
Successful stereotactic radiotherapy of meningiomas in a patient with Cowden syndrome: a case report
Introduction
Cowden’s Syndrome (CS) is an autosomal dominant, hereditary, and multisystem disease resulting in multiple hamartomas throughout the body with an increased risk of several carcinomas (1,2). It has incomplete penetrance and variable expressivity with a germline mutation in the phosphatase and tensin homolog (PTEN) tumor suppressor gene on chromosome 10q23.3 (3,4). CS causes an increased risk of breast, thyroid, endometrial, and renal cell cancers with lifetime risk as high as 85%, 35%, 28%, and 35%, respectively (5,6). Increased rate and number of meningiomas in CS has been described in case reports. However meningiomas do not fall under the syndrome’s diagnostic criteria due to lack of data and high prevalence in the general population (7,8). CS is rare with an estimated prevalence of 1:200,000 patients; but this figure is likely an underestimation due to under diagnosis (9).
There is a lack of evidence describing the effects of radiation as a treatment modality in this patient population. Experimental studies have shown radiation sensitivity following PTEN inhibition in cell lines (10). Limited case reports have also described the negative effects of treating CS patients with radiation therapy (RT) including secondary malignancies, radiation dermatitis, and neurotoxicity (10,11). Patients with CS and unresectable meningiomas pose a conundrum: is RT safe in this population? We present the following case of a 35-year-old woman with CS and multiple meningiomas treated with multiple bouts of RT in accordance with the CARE-Guideline (12).
Case presentation
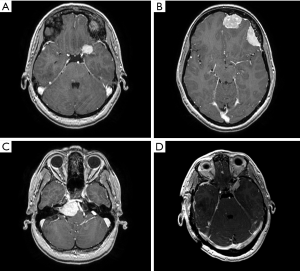
A 35-year-old woman presented to our institution with a recent diagnosis of CS and a magnetic resonance imaging (MRI) of the brain was performed as part of her evaluation. MRI demonstrated a lesion in the cerebellum suggesting dysplastic gangliocytoma consistent with Lhermitte-Duclos disease (LDD), considered part of CS, as well as five intracranial, homogeneously enhancing masses of the meninges consistent with meningiomas. The meningeal lesions were left frontal parafalcine, left frontal convexity, left paraclinoid, right petroclival, and right olfactory groove, measuring 3.9, 3.4, 2.2, 3.3, and 0.4 cm in the longest dimension, respectively (Figure 1). There was 1.6 cm of brainstem compression in the region of the pons from the petroclival lesion, and optic nerve abutment from the paraclinoid mass. She was otherwise asymptomatic, and her neurological exam was normal.
First treatment
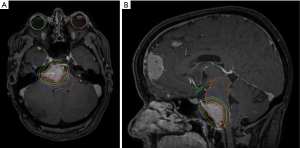
The patient underwent a right middle fossa and retrosigmoid craniotomy for resection of the right petroclival meningioma. Subtotal resection (Simpson grade IV) was achieved due to the fibrous nature of the lesion and risk of cranial neuropathy. Pathology revealed low-grade spindle cell processes, Ki-67 <1%, with positive immunohistochemical stains for EMA and vimentin, consistent with World Health Organization (WHO) grade I meningioma. Post-operative MRI of the brain showed surgical changes with significant residual petroclival disease and stability of the remaining meningiomas. Her recovery was complicated by mild right sixth nerve palsy and hoarseness which improved with follow-up. She was referred to radiation oncology, and adjuvant RT was recommended to the residual disease. Active surveillance was elected for the remaining lesions as they were asymptomatic, the patient did not want surgery, and concern for malignant transformation from RT in the setting of CS. The patient underwent fractionated stereotactic radiation therapy (FSRT) 50 Gy in 25 fractions with a 2 mm margin, prescribed to the 97% isodose line, using four arcs and five modulated static beams (Figure 2). Mean dose to the brainstem was 21.8 Gy, with maximal point dose of 51.7 Gy. She completed adjuvant therapy four months postoperatively with only grade 1 fatigue, headaches, and nausea that self-resolved.
