
Review on gall bladder myeloid sarcoma: a great masquerader
Introduction
Acute abdomen is one of the frequently encountered scenario in emergency room. Cholecystitis and cholelithiasis are amongst the common causes of acute abdomen. Approximately 5% of patients with acute leukemia can have acute abdomen due to variety of reasons (1). Term “Granulocytic sarcoma (GS)” was coined by Rappaport et al. in 1966, also commonly known as myeloid sarcoma (MS) and referred to as extramedullary solid deposits, found either in isolation or in association with leukemias. Most commonly, it has been reported to be associated with acute myeloid leukemia (AML). Other hematological conditions like myelodysplastic syndrome (MDS), chronic myelogenous leukemia (CML), acute lymphoblastic leukemia (ALL), and myelofibrosis (MF) are also reported to have leukemic deposits. MS commonly involves lymph nodes, skin, head and neck region, and orbit although almost any site may be involved (2-13). Gastrointestinal involvement is very rare site of MS. Gallbladder is even more rare to involve and often confused with other common gallbladder pathologies like cholecystitis, gallbladder cancer etc.
Clinically, depending on the time of occurrence, MS is categorized in three different groups as follows: (I) concurrent, when primary hematological disease and MS is diagnosed at the same time; (II) isolated, when MS is the sole finding without any evidence of leukemia/MPN etc.; (III) secondary, when MS develops in a known case of leukemia/MPN signifying either as a relapse or extramedullary blast crisis.
Methodology
A comprehensive search strategy was devised by two independent researchers. By using a combination of the medical subject heading (MeSH) terms “gallbladder and myeloid sarcoma”, “gallbladder and Chloroma”, “gallbladder and granulocytic sarcoma”, we searched the Ovid MEDLINE and Ovid Embase along with relevant citations between 1946 and 2019. English as a language restriction was applied and abstract and title of the all the citations were screened. Ultimately, the search showed in total 17 cases of myeloid leukemic infiltration of gallbladder.
Results
Patient characteristics
In total, we included 17 cases in our study cohort. Patient’s age, sex, clinical presentations, and laboratory data are shown in Table 1. Of the 17 total patients, the median age was 52 years old with age range of 23 to 84 years.
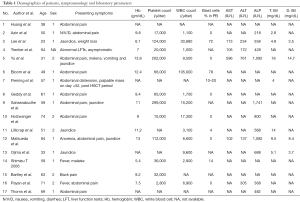
Full table
Diagnostic challenges
Based on imaging or pathological studies, 3 cases were initially confused with gallbladder lymphoma or cancer. Later, review of the case confirmed them as gallbladder myeloid sarcoma (GB-MS) (14-16).
Other sites of involvement by leukemic infiltrates
We also reviewed other sites of involvement by leukemic infiltrates apart from gallbladder. We found MS involvement in pancreas, stomach, common bile duct, omental bursa, liver, cystic duct, lymph node and so on (Table S1).
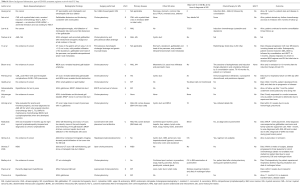
Full table
Categorizations of TMS
By analyzing the clinical data, we divided the patients to either concurrent GB-MS cases (4 cases), isolated GB-MS cases (5 cases) and secondary GB-MS cases (8 cases). In patients with secondary and concurrent GB-MS, the underlying diseases were AML (5 cases), MF (4 cases), 2 CML (2 cases) and MDS (1 case).
Treatment strategies and outcome
Different authors treated their patients with different regimens including various combinations of cholecystectomy, chemotherapy and radiotherapy. Table S1 mentions different regimens used for treatment of GB-MS which clearly points to the fact that there is no unifying protocol driven treatment guidelines till now. Table S1 also mentions in detail the follow up details about the patients. Analysis also showed that 56.25% patients died during the same hospitalization or at follow up. Reasons were variable, sepsis, multiorgan failure, DIC, or disease progression.
Discussion
The available literature with regards to leukemic involvement of gastrointestinal tract has been known since long (16). The gastrointestinal tract is otherwise a rare site of MS involvement. Due to rarity, the exact frequency is relatively unknown. However, few autopsy series evaluating patients dying during acute phase of acute leukemia (both lymphocytic and myelocytic) have reported gastrointestinal tract involvement to be variable and anywhere ranging from 13% to 63% (17).
