
Adjuvant endocrine treatment for estrogen receptor (ER)-positive/HER2-negative breast cancer
Introduction
The incidence of breast cancer in East Asia has been increasing over the past decade compared with the United States (US) and Western Europe, which have decreasing incidences but rates that remain high (1-3). Rates in Japan, Korea, Singapore, and China have doubled or tripled during this time (1,3,4). Moreover, East Asian female breast cancer has the epidemiological characteristic of higher breast cancer incidence in younger women compared with the US (3), as well as the clinicopathological characteristic of higher hormone receptor (HR)-positivity in younger women compared with older (>50 years) women (5). Therefore, an endocrine therapy strategy is essential for East Asian breast cancer patients, especially for premenopausal and perimenopausal woman.
In premenopausal women, estrogen is mainly supplied from the ovaries. Endocrine therapy for breast cancer began with the efficacy of bilateral oophorectomy for advanced breast cancer performed by Beatson, as reported by Stockwell in 1896 (6). In recent years, the use of surgery for ovarian function suppression (OFS) has been surpassed by the use of drug therapies such as luteinizing hormone-releasing hormone (LH-RH) agonists. After the discovery of the estrogen receptor (ER) in the 1960s and the development of tamoxifen, endocrine therapy with drugs became the mainstay of treatment. Tamoxifen, a critical drug in breast cancer endocrine therapy, suppresses the estrogen-dependent growth of breast cancer by competitively inhibiting the binding of estrogen to ER in the nuclei of breast cancer cells (7). Therefore, the accurate assessment and interpretation of ER and progesterone receptor (PR) expression by immunohistochemistry (IHC) are fundamentally important. According to the American Society of Clinical Oncology/College of American Pathologists (ASCO/CAP), breast cancer samples with 1% to 100% ER-positive tumor nuclei should be interpreted as ER-positive (8). Among patients with ER-positive disease, the reductions in recurrence and breast cancer mortality rates were found to be highly significant in some trials of approximately 5 years of tamoxifen (15-year gain: 11.8% and 9.2%, respectively), regardless of tamoxifen dose, use of chemotherapy, entry age, and nodal status (9), and thus the efficacy of tamoxifen for HR-positive breast cancer is established.
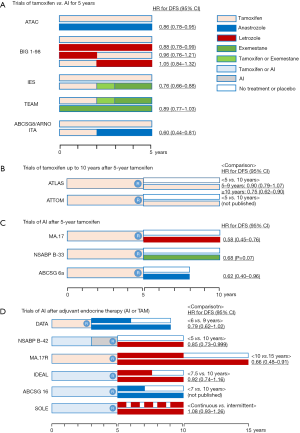
In postmenopausal women, androgen secreted from the adrenal glands is converted to estrogen by aromatase in peripheral tissues and is supplied throughout the body. In adjuvant endocrine therapy for postmenopausal patients with HR-positive breast cancer, 5 years of treatment with third-generation aromatase inhibitors (AIs) such as anastrozole (ATAC trial) (10), letrozole (BIG 1-98 trial) (11), and exemestane (TEAM trial) (12) improved disease-free survival (DFS) compared with 5 years of tamoxifen (Figure 1A). In addition, combined analyses (n=9,856) based on the ATAC and BIG1-98 trials showed that AIs reduced recurrence by 23% compared with tamoxifen but failed to reduce breast cancer mortality (13). Meanwhile, meta-analyses (n=31,920) based on randomized trials of 5 years of tamoxifen vs. 5 years of AIs reported that the latter reduced 10-year recurrence by 20% and mortality by 15% compared with tamoxifen (14), and hence the superior efficacy of AIs over tamoxifen for HR-positive postmenopausal breast cancer is established.
In this review, the rationale for adjuvant endocrine treatment is described by summarizing the results of clinical trials and the updated guidelines from ASCO, the National Comprehensive Cancer Network (NCCN), and the European Society of Medical Oncology (ESMO).
Premenopausal patients
According to the updated meta-analyses of randomized trials of the efficacy of adjuvant tamoxifen, 5 years of tamoxifen for HR-positive disease (n=10,645) safely reduces 15-year risks of breast cancer recurrence [rate ratio (RR) 0.61, 95% confidence interval (CI): 0.57–0.65] and breast cancer death (RR 0.70, 95% CI: 0.64–0.75), regardless of quantitative ER and PR measurement, dose of tamoxifen, use of chemotherapy, entry age, nodal status, tumor differentiation, and diameter and site of first recurrence (15). For entry ages younger than 45 and 55–69 years, significant reductions in breast cancer mortality of 10.6% and 12.7% were observed (RR 0.71, 95% CI: 0.61–0.83 and RR 0.63, 95% CI: 0.56–0.71, respectively). However, there is not enough evidence to show the efficacy of endocrine therapy for HR-positive, node-negative breast cancer with a tumor diameter of 5 mm or less (T1aN0). The NCCN guidelines recommend “considering” adjuvant endocrine therapy for HR-positive, node-negative breast cancer (T1aN0) (16).
