with olaparib and abiraterone compared with 8.2 months with abiraterone alone (35).
Platinum-based chemotherapy
Platinum-based chemotherapies are currently being investigated as concurrent therapies with PARPi They have a similar mechanism of action to PARPi by causing double-strand DNA breaks, and the combination could potentially increase tumor cell death. A study used the cisplatin-olaparib combination in breast and ovarian tumors carrying BRCA1/BRCA2 mutations with promising results, however significant hematologic toxicities limited the further use of this combination (36). So far, FDA has not approved platinum-based chemotherapies for PCa, and further studies are needed to explore their possible clinical benefit.
Immunotherapy
The anti-PD1 immune checkpoint inhibitor, pembrolizumab, is FDA-approved for several solid tumors with microsatellite instability (MSI). Impaired DNA mismatch repair (MMR) predisposes the DNA to genetic hypermutability which leads to an MSI phenotype and can be the result of germline or somatic mutations. Le et al. reported favorable responses to anti PD-1 immunotherapy in different tumors with MMR deficiencies in a phase II clinical trial. 53% of patients achieved objective radiographic response, and 21% of patients had complete response. These data suggest that hypermutability in mismatch repair–deficient cancers make them sensitive to immune checkpoint blockade, regardless of the cancers’ tissue of origin (37). This has important implications in PCa as a good percentage of patients demonstrate such mutations. Pritchard et al. reported that 12% of advanced PCa patients have a hypermutated subtype which are associated with MMR mutations (MSH2, MSH6) (3). Abida and colleagues recruited 1,033 patients who underwent tumor molecular profiling, 3.2% (33 patients) carried a mutation for MSI-H/dMMR, and 11 patients received pembrolizumab with promising results (38). The NCCN now recommend pembrolizumab therapy as a second-line therapy for patients with metastatic disease found to have the MSI-H/dMMR phenotype regardless of histology (4).
Genetic counseling and barriers to genetic testing
Genetic counseling should ideally be provided to all individuals undergoing germline genetic testing. Counseling can aid patients understand the medical, legal, ethical and psychological aspects of genetic testing and make informed decisions regarding treatment options. “Cascade genetic testing” is the systematic process which ensures appropriate education and testing of family members, and, if positive, enhanced cancer screening or risk-reduction strategies (20). Genetic testing performed without proper genetic counseling by qualified physicians or specialists can lead to misinterpretation of results, psychological distress, and inappropriate management.
Barriers to genetic testing have been described in several familial malignancies including breast and ovarian cancers, the most common barriers being technical issues (referral to counselors, access, cost and insurance coverage); physician knowledge and comfort with the process; patients’ lack of awareness (39). The Germline Genetics Working Group (GGWG) of the Prostate Cancer Clinical Trials Consortium (PCCTC) was established in June 2017 in order to collaborate with clinicians and researchers to guide and better inform physicians of genetic testing in PCa. In June 2018, the GGWG introduced a framework to address challenges related to germline testing in patients with advanced PCa. The GGWG developed a survey to which 26 genitourinary oncologists in 19 US institutions responded. Similar to genetic testing in other malignancies, the three most common barriers were found to be: limited access to genetic counseling, lack of effective workflow systems, and no insurance coverage (40). More than half of the participating oncologists reported taking personal responsibility for some or all genetic assessment (40) and only 10 of 26 oncologists (38%) reported referring patients to a separate department for this matter. A worsening shortage of genetic counselors is being recognized, which is paving way for oncologists to pursue further subspecialty training in genetics. Assessing physician practice patterns and barriers regarding genetic testing is fundamental in order to make gene assays a part of everyday clinical practice, and appropriate patient education may allow patients to be more interested in pursuing testing. Rigorous efforts are needed to ensure that genetic counseling and testing is available to underserved populations, including ethnic and racial minorities, people of low income, and in limited resource environments.
Conclusions
DRG mutations are present in a significant minority of PCa patients and cannot be predicted from clinical features. Gene assays in everyday clinical practice have the potential to stratify patients into defined subgroups, aiding therapeutic decision-making in the era of precision medicine. To date, the tailored therapies that are being studied in prostate cancer include PARPi, immunotherapy and platinum-based chemotherapy among many others. Response rates to PARPi and other novel treatments differ between subgroups, and currently no specific genomic assay can be recommended over another. Large-scale multi-center studies are needed, in addition to head-to-head comparisons of the different available treatment options. The positive results of the phase III PROfound study showing the benefit of olaparib in PCa patients with BRCA and ATM mutations has changed treatment algorithms and expanded treatment options for a defined subgroup of patients. Close collaboration between oncologists, urologists, clinical geneticists, researchers, and patients along with their families, will ensure the best long-term management of PCa.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Shaheenah Dawood) for the series “Targeting the DNA Damaging Pathway: PARPi and Beyond” published in Chinese Clinical Oncology. The article was sent for external peer review organized by the Guest Editor and the editorial office.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/cco-19-207). The series “Targeting the DNA Damaging Pathway: PARPi and Beyond” was commissioned by the editorial office without any funding or sponsorship. DM reports grants and personal fees from Astellas, grants and personal fees from Jannsen, grants and personal fees from Merck, grants and personal fees from Roche, personal fees from Astra-Zeneca, personal fees from Sanofi, personal fees from BMS, personal fees from MSD, outside the submitted work. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cancer Facts & Figures 2020. American Cancer Society. Atlanta, GA 2020.