Diagnostic dilemma for ER physicians
As depicted in our study, GB-MS can be easily confused with benign conditions like cholecystitis, cholangitis or malignant conditions like adenocarcinoma. Though GB-MS is a rare entity, but ER physicians should always keep it in mind while evaluating any case of right upper quadrant pain or obstructive jaundice especially with a background history of myeloid leukemia or abnormal cells in peripheral circulation. Menasce et al. reported that almost in 75–86% nonleukemic patients, MS were initially misdiagnosed. Our review also shows the similar results which underscores the importance of educating ER physicians about this rare entity (18).
Other malignant differential diagnosis for acute abdomen/cholecystitis
There are various close differentials to GB-MS like: non-Hodgkin’s lymphoma (NHL), Ewing’s sarcoma, primitive neuroectodermal tumors, high risk small cell type stromal tumors, eosinophilic granuloma, and undifferentiated small cell lung cancer (18). Microscopically, MS cells can be easily confused with lymphoma cells. MS blast cells have acidophilic cytoplasm and are positive for CD 117, MPO and CD 43. Cytogenetic abnormalities like inv chromosome 16, t (8:21), lack of Auer rods, FAB M2, M4, M5 and CBFB/MYH 11 fusion gene have been associated with high incidence of MS (19).
Clinical presentation
Basically, the symptomatology in GI based MS is variable and depends on the site of location. As evident in our study, in all cases, GB-MS presented with non-specific symptoms of cholecystitis. It is almost impossible to diagnose GB-MS without histopathological evaluation in naïve cases except in conditions when there is already a known history of hematological malignancy or presence of frank circulating blast cells to lead the physicians. This fact emphasizes the need for ER physicians to quickly scan patient’s history for hematological malignancy which will help start evaluating the patient from day 1 itself for GB-MS which will save previous time.
Ultrasound of abdomen (USG) and CT abdomen are two major supplementary diagnostic modalities. Best specimen to diagnose GB-MS would be histopathological study of the resected gall bladder. In case of simultaneous biliary tract involvement, ERCP and cytology of exfoliated cells may assist in diagnosing additional sites of MS involvement. Recently, Matsueda et al. reviewed MS cases presenting with obstructive jaundice (15). In all cases, the site of infiltration was reported as biliary tract or head of pancreas. Azin et al.’s case had multiple hospitalizations for recurrent cholecystitis (20). After approximately 7 weeks of the initial presentation, patient underwent elective cholecystectomy and was diagnosed with GB-MS. Although, the course of therapy did not change as patient denied any further chemotherapy however an early diagnosis and surgical interventions could have at least improved the quality of life of the patient (20). Hunter et al. studied gastrointestinal complications of leukemia (142 patients) while undergoing chemotherapy and reported that 9% had abdominal symptoms with only 1 case of MS involving common bile duct (21).
Hence for a comprehensive and timely diagnosis, a combined diagnosed strategy including imaging studies, ERCP, histopathology, and immunohistochemistry are the necessary requirements. Accurate diagnosis becomes more daunting in post HSCT patients due to other concomitant complexities like graft versus host disease (GVHD), immunosuppressive drug related adversities like cholestasis, opportunistic infections, sinusoidal obstruction syndrome (SOS), acalculous cholecystitis and so on (22). Approximately 5% of patient’s undergoing chemotherapy for leukemia developed cholecystitis in one series (23).
Background information can play a crucial role especially in emergency room. In our review, there were 12 cases (70.58% of study cohort) who had associated hematological malignancy (5 AML, 4 MF, 2 CML and 1 MDS case). It is expected to have a higher probability of having GB-MS with cancer background when compared to those without cancer history. Scully et al.’s case was challenging as the 47-year-old had history of both colon cancer and AML thereby keeping both differentials as the possibility during evaluation of the abdominal pain and obstructive jaundice in their case (24). Microscopic evaluation of the surgical specimen confirmed it to be leukemic infiltration of common bile duct (24). Hence, sometimes despite the best clinical judgement, only the specimen examination confirms the diagnosis. Our review showed 5 cases out of 17 to have isolated GB-MS without any bone marrow involvement (14,25-28). Bartley et reported an interesting case of disseminated extramedullary myeloid tumor of the gallbladder but without involvement of the bone marrow (28).