It is well-known that amenorrhea after chemotherapy in premenopausal women with HR-positive, node-positive breast cancer is associated with a significant improvement in both DFS and overall survival (OS) [hazard ratio (HR) 0.70 and HR 0.76, respectively] based on the results of the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-30 (17). This phenomenon revealed the efficacy of OFS for premenopausal women with HR-positive breast cancer. In the integrated analyses of the Suppression of Ovarian Function Trial (SOFT, n=3,066) and the Tamoxifen and Exemestane Trial (TEXT, n=2,672), the addition of OFS to tamoxifen significantly improved both 8-year DFS (HR 0.76, 95% CI: 0.62–0.93) and OS (HR 0.67, 95% CI: 0.48–0.92) compared with tamoxifen alone for premenopausal patients with HR-positive breast cancer (18). Moreover, it was reported that younger age at random assignment years, a high number of positive nodes, larger tumor size, weaker ER expression, weaker PR expression, higher tumor grade, and stronger Ki-67 expression were risk factors (19), and thus the treatment strategy should be selected considering these factors. The frequency of adverse events such as hot flashes, insomnia, and hypertension was higher in the OFS group than in the tamoxifen alone group. Next, the use of exemestane plus OFS resulted in significantly higher 8-year rates of freedom from recurrence than tamoxifen plus OFS (HR 0.73, 95% CI: 0.55–0.96), but did not improve OS (18).
In the St. Gallen Consensus Conference 2019 on early breast cancer treatment standards in Vienna, for a 33-year-old patient (pN+ ER+ PR+ G3, adjuvant chemotherapy planned), 57.1% of all panelists would select OFS plus either tamoxifen or an AI (20). A total of 55.1% considered that 5 years was an appropriate duration for OFS, based on the SOFT and TEXT trials. The ESMO practice guidelines ‘strongly’ recommend that the addition of OFS to adjuvant endocrine therapy should be considered for premenopausal women with ER-positive/HER2-negative and higher-risk tumors (21), as well as the ASCO clinical practice guidelines (22). Also, the ASCO guidelines state that 5 years is the standard duration for OFS with either tamoxifen or an AI such as exemestane.
Postmenopausal patients
Five years of third generation AIs has become the standard adjuvant therapy for postmenopausal patients with HR-positive breast cancer, as described above (Figure 1A). There is no significant difference in the frequency of serious adverse events, but the profiles for individual adverse events are different. In the ATAC trial (10), there were fewer endometrial cancers [odds ratio (OR) 0.25, 95% CI: 0.08–0.63] and more fractures (OR 1.33, 95% CI: 1.15–1.55) in the anastrozole group than in the tamoxifen group. However, there was no significant difference in the incidence of fractures between the two groups after treatment completion, which indicates that the effect of anastrozole on bone mineral density and fracture risk was promptly attenuated after the administration of anastrozole had been finished. Meanwhile, in the BIG 1-98 trial (11), there was a higher incidence of thromboembolic events in the tamoxifen-included regimen groups than in the letrozole monotherapy group (4.1–4.9% vs. 2.4%, P<0.001). Hot flashes and night sweats occurred more frequently in the tamoxifen-included regimen groups than in the letrozole monotherapy group (hot flashes: 41.7–44.0% vs. 37.7%, P=0.003; night sweats: 17.8–19.4% vs. 15.6%, P=0.04). Arthralgia, myalgia, or both were more frequent in the letrozole-included regimen groups than in the tamoxifen monotherapy group (31.9–34.7% vs. 30.1%, P=0.05).