- Sumanasuriya S, De Bono J. Treatment of Advanced Prostate Cancer-A Review of Current Therapies and Future Promise. Cold Spring Harb Perspect Med 2018;8:a030635. [Crossref] [PubMed]
- Pritchard CC, Mateo J, Walsh MF, et al. Inherited DNA-Repair Gene Mutations in Men with Metastatic Prostate Cancer. N Engl J Med 2016;375:443-53. [Crossref] [PubMed]
- Mohler JL, Antonarakis ES, Armstrong AJ, et al. Prostate Cancer, Version 2.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2019;17:479-505. [Crossref] [PubMed]
- Wei Y, Wu J, Gu W, et al. Germline DNA Repair Gene Mutation Landscape in Chinese Prostate Cancer Patients. Eur Urol 2019;76:280-3. [Crossref] [PubMed]
- Castro E, Goh C, Olmos D, et al. Germline BRCA Mutations Are Associated With Higher Risk of Nodal Involvement, Distant Metastasis, and Poor Survival Outcomes in Prostate Cancer. J Clin Oncol 2013;31:1748-57. [Crossref] [PubMed]
- Jonsson P, Bandlamudi C, Cheng ML, et al. Tumour lineage shapes BRCA-mediated phenotypes. Nature 2019;571:576-9. [Crossref] [PubMed]
- Robinson D, Van Allen EM, Wu YM, et al. Integrative Clinical Genomics of Advanced Prostate Cancer. Cell 2015;162:454. [Crossref] [PubMed]
- Leongamornlert DA, Saunders EJ, Wakerell S, et al. Germline DNA Repair Gene Mutations in Young-onset Prostate Cancer Cases in the UK: Evidence for a More Extensive Genetic Panel. Eur Urol 2019;76:329-37. [Crossref] [PubMed]
- Bhaskaran SP, Chandratre K, Gupta H, et al. Germline variation in BRCA1/2 is highly ethnic-specific: Evidence from over 30,000 Chinese hereditary breast and ovarian cancer patients. Int J Cancer 2019;145:962-73. [Crossref] [PubMed]
- Mateo J, Carreira S, Sandhu S, et al. DNA-Repair Defects and Olaparib in Metastatic Prostate Cancer. N Engl J Med 2015;373:1697-708. [Crossref] [PubMed]
- Carter HB, Helfand B, Mamawala M, et al. Germline Mutations in ATM and BRCA1/2 Are Associated with Grade Reclassification in Men on Active Surveillance for Prostate Cancer. Eur Urol 2019;75:743-9. [Crossref] [PubMed]
- Hemminki K. Familial risk and familial survival in prostate cancer. World J Urol 2012;30:143-8. [Crossref] [PubMed]
- Bruner DW, Moore D, Parlanti A, et al. Relative risk of prostate cancer for men with affected relatives: systematic review and meta-analysis. Int J Cancer 2003;107:797-803. [Crossref] [PubMed]
- Cancer risks in BRCA2 mutation carriers. J Natl Cancer Inst 1999;91:1310-6. [Crossref] [PubMed]
- Lecarpentier J, Silvestri V, Kuchenbaecker KB, et al. Prediction of Breast and Prostate Cancer Risks in Male BRCA1 and BRCA2 Mutation Carriers Using Polygenic Risk Scores. J Clin Oncol 2017;35:2240-50. [Crossref] [PubMed]
- Welch HG, Gorski DH, Albertsen PC. Trends in Metastatic Breast and Prostate Cancer--Lessons in Cancer Dynamics. N Engl J Med 2015;373:1685-7. [Crossref] [PubMed]
- Final Update Summary: Prostate Cancer: Screening. U.S. Preventive Services Task Force. Available online: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/prostate-cancer-screening1. April 2019.