Management and prognosis
Conventionally the prognosis of MS is extremely poor. Untreated MS cases usually transform to frank leukemia within 6–12 months (29). With regards to the treatment, surgical resection like cholecystectomy serves purpose for not only immediate relief to the patient but also provides specimen for definitive diagnosis. However, cholecystectomy is not a definitive treatment for GB-MS and cannot delay the transformation to leukemia or prevent the progression of disease unless chemotherapy is initiated soon (30). This is based on the expert opinion that suggests treating primary or isolated GB-MS just like any systemic AML disease. Hence, hematology opinion should be sorted for the patient care as soon as the diagnosis is established. Also, important to note that in general any surgery in patients with leukemia are associated with high mortality rates (31). Although, there has been significant improvement in survival in last few decades, most of the non-cancer related deaths are reported to result from uncontrolled sepsis (32-34). The current literature supports the use of systemic anti-leukemic therapy followed by HSCT as soon as possible in order to control the progression and improve the prognosis (26,35,36).
Conclusions
In conclusion, MS involving gallbladder and nearby organs is extremely rare and tends to be misdiagnosed. Awareness of this entity amongst the ER physicians, surgeons and hospitalists is of utmost importance. Despite the exponential advancement in the management, prognosis is still dismal and further RCTs are need of hour to improve survival of patients.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/cco-19-250). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hawkins JA, Mower WR, Nelson EW. Acute abdominal conditions in patients with leukemia. Am J Surg 1985;150:739-42. [Crossref] [PubMed]
- Sahu KK, Mishra AK, Lal A. Advancements in Treatment of Refractory and Relapsed Myeloid Sarcoma. J Oncol Pract 2019;15:622-3. [Crossref] [PubMed]
- Sahu KK, Lal A, Mishra AK. Myeloid sarcoma of central nervous system: Approach and management. J Clin Neurosci 2019;70:267-8. [Crossref] [PubMed]
- Sahu KK, Sherif AA, Mishra AK, et al. Testicular Myeloid Sarcoma: A Systematic Review of the Literature. Clin Lymphoma Myeloma Leuk 2019;19:603-18. [Crossref] [PubMed]
- Sahu KK, Thakur K. Role of Positron Emission Tomography Imaging in Myeloid Sarcoma. Indian J Nucl Med 2018;33:90. [PubMed]
- Gautam A, Jalali GK, Sahu KK, et al. Cardiac Myeloid Sarcoma: Review of Literature. J Clin Diagn Res 2017;11:XE01-4. [PubMed]
- Sahu KK, Gautam A, Ailawadhi S. Re: FDG PET/CT Findings of Intracardiac Myeloid Sarcoma. Clin Nucl Med 2017;42:242-5. [Crossref] [PubMed]
- Sahu KK, Dhibar DP, Malhotra P. Isolated myeloid sarcoma. Orbit 2016;35:351. [Crossref] [PubMed]
- Sahu KK, Jain A, Yanamandra U, et al. Myeloid Sarcoma of Vulva: A Short Update. Indian J Hematol Blood Transfus 2016;32:69-71. [Crossref] [PubMed]
- Sahu KK, Yanamandra U, Malhotra P. Orbital myeloid sarcoma: Rare presentation of AML. Orbit 2016;35:157-8. [Crossref] [PubMed]
- Sahu KK, Malhotra P. Re: "Granulocytic Sarcoma of the Orbit Presenting as a Fulminant Orbitopathy in an Adult with Acute Myeloid Leukemia Ophthalmic Plast Reconstr Surg 2015;31:421. [Crossref] [PubMed]
- Jain A, Sahu KK, Sharma S, et al. Shoulder Myeloid Sarcoma: An Initial Presentation of CML Blast Crisis. Indian J Hematol Blood Transfus 2016;32:361-3. [Crossref] [PubMed]
- Sahu KK, Tyagi R, Law AD, et al. Myeloid Sarcoma: An Unusual Case of Mediastinal Mass and Malignant Pleural Effusion with Review of Literature. Indian J Hematol Blood Transfus 2015;31:466-71. [Crossref] [PubMed]
- Ojima H., Hasegawa T., Matsuno Y, et al. Extramedullary myeloid tumour (EMMT) of the gallbladder. J Clin Pathol 2005;58:211-3. [Crossref] [PubMed]