According to meta-analyses (n=31,920) based on the randomized trials of 5 years of adjuvant endocrine therapy including tamoxifen and AIs, 2–3 years of tamoxifen followed by AIs up to 5 years was similar to 5 years of AIs in terms of 7-year recurrence and breast cancer mortality, and significantly superior to 5 years of tamoxifen in terms of 10-year recurrence (RR 0.82, 95% CI: 0.75–0.91, 10-year gain 2.0%) and breast cancer mortality (RR 0.84, 95% CI: 0.72–0.96, 10-year gain 1.5%) (15). In the BIG 1-98 trial (23) for postmenopausal women with HR-positive early breast cancer, sequential “switch” therapies including tamoxifen and letrozole did not improve outcome compared with letrozole monotherapy, but might be useful strategies when considering an individual patient’s treatment tolerability because sequential regimens did not mean inferiority to letrozole monotherapy.
Duration of adjuvant endocrine therapy
Five randomized clinical trials, ATLAS (24), NSABP B-14 (25), Scottish (26), ECOG (27), and ATTOM, examined the extended administration of tamoxifen after 5 years of adjuvant tamoxifen (Figure 1B). Of these, the ATTOM trial was a large-scale study but was not published. The NSABP B-14 study included only node-negative patients and was not examined with an intention to treat (ITT) analysis. The Scottish trial and the ECOG trial included HR-negative cases. The ATLAS trial did not have a placebo control but was a large-scale study and reported the results of treatment for HR-receptor positive patients. In the ATLAS trial, patients with HR-positive breast cancer, who had received 5 years of adjuvant tamoxifen, were randomly assigned to continue tamoxifen up to 10 years or to stop at 5 years. ATLAS showed that, compared with 5 years of tamoxifen, 10 years of tamoxifen provided significantly further benefit to women with HR-positive breast cancer in recurrence (5–9 years: RR 0.90; ≥10 years: RR 0.75; and all years: P=0.002) and breast cancer mortality (5–9 years: RR 0.97; ≥10 years: RR 0.71; and all years: P=0.01), and its benefit with endocrine therapy continued particularly after reaching 10 years, which is considered a “carry over” effect (24). For the incidence (hospitalization or death) rates of specific diseases, RRs were pulmonary embolus 1.87 (95% CI: 1.13–3.07, P=0.01), ischemic heart disease 0.75 (95% CI: 0.60–0.95, P=0.02), and endometrial cancer 1.74 (95% CI: 1.30–2.34, P=0.0002). The cumulative risk of endometrial cancer during years 5–14 was 3.1% (mortality 0.4%) for women assigned to extend tamoxifen vs. 1.6% (mortality 0.2%) for controls (24). Therefore, the absolute mortality increased by only 0.2%. Also, ASCO guidelines strongly recommended that premenopausal or perimenopausal women with HR-positive breast cancer should receive additional endocrine therapy after 5 years of adjuvant tamoxifen (28).
Three randomized clinical trials, MA.17 (29), NSABP B-33 (30), and ABCSG 6a (31) investigated the extended administration of AIs after 5 years of tamoxifen (Figure 1C). Of these, the MA.17 trial was a large-scale, randomized, double-blind trial of 5 years of letrozole (n=2,583) vs. placebo (n=2,587) after 4.5–6 years of adjuvant tamoxifen for postmenopausal women with HR-positive breast cancer. This study was stopped early after an interim analysis showed improved DFS with letrozole. After a median follow-up of 30 months (range, 1.5–61.4 months) women in the letrozole group had significantly better DFS (HR 0.58, 95% CI: 0.45–0.76) than women in the placebo group. Although OS was similar in both groups, OS in node-positive patients was significantly improved with letrozole (HR 0.61, 95% CI: 0.38–0.98) (29). Meanwhile, hot flashes, anorexia, arthralgia, myalgia, and alopecia occurred significantly more frequent with letrozole. Also, osteoporosis was newly diagnosed in 8.1% of the letrozole group and 6.0% of the placebo group (P=0.003), but the incidence of bone fractures was similar in the two groups. The NSABP B-33 study was a double-blind trial of 5 years of exemestane (n=783) vs. placebo (n=779) after 5 years of adjuvant tamoxifen for postmenopausal women with HR-positive breast cancer (30). Results of the MA.17 trial revealed that the benefit of extended letrozole affected the progression of NSABP B-33 study. After unblinding, 560 (72%) in the exemestane group continued exemestane and 344 (44%) in the placebo group switched to exemestane. The original exemestane group showed an improvement in 4-year DFS (91% for exemestane vs. 89% for placebo; RR 0.68, P=0.07) and relapse-free survival (96% for exemestane vs. 94% for placebo; RR 0.44, P=0.004) at 30 months of median follow-up. The ABCSG 6a trial is an extension of the ABCSG 6 trial for postmenopausal women, with HR-positive breast cancer receiving 5 years of adjuvant tamoxifen. For the ABCSG 6a trial, disease-free patients were randomly assigned to receive either 3 years of anastrozole or no further treatment. At 62.3 months of median follow-up, patients with 3 years of anastrozole had a 38% risk reduction of recurrence (HR 0.62, 95% CI: 0.40–0.96). ASCO guidelines strongly recommend that postmenopausal women with HR-positive breast cancer should continue tamoxifen or switch to an AI for a total duration of 10 years of adjuvant endocrine therapy (28).