- Mitra AV, Bancroft EK, Barbachano Y, et al. Targeted prostate cancer screening in men with mutations in BRCA1 and BRCA2 detects aggressive prostate cancer: preliminary analysis of the results of the IMPACT study. BJU Int 2011;107:28-39. [Crossref] [PubMed]
- Cheng HH, Sokolova AO, Schaeffer EM, et al. Germline and Somatic Mutations in Prostate Cancer for the Clinician. J Natl Compr Canc Netw 2019;17:515-21. [Crossref] [PubMed]
- Dudbridge F. Power and predictive accuracy of polygenic risk scores. PLoS Genet 2013;9:e1003348. [Crossref] [PubMed]
- Al Olama AA, Kote-Jarai Z, Berndt SI, et al. A meta-analysis of 87,040 individuals identifies 23 new susceptibility loci for prostate cancer. Nat Genet 2014;46:1103-9. [Crossref] [PubMed]
- Pashayan N, Duffy SW, Neal DE, et al. Implications of polygenic risk-stratified screening for prostate cancer on overdiagnosis. Genet Med 2015;17:789-95. [Crossref] [PubMed]
- Wu L, Wang J, Cai Q, et al. Identification of Novel Susceptibility Loci and Genes for Prostate Cancer Risk: A Transcriptome-Wide Association Study in Over 140,000 European Descendants. Cancer Res 2019;79:3192-204. [Crossref] [PubMed]
- Aly M, Wiklund F, Xu J, et al. Polygenic risk score improves prostate cancer risk prediction: results from the Stockholm-1 cohort study. Eur Urol 2011;60:21-8. [Crossref] [PubMed]
- Seibert TM, Fan CC, Wang Y, et al. Polygenic hazard score to guide screening for aggressive prostate cancer: development and validation in large scale cohorts. BMJ 2018;360:j5757. [Crossref] [PubMed]
- Hampel H, Bennett RL, Buchanan A, et al. A practice guideline from the American College of Medical Genetics and Genomics and the National Society of Genetic Counselors: referral indications for cancer predisposition assessment. Genet Med 2015;17:70-87. [Crossref] [PubMed]
- Oliva L, Lozano R, Llácer C, et al. Risk Prediction Tools Available for Germline BRCA1/2Mutations Underperform in Prostate Cancer Patients. Eur Urol Oncol 2019. [Epub ahead of print]. [Crossref] [PubMed]
- Mateo J, Porta N, McGovern UB, et al. TOPARP-B: A phase II randomized trial of the poly(ADP)-ribose polymerase (PARP) inhibitor olaparib for metastatic castration resistant prostate cancers (mCRPC) with DNA damage repair (DDR) alterations. J Clin Oncol 2019;37:5005. [Crossref]
- Marshall CH, Fu W, Wang H, et al. Prevalence of DNA repair gene mutations in localized prostate cancer according to clinical and pathologic features: association of Gleason score and tumor stage. Prostate Cancer Prostatic Dis 2019;22:59-65. [Crossref] [PubMed]
- de Bono J, Mateo J, Fizazi K, et al. Olaparib for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med 2020;382:2091-102. [Crossref] [PubMed]
- FDA approves olaparib for HRR gene-mutated metastatic castration-resistant prostate cancer. FDA Drug Approvals And Databases. 2020. Available online: https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-olaparib-hrr-gene-mutated-metastatic-castration-resistant-prostate-cancer#:~:text=On%20May%2019%2C%202020%2C%20the,(mCRPC)%2C%20who%20have%20progressed. Accessed 10/06/2020.
- FDA grants accelerated approval to rucaparib for BRCA-mutated metastatic castration-resistant prostate cancer U.S. Food and Drug Administration. 2020. Available online: https://www.fda.gov/drugs/fda-grants-accelerated-approval-rucaparib-brca-mutated-metastatic-castration-resistant-prostate#:~:text=FDA%20grants%20accelerated%20approval%20to%20rucaparib%20for%20BRCA,metastatic%20castration%2Dresistant%20prostate%20cancer&text=On%20May%2015%2C%202020%2C%20the,%2C%20Clovis%20Oncology%2C%20Inc.
- Schiewer MJ, Goodwin JF, Han S, et al. Dual roles of PARP-1 promote cancer growth and progression. Cancer Discov 2012;2:1134-49. [Crossref] [PubMed]
- Clarke N. Is combining PARP and androgen receptor inhibition really a winning strategy in metastatic castration-resistant prostate cancer? - Authors' reply. Lancet Oncol 2018;19:e438. [Crossref] [PubMed]
- Balmaña J, Tung NM, Isakoff SJ, et al. Phase I trial of olaparib in combination with cisplatin for the treatment of patients with advanced breast, ovarian and other solid tumors. Ann Oncol 2014;25:1656-63. [Crossref] [PubMed]
- Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017;357:409-13. [Crossref] [PubMed]
- Abida W, Cheng ML, Armenia J, et al. Analysis of the Prevalence of Microsatellite Instability in Prostate Cancer and Response to Immune Checkpoint Blockade. JAMA Oncol 2019;5:471-8. [Crossref] [PubMed]
- Carlo MI, Giri VN, Paller CJ, et al. Evolving Intersection Between Inherited Cancer Genetics and Therapeutic Clinical Trials in Prostate Cancer: A White Paper From the Germline Genetics Working Group of the Prostate Cancer Clinical Trials Consortium. JCO Precis Oncol 2018;2018:. [Crossref]
- Paller CJ, Antonarakis ES, Beer TM, et al. Germline Genetic Testing in Advanced Prostate Cancer; Practices and Barriers: Survey Results from the Germline Genetics Working Group of the Prostate Cancer Clinical Trials Consortium. Clin Genitourin Cancer 2019;17:275-82.e1. [Crossref] [PubMed]