- Matsueda K, Yamamoto H, Doi I. An autopsy case of granulocytic sarcoma of the porta hepatis causing obstructive jaundice. J Gastroenterol 1998;33:428-33. [Crossref] [PubMed]
- Bloom SH, Coad JE, Greeno EW, et al. Cholecystitis as the presenting manifestation of acute myeloid leukemia: report of a case. Am J Hematol 2002;70:254-6. [Crossref] [PubMed]
- Kirshbaum JD, Preuss FS. Leukemia; clinical and pathologic study of 123 fatal cases in a series of 14,400 necropsies. Arch Intern Med 1943;71:777-92. [Crossref]
- Menasce LP, Banerjee SS, Beckett E, et al. Extra-medullary myeloid tumor (granulocytic sarcoma) is often misdiagnosed: a study of 26 cases. Histopathology 1999;34:391-8. [Crossref] [PubMed]
- Shimizu T, Tajiri T, Akimaru K, et al. Cholecystitis caused by infiltration of immature myeloid cells: a case report. J Nippon Med Sch 2006;73:97-100. [Crossref] [PubMed]
- Azin A, Racz JM, Jimenez MC, et al. Relapse of acute myeloid leukemia manifested by cholecystitis: A case report and review of the literature. Int J Surg Case Rep 2014;5:302-5. [Crossref] [PubMed]
- Hunter TB, Bjelland JC. Gastrointestinal complications of leukemia and its treatment. AJR Am J Roentgenol 1984;142:513-8. [Crossref] [PubMed]
- Fleming DR, Slone SP. CML blast crisis resulting in biliary obstruction following BMT. Bone Marrow Transplant 1997;19:853-4. [Crossref] [PubMed]
- Gorschlüter M, Marklein G, Hofling K, et al. Abdominal infections in patients with acute leukaemia: a prospective study applying ultrasonography and microbiology. Br J Haematol 2002;117:351-8. [Crossref] [PubMed]
- Case Records of the Massachusetts General Hospital. (Case 32-1988). N Engl J Med 1988;319:356-64. [PubMed]
- Huang XL, Tao J, Li JZ, et al. Gastric myeloid sarcoma without acute myeloblastic leukemia. World J Gastroenterol 2015;21:2242-8. [Crossref] [PubMed]
- Yu T, Xu G, Xu X, et al. Myeloid sarcoma derived from the gastrointestinal tract: A case report and review of the literature. Oncol Lett 2016;11:4155-9. [Crossref] [PubMed]
- Holzwanger EA, Alam Z, Hsu E, et al. A Case of Granulocytic Sarcoma or Extramedullary Acute Myelomonocytic Leukemia of the Gallbladder. Am J Case Rep 2018;19:1262-6. [Crossref] [PubMed]
- Bartley AN, Nelson CL, Nelson DH, et al. Disseminated extramedullary myeloid tumor of the gallbladder without involvement of the bone marrow. Am J Hematol 2007;82:65-8. [Crossref] [PubMed]
- Yamauchi K, Yasuda M. Comparison in treatments of nonleukemic granulocytic sarcoma: report of two cases and a review of 72 cases in the literature. Cancer 2002;94:1739-46. [Crossref] [PubMed]
- He J, Zhu L, Ye X, et al. Clinical characteristics and prognosis of nonleukemic myeloid sarcoma. Am J Med Sci 2014;347:434-8. [Crossref] [PubMed]
- Björnsson S, Yates JW, Mittelman A, et al. Major surgery in acute leukemia. Cancer 1974;34:1272-5. [Crossref] [PubMed]
- Vaughn EA, Key CR, Sterling WA Jr. Intraabdominal operations in patients with leukemia. Am J Surg 1988;156:51-3. [Crossref] [PubMed]
- Yanamandra U, Sahu KK, Khadwal A, et al. Recurrent Sweet’s Syndrome in a Case of AML. Indian J Hematol Blood Transfus 2016;32:82-5. [Crossref] [PubMed]
- Sahu KK, Mishra K, Malhotra P. Extramedullary deposits in leukemia: Out of blood but not out of mind. J Microsc Ultrastruct 2019;8:35-6. [PubMed]
- Sahu KK, Prakash G, Sanamandra P, et al. An Unusual Site of Acute Lymphoblastic Leukaemia Relapse: Challenge for Gynaecologists. J Obstet Gynaecol India 2016;66:656-61. [Crossref] [PubMed]
- Sahu KK, Malhotra P, Uthamalingam P, et al. Chronic Myeloid Leukemia with Extramedullary Blast Crisis: Two Unusual Sites with Review of Literature. Indian J Hematol Blood Transfus 2016;32:89-95. [Crossref] [PubMed]