Six randomized clinical trials, MA.17R (32), NSABP B-42 (33), DATA (34), ABCSG 16 (35), IDEAL (36), and SOLE (37) studied the extended administration of AIs after adjuvant endocrine therapy for postmenopausal women with stage I–III HR-positive breast cancer (Figure 1D). Of these, the ABCSG 16 trial, which included 3,484 disease-free women after adjuvant therapy with tamoxifen who received an AI or tamoxifen followed by an AI assigned to either 2 or 5 years of extended anastrozole (35), was presented at the San Antonio Breast Cancer Symposium 2017, but was not published. The purpose of this trial was to compare 7 years of adjuvant endocrine therapy including anastrozole with 10 years. The MA.17R trial studied extended letrozole compared with placebo for 5 years in 1,918 patients after adjuvant AI therapy, which was preceded in most women by treatment with tamoxifen (32). The NSABP B-42 study compared extended letrozole with placebo for 5 years in 3,923 patients after 5 years of adjuvant endocrine therapy (33). The DATA trial compared extended anastrozole for either 3 or 6 years in 1,660 patients after 2 to 3 years of adjuvant tamoxifen (34). The IDEAL trial compared extended letrozole for either 2.5 or 5 years in 1,824 patients after 5 years adjuvant endocrine therapy (36). The SOLE trial compared extended letrozole for 5 years of a continuous or an intermittent schedule in 4,884 women with node-positive breast cancer after 5 years of adjuvant endocrine therapy. In all trials, DFS was considered the primary outcome. The MA.17R trial reported significantly higher 5-year rates of DFS (HR 0.66, 95% CI: 0.48–0.91) in the extended letrozole group compared with the placebo group at a median follow-up of 6.3 years (32). The rate of 5-year OS was the same in the two groups. The NSABP B-42 (33), DATA (34), and IDEAL (36) trials resulted in a trend toward better DFS with extended AI therapy, but there was no statistical significance (38). There was no difference in DFS shown in the ABCSG 16 trial (35) of 10 vs. 7 years of extended AI therapy and in the SOLE study (37) of intermittent vs. continuous extended AI therapy up to a total of 10 years. Finally, extended AI therapy did not improve OS in any of the six trials (38). However, the MA.17R (32) and NSABP B-42 (33) trials reported that the risk of breast cancer recurrence or contralateral breast cancer was significantly reduced by 34% and 28%, respectively. Extended AI therapy increased bone-related AEs in the six trials. In the MA.17R trial, bone pain, bone fracture, and new-onset osteoporosis occurred significantly more frequently with extended AI therapy. Therefore, ASCO guidelines recommended that node-positive or high risk/node-negative, postmenopausal women with stages I–III HR-positive breast cancer should extend AI therapy for up to a total of 10 years after adjuvant endocrine therapy (38). A major benefit of extended AI therapy is the prevention of secondary or contralateral breast cancer (38).