Follow-up brain MRI six months post adjuvant RT showed interval decrease in the size of the right petroclival meningioma from 2.4×3.0 to 1.9×1.8 cm2 with decreased mass effect on the brainstem, and no evidence of brainstem edema. There was interval progression of the left frontal parafalcine meningioma from 3.7×2.4 to 3.8×2.6 cm2 and stability of the remaining three lesions. She continued to be asymptomatic with no visual deficits. Surgical resection was recommended due to progression. However, the patient elected for continued surveillance.
Second treatment
At 18 months post-operatively the patient presented with new onset of mild right arm weakness and headaches. MRI at this time revealed progression of left parafalcine and left frontal convexity meningiomas, significantly increased cerebral edema, and increased mass effect with abutment of the sagittal sinus. Surgical intervention for these two lesions, the left clinoid, and right olfactory groove meningiomas was recommended but was delayed due to patient preference.
Two months later the patient underwent image-guided left frontotemporal craniotomy and achieved gross total resection (Simpson Grade II) for all sites of disease. Post-operative MRI confirmed these changes as well as new small meningiomas in the right anterior cranial fossa and left tentorium, 3 and 5 mm respectively. Pathology of the left frontal specimen was WHO grade II atypical meningioma with brain invasion, reactive gliosis, and bone invasion with KI67 of 2%, and the remaining lesions were WHO grade I. The patient recovered without complication.
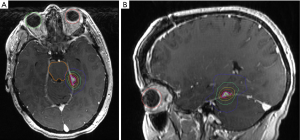
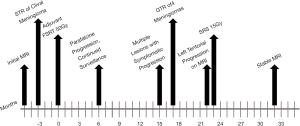
MRI of the brain five months after surgery demonstrated improvement in postoperative changes, enlargement of the left tentorial meningioma, and increased enhancement over the left frontal convexity thought to be due to surgical changes. The patient complained of occasional forgetfulness and fatigue but was otherwise doing well. Review of prior radiation plan did not reveal field overlap with new meningiomas, and definitive RT was recommended to the progressing lesion. She underwent stereotactic radiosurgery (SRS) to 15 Gy using four stereodynamic arcs prescribed to the 81% isodose line without margin, and minimal dose to critical structures (Figure 3). Neurosurgery reported thorough resection of the left frontal WHO grade II meningioma, therefore observation was elected. The patient tolerated treatment well and is currently being followed with ongoing surveillance. Most recent MRI showed no significant changes 32 months from original FSRT and 7 months post-SRS. She has reported no long term side effects or toxicity as a result of her radiation treatments. A timeline of care received by the patient is available in Figure 4.
Discussion
There are few publications describing the use of RT in patients with CS. One case report (abstract) describes the discontinuation of adjuvant radiation to the breast due to severe skin desquamation (10). This same patient experienced near total hair loss in the first week of whole brain radiotherapy (WBRT) despite the use of hippocampal sparing, intensity modulated radiotherapy technique with the scalp receiving <10 Gy. Four months after completing WBRT the patient developed emesis, ataxia, and headache with MRI showing increased intracranial edema and nodular enhancement believed to be treatment-induced changes (10). In another report, a patient with CS was diagnosed with undifferentiated pleomorphic sarcoma (UPS) of the breast, thought to be from adjuvant RT for breast cancer seven years prior (11).
Our patient underwent multiple bouts of high dose, definitive RT. However, unlike the case report by Tatebe et al. our patient developed minimal toxicity or post-RT MRI changes (10). There are several potential explanations for the difference in outcomes. Firstly, we utilized stereotactic techniques with minimal margins. Given the potential for increased toxicity in this patient group, maximizing the therapeutic ratio is critical, reinforcing the principle of “as low as reasonably achievable” (ALARA). The various levels of penetrance and expressivity inherent to CS may also provide explanation, with some patients possibly being more sensitive to RT than others. In the case report by Chandhanayingyong et al. they described the treatment of a patient with CS and Ewing sarcoma of the pelvis. The patient underwent neoadjuvant RT to 45 Gy prior to hemipelvectomy with no significant toxicity reported (13). There is no reliable way to identify patients that will develop significant treatment toxicity. Indeed, given that our patient had brain parenchymal manifestation of her disease via LDD, it would have been reasonable to presume increased risk of toxicity. Ultimately, the risk and benefits of RT should always be considered and catered to each patient individually.