Treatment implications of multigene expression assays
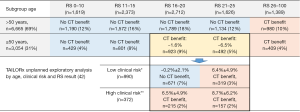
It is important to select which patients with early breast cancer could avoid the administration of cytotoxic chemotherapy. Unfortunately, limited information is available to answer this important question, although there is some understanding of the clinical and pathological features of these tumors. The Oncotype DX Breast Recurrence Score® (RS) test (OTDX/RS, Exact Sciences Corporation, Madison, WI, USA), a multigene expression assay, provides a genomic-based risk assessment for HR-positive, HER2-negative breast cancer in adjuvant settings. Recently, OTDX/RS was included in the American Joint Committee on Cancer (AJCC) breast cancer staging criteria to assign the prognostic stage groups (39). Because of the incorporation of the RS result, the following patients are down-staged to pathological stage IA if they have an RS result 0–10: T1/2N0M0, grade any; HER2−, ER+, PR any. The NCCN guidelines recognized the OTDX as the only “preferred” multigene assay to guide adjuvant chemotherapy decisions for node-negative disease (16), and is backed by level 1 evidence per NCCN Category of Evidence and Consensus, and level 1 evidence of clinical utility for prognostic and predictive tumor biomarkers (40), demonstrating its ability to predict response to adjuvant chemotherapy. The ASCO updated the clinical practice guidelines for patients with HR-positive, HER2-negative, node-negative early breast cancer, based on the results from the Trial Assigning Individualized Options for Treatment (TAILORx) (41-44). The 10,273 registered women were assigned to each treatment group: endocrine therapy alone if an RS of less than 10, randomly endocrine therapy alone of chemoendocrine therapy if an RS of less than 11–25, and chemoendocrine therapy if an RS of more than 26 (41). According to recent findings, for patients older than 50 years with an RS of less than 26, or patients aged 50 years or younger with an RS of less than 16, there is little benefit from chemotherapy (41,42). For patients aged 50 years or younger with an RS of 16 to 25, chemoendocrine therapy may be beneficial. For an RS of 26 to 30 regardless of age, chemoendocrine therapy may be beneficial. For an RS of greater than 30, chemoendocrine therapy should be considered (Figure 2). A chemotherapy benefit was observed in women ≤50 years with RS results of 16–20 and a high clinical risk, which meant all other cases except for those with low risk, regardless of clinical risk: tumor size ≤3 cm and grade 1; tumor size ≤2 cm and grade 2; tumor size ≤1cm and grade 3; or RS result 21–25 (Figure 2). Furthermore, there was no chemotherapy benefit (approximately 0.2%±2.1%) in women ≤50 years with RS results 16–20 and low clinical risk (n=671, 7% of all patients) (42). Also, the ASCO strongly recommend that the MammaPrint assay (Agendia, Irvine, CA, USA) may be used for those who have HR-positive, HER2-negative, node-negative breast cancer with high clinical risk per MINDACT categorization for decisions of adjuvant chemotherapy (44). The ESMO guidelines for early breast cancer noted that first-generation signatures such as MammaPrint and OTDX were biomarkers used in treatment decision-making for ER-positive and HER2-negative tumors (21). Adjuvant chemotherapy is indicated if high risk or high scoring. Moreover, the NCCN also added that the OTDX should be considered for patients with 1–3 positive nodes as category 2A (16), based on predictive data from SWOG 8814 (45) as well as supportive data from the prospective Clalit registry (46) and prospective data from the West German Plan B study (47), but the results of the RxPONDER trial (NCT01272037) remain unavailable and are awaited.
Adjuvant endocrine therapy for ductal carcinoma in situ (DCIS)
Two randomized controlled trials, the NSABP B-24 study (48) and the UK/ANZ DCIS trial (49), have been reported on the administration of tamoxifen for patients with DCIS after breast-conserving surgery (BCS). The NSABP B-24 study (n=1,804) retrospectively evaluated the relevance of the HR receptor and the response to tamoxifen. ER was classified as positive in 76% of patients. This study showed a significant benefit for adjuvant tamoxifen in patients with ER-positive DCIS after BCS and local radiation at 10 years (HR 0.49, P<0.001). The results of the NSABP B-24 study suggested that this effect of adjuvant tamoxifen is likely to be limited to patients with HR-positive tumors. The UK/ANZ DCIS trial (n=1,701) was a randomized 2×2 factorial trial of radiotherapy, tamoxifen, or both to confirm whether they reduced the incidence of breast cancer. Adjuvant tamoxifen significantly reduced all new breast cancer events (HR 0.71, 95% CI: 0.58–0.88), regardless of radiotherapy, at a median follow-up of 12.7 years (49). However, no reduction in breast cancer death was observed. The NSABP B-35 study (n=3,104) comparing tamoxifen vs. anastrozole in postmenopausal women with HR-positive DCIS after excision and radiotherapy, showed a significant improvement with anastrozole to tamoxifen (HR 0.73, 95% CI: 0.56–0.96) in breast cancer-free intervals, mainly in women younger than 60 years of age (50). The ESMO guidelines recommend in patients who underwent BCS for HR-positive DCIS that both tamoxifen and AIs show reduced risk of invasive and non-invasive recurrences and secondary (contralateral) breast cancer, without an effect on OS (21).
Conclusions
Most breast cancer cases are HR-positive/HER2-negative, for example those in East Asia, which are reported at a rate of approximately 70% in the Japanese Breast Cancer Society registry (51). Therefore, adjuvant endocrine treatment strategies for ER-positive/HER2-negative breast cancer are very important. The balance of evidence-based information and patients’ preferences needs to be considered. This review article provides important information to guide the daily medical care of breast cancer patients.
Acknowledgments
We thank H. Nikki March, PhD, from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Yutaka Yamamoto and Takayuki Ueno) for the series “Neoadjuvant/Adjuvant Treatment for Early Breast Cancer” published in Chinese Clinical Oncology. The article was sent for external peer review organized by the Guest Editors and the editorial office.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/cco-20-125). The series “Neoadjuvant/Adjuvant Treatment for Early Breast Cancer” was commissioned by the editorial office without any funding or sponsorship. MK reports receiving honorariums as a speaker or consultant/advisory role from Chugai Pharmaceutical Co. (Tokyo, Japan).
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Porter P. "Westernizing" women's risks? Breast cancer in lower-income countries. N Engl J Med 2008;358:213-6. [Crossref] [PubMed]
- Clarke M, Collins R, Darby S, et al. Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005;366:2087-106. [Crossref] [PubMed]
- Lin CH, Yap YS, Lee KH, et al. Asian Breast Cancer Cooperative Group. Contrasting Epidemiology and Clinicopathology of Female Breast Cancer in Asians vs the US Population. J Natl Cancer Inst 2019;111:1298-306. [Crossref] [PubMed]
- Kurebayashi J, Miyoshi Y, Ishikawa T, et al. Clinicopathological characteristics of breast cancer and trends in the management of breast cancer patients in Japan: Based on the Breast Cancer Registry of the Japanese Breast Cancer Society between 2004 and 2011. Breast Cancer 2015;22:235-44. [Crossref] [PubMed]
- Lin CH, Chen YC, Chiang CJ, et al. The emerging epidemic of estrogen-related cancers in young women in a developing Asian country. Int J Cancer 2012;130:2629-37. [Crossref] [PubMed]
- Stockwell S. Classics in oncology. George Thomas Beatson, M.D. (1848-1933). CA Cancer J Clin 1983;33:105-21. [Crossref] [PubMed]
- Ingle JN, Ahmann DL, Green SJ, et al. Randomized clinical trial of diethylstilbestrol versus tamoxifen in postmenopausal women with advanced breast cancer. N Engl J Med 1981;304:16-21. [Crossref] [PubMed]
- Allison KH, Hammond MEH, Dowsett M, et al. Estrogen and Progesterone Receptor Testing in Breast Cancer: ASCO/CAP Guideline Update. J Clin Oncol 2020;38:1346-66. [Crossref] [PubMed]
- Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005;365:1687-717. [Crossref] [PubMed]
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG), Davies C, Godwin J, et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet 2011;378:771-84. [Crossref] [PubMed]
- Cuzick J, Sestak I, Baum M, et al. ATAC/LATTE investigators. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 10-year analysis of the ATAC trial. Lancet Oncol 2010;11:1135-41. [Crossref] [PubMed]
- Breast International Group (BIG) 1-98 Collaborative Group, Thürlimann B, Keshaviah A, et al. A comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer. N Engl J Med 2005;353:2747-57. Erratum in: N Engl J Med. 2006 May 18;354(20):2200. Wardly, Andrew [corrected to Wardley, Andrew].
- van de Velde CJ, Rea D, Seynaeve C, et al. Adjuvant tamoxifen and exemestane in early breast cancer (TEAM): a randomised phase 3 trial. Lancet 2011;377:321-31. [Crossref] [PubMed]
- Dowsett M, Cuzick J, Ingle J, et al. Meta-analysis of breast cancer outcomes in adjuvant trials of aromatase inhibitors versus tamoxifen. J Clin Oncol 2010;28:509-18. [Crossref] [PubMed]
- Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet 2015;386:1341-52. [Crossref] [PubMed]
- NCCN Guidelines Version 3.2019. Accessed January 6, 2020. Available online: https://www.nccn.org/store/login/login.aspx?ReturnURL=https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf
- Swain SM, Jeong JH, Geyer CE Jr, et al. Longer therapy, iatrogenic amenorrhea, and survival in early breast cancer. N Engl J Med 2010;362:2053-65. [Crossref] [PubMed]
- Francis PA, Pagani O, Fleming GF, et al. Tailoring Adjuvant Endocrine Therapy for Premenopausal Breast Cancer.; SOFT and TEXT Investigators and the International Breast Cancer Study Group. N Engl J Med 2018;379:122-37. [Crossref] [PubMed]
- Pagani O, Francis PA, Fleming GF, et al. Absolute Improvements in Freedom From Distant Recurrence to Tailor Adjuvant Endocrine Therapies for Premenopausal Women: Results From TEXT and SOFT. J Clin Oncol 2020;38:1293-303. [Crossref] [PubMed]
- Balic M, Thomssen C, Würstlein R, et al. St. Gallen/Vienna 2019: A Brief Summary of the Consensus Discussion on the Optimal Primary Breast Cancer Treatment. Breast Care (Basel) 2019;14:103-10. [Crossref] [PubMed]
- Cardoso F, Kyriakides S, Ohno S, et al. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2019;30:1674. [Crossref] [PubMed]
- Burstein HJ, Lacchetti C, Anderson H, et al. Adjuvant Endocrine Therapy for Women With Hormone Receptor-Positive Breast Cancer: American Society of Clinical Oncology Clinical Practice Guideline Update on Ovarian Suppression. J Clin Oncol 2016;34:1689-701. [Crossref] [PubMed]
- BIG 1-98 Collaborative Group, Mouridsen H, Giobbie-Hurder A, et al. Letrozole therapy alone or in sequence with tamoxifen in women with breast cancer. N Engl J Med 2009;361:766-76.