Due to limited evidence and few reports describing these negative effects, RT has frequently been avoided in CS patients. The reluctance to use radiation in CS patients with intracranial disease leaves providers with limited therapeutic options. When treating CS patients, non-RT methods should be exhausted. If RT is necessary, highly conformal, stereotactic techniques with minimal dose to normal structures should be utilized to decrease potential toxicity. Our case demonstrates the first successful use of intracranial stereotactic RT in a CS patient. While more evidence is needed, this offers a potential treatment option for this patient population.
In conclusion, we report the first case, to the best of our knowledge, of intracranial RT using stereotactic techniques in a patient with CS who has not demonstrated significant treatment toxicity to date. Previous reports of toxicity may be due to the variable penetrance of the CS and large RT fields used in prior reports. While RT has historically been avoided in CS patients due to concern for secondary malignancies and increased toxicity, consideration in select patients utilizing stereotactic techniques may be viable after exhaustion of non-RT interventions.
Acknowledgments
We thank Kinga Skowron MD for her critical proof readings.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/cco.2020.03.04). WS reports Associate Editor-in-Chief of Chinese Clinical Oncology. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Patient information reported as per approved IRB #18D.480. Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hobert JA, Eng C. PTEN hamartoma tumor syndrome: an overview. Genet Med 2009;11:687-94. [Crossref] [PubMed]
- Pilarski R, Burt R, Kohlman W, et al. Cowden syndrome and the PTEN hamartoma tumor syndrome: systematic review and revised diagnostic criteria. J Natl Cancer Inst 2013;105:1607-16. [Crossref] [PubMed]
- Liaw D, Marsh DJ, Li J, et al. Germline mutations of the PTEN gene in Cowden disease, an inherited breast and thyroid cancer syndrome. Nat Genet 1997;16:64-7. [Crossref] [PubMed]
- Nelen MR, van Staveren WC, Peeters EA, et al. Germline mutations in the PTEN/MMAC1 gene in patients with Cowden disease. Hum Mol Genet 1997;6:1383-7. [Crossref] [PubMed]
- Bubien V, Bonnet F, Brouste V, et al. High cumulative risks of cancer in patients with PTEN hamartoma tumour syndrome. J Med Genet 2013;50:255-63. [Crossref] [PubMed]
- Nieuwenhuis MH, Kets CM, Murphy-Ryan M, et al. Cancer risk and genotype-phenotype correlations in PTEN hamartoma tumor syndrome. Fam Cancer 2014;13:57-63. [Crossref] [PubMed]
- Lok C, Viseux V, Avril MF, et al. Brain magnetic resonance imaging in patients with Cowden syndrome. Medicine (Baltimore) 2005;84:129-36. [Crossref] [PubMed]
- Gosein MA, Narinesingh D, Nixon CA, et al. Multi-organ benign and malignant tumors: recognizing Cowden syndrome: a case report and review of the literature. BMC Res Notes 2016;9:388. [Crossref] [PubMed]
- Kufe DW, Pollock RE, Weichselbaum RR, et al. Holland-Frei Cancer Medicine [Internet]. 6th ed. BC Decker Inc; 2003. Available online: https://www.ncbi.nlm.nih.gov/books/NBK12354/?term=NBK12354%20AND%20cmed6%5Bbook%5D
- Tatebe K, Chmura SJ, Connell PP. Severe Radiation Toxicity Associated with a Germline PTEN Mutation. Int J Radiat Oncol 2017;99:E620. [Crossref]
- Hatta N, Horita Y. Undifferentiated pleomorphic sarcoma in a patient with Cowden syndrome after radiotherapy for breast cancer. J Dermatol 2019;46:e73-5. [Crossref] [PubMed]
- Riley DS, Barber MS, Kienle GS, et al. CARE guidelines for case reports: explanation and elaboration document. J Clin Epidemiol 2017;89:218-35. [Crossref] [PubMed]
- Chandhanayingyong MC, Bernthal NM, Ungarreevittaya P, et al. Ewing Sarcoma in a Patient With Cowden Syndrome. J Natl Compr Canc Netw 2015;13:1310-4. [Crossref] [PubMed]