- Davies C, Pan H, Godwin J, et al. Adjuvant Tamoxifen: Longer Against Shorter (ATLAS) Collaborative Group. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet 2013;381:805-16. [Crossref] [PubMed]
- Fisher B, Dignam J, Bryant J, et al. Five versus more than five years of tamoxifen therapy for breast cancer patients with negative lymph nodes and estrogen receptor-positive tumors. J Natl Cancer Inst 1996;88:1529-42. [Crossref] [PubMed]
- Stewart HJ, Forrest AP, Everington D, et al. Randomised comparison of 5 years of adjuvant tamoxifen with continuous therapy for operable breast cancer. The Scottish Cancer Trials Breast Group. Br J Cancer 1996;74:297-9. [Crossref] [PubMed]
- Tormey DC, Gray R, Falkson HC. Postchemotherapy adjuvant tamoxifen therapy beyond five years in patients with lymph node-positive breast cancer. Eastern Cooperative Oncology Group. J Natl Cancer Inst 1996;88:1828-33. [Crossref] [PubMed]
- Burstein HJ, Temin S, Anderson H, et al. Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: American society of clinical oncology clinical practice guideline focused update. J Clin Oncol 2014;32:2255-69. [Crossref] [PubMed]
- Goss PE, Ingle JN, Martino S, et al. Randomized trial of letrozole following tamoxifen as extended adjuvant therapy in receptor-positive breast cancer: updated findings from NCIC CTG MA.17. J Natl Cancer Inst 2005;97:1262-71. [Crossref] [PubMed]
- Mamounas EP, Jeong JH, Wickerham DL, et al. Benefit from exemestane as extended adjuvant therapy after 5 years of adjuvant tamoxifen: intention-to-treat analysis of the National Surgical Adjuvant Breast And Bowel Project B-33 trial. J Clin Oncol 2008;26:1965-71. [Crossref] [PubMed]
- Jakesz R, Greil R, Gnant M, et al. Austrian Breast and Colorectal Cancer Study Group. Extended adjuvant therapy with anastrozole among postmenopausal breast cancer patients: results from the randomized Austrian Breast and Colorectal Cancer Study Group Trial 6a. J Natl Cancer Inst 2007;99:1845-53. [Crossref] [PubMed]
- Goss PE, Ingle JN, Pritchard KI, et al. Extending aromatase-inhibitor adjuvant therapy to 10 years. N Engl J Med 2016;375:209-19. [Crossref] [PubMed]
- Mamounas EP, Bandos H, Lembersky BC, et al. Use of letrozole after aromatase inhibitor-based therapy in postmenopausal breast cancer (NRG Oncology/NSABP B-42): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2019;20:88-99. [Crossref] [PubMed]
- Tjan-Heijnen VCG, van Hellemond IEG, Peer PGM, et al. Extended adjuvant aromatase inhibition after sequential endocrine therapy (DATA): a randomised, phase 3 trial. Lancet Oncol 2017;18:1502-11. Erratum in: Correction to Lancet Oncol 2017;18:1502-11. Lancet Oncol. 2017 Nov;18(11):e642. [Crossref] [PubMed]
- Gnant M, Steger G, Greil R, et al: A prospective randomized multi-center phase-III trial of additional 2 versus additional 5 years of anastrozole after initial 5 years of adjuvant endocrine therapy – results from 3,484 postmenopausal women in the ABCSG-16 trial. San Antonio Breast Cancer Symposium, San Antonio, TX, Dec 5-9, 2017: abstr GS3-1.
- Blok EJ, Kroep JR, Meershoek-Klein Kranenbarg E, et al. Optimal Duration of Extended Adjuvant Endocrine Therapy for Early Breast Cancer; Results of the IDEAL Trial (BOOG 2006-05). J Natl Cancer Inst 2018. [Crossref] [PubMed]
- Colleoni M, Luo W, Karlsson P, et al. SOLE Investigators. Extended adjuvant intermittent letrozole versus continuous letrozole in postmenopausal women with breast cancer (SOLE): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol 2018;19:127-38. [Crossref] [PubMed]
- Burstein HJ, Lacchetti C, Anderson H, et al. Adjuvant Endocrine Therapy for Women With Hormone Receptor-Positive Breast Cancer: ASCO Clinical Practice Guideline Focused Update. J Clin Oncol 2019;37:423-38. [Crossref] [PubMed]
- AJCC Cancer Staging Manual. 8th edition. Accessed January 6, 2020. Available online: https://cancerstaging.org/references-tools/deskreferences/Pages/Breast-Cancer-Staging.aspx
- Simon RM, Paik S, Hayes DF. Use of archived specimens in evaluation of prognostic and predictive biomarkers. J Natl Cancer Inst 2009;101:1446-52. [Crossref] [PubMed]
- Sparano JA, Gray RJ, Makower DF, et al. Adjuvant Chemotherapy Guided by a 21-Gene Expression Assay in Breast Cancer. N Engl J Med 2018;379:111-21. [Crossref] [PubMed]
- Sparano JA, Gray RJ, Ravdin PM, et al. Clinical and Genomic Risk to Guide the Use of Adjuvant Therapy for Breast Cancer. N Engl J Med 2019;380:2395-405. [Crossref] [PubMed]
- Sparano JA, Gray RJ, Makower DF, et al. Clinical Outcomes in Early Breast Cancer With a High 21-Gene Recurrence Score of 26 to 100 Assigned to Adjuvant Chemotherapy Plus Endocrine Therapy: A Secondary Analysis of the TAILORx Randomized Clinical Trial. JAMA Oncol 2019;6:367-74. [Crossref] [PubMed]
- Andre F, Ismaila N, Henry NL, et al. Use of Biomarkers to Guide Decisions on Adjuvant Systemic Therapy for Women With Early-Stage Invasive Breast Cancer: ASCO Clinical Practice Guideline Update-Integration of Results From TAILORx. J Clin Oncol 2019;37:1956-64. [Crossref] [PubMed]
- Albain KS, Barlow WE, Shak S, et al. Breast Cancer Intergroup of North America. Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. Lancet Oncol 2010;11:55-65. [Crossref] [PubMed]
- Stemmer SM, Steiner M, Rizel S, et al. Clinical outcomes in patients with node-negative breast cancer treated based on the recurrence score results: evidence from a large prospectively designed registry. NPJ Breast Cancer 2017;3:33. [Crossref] [PubMed]
- Nitz U, Gluz O, Christgen M, et al. Reducing chemotherapy use in clinically high-risk, genomically low-risk pN0 and pN1 early breast cancer patients: five-year data from the prospective, randomised phase 3 West German Study Group (WSG) PlanB trial. Breast Cancer Res Treat 2017;165:573-83. [Crossref] [PubMed]
- Allred DC, Anderson SJ, Paik S, et al. Adjuvant tamoxifen reduces subsequent breast cancer in women with estrogen receptor-positive ductal carcinoma in situ: a study based on NSABP protocol B-24. J Clin Oncol 2012;30:1268-73. [Crossref] [PubMed]
- Cuzick J, Sestak I, Pinder SE, et al. Effect of tamoxifen and radiotherapy in women with locally excised ductal carcinoma in situ: long-term results from the UK/ANZ DCIS trial. Lancet Oncol 2011;12:21-9. [Crossref] [PubMed]
- Margolese RG, Cecchini RS, Julian TB, et al. Anastrozole versus tamoxifen in postmenopausal women with ductal carcinoma in situ undergoing lumpectomy plus radiotherapy (NSABP B-35): a randomised, double-blind, phase 3 clinical trial. Lancet 2016;387:849-56. [Crossref] [PubMed]
- Kubo M, Kumamaru H, Isozumi U, et al. Annual report of the Japanese Breast Cancer Society registry for 2016. Breast Cancer 2020. [Crossref] [PubMed]


