
Neoadjuvant endocrine therapy for estrogen receptor-positive primary breast cancer
Introduction
Hormone receptor (HR)-positive primary breast cancer accounts for 70–80% of all breast cancers (1). Adjuvant endocrine therapy significantly contributes to reducing recurrence and improving survival and has high tolerability in patients with HR-positive breast cancer (2). Neoadjuvant endocrine therapy (NAE) was initially used for inoperable HR-positive locally advanced breast cancer in elderly patients with poor performance status for whom chemotherapy was not tolerable. Recently, NAE has also been used for selected patients with operable HR-positive/human epidermal growth factor receptor 2 (HER2)-negative cancer who opt for breast-conserving surgery (BCS) (3). NAE and neoadjuvant chemotherapy (NAC) require a quite different administration schedule. NAC is often given during the same treatment period as adjuvant chemotherapy. On the other hands, in NAE, a portion (generally 2 weeks to 6 months) of the total (5 to 10 years) endocrine therapy period is given preoperatively and endocrine therapy is given again after surgery for the remainder of the period.
Neoadjuvant systemic treatment is given for several purpose such as improving surgical outcomes through inducing tumor shrinkage, prediction of prognosis based on treatment response or clinicopathological characteristics in residual tumors, improving prognosis by adding postoperative treatment, improving prognosis by starting systemic treatment early and evaluating new drugs early. In this article, we review the current status of NAE for operable breast cancer and discuss its future prospects.
Clinical significance of NAE
Does NAE improve surgical outcome in operable breast cancer?
Table 1 shows the tumor response rates and BCS rates for NAE. The rate of clinical response by palpation, radiological response by imaging, and BCS, with NAE were 26% to 75%, 24% to 64%, and 29% to 89%, respectively (4-9). Clinical response and BCS rates were higher following treatment with aromatase inhibitors (AIs) than with tamoxifen (4,9,10). Clinical response and BCS rates in patients receiving NAE were similar to those receiving anthracycline- and taxane-based NAC among patients with HR-positive primary breast cancer (10-13) (Table 2). In addition, NAE reduced adverse events such as febrile neutropenia, nausea, vomiting, stomatitis, alopecia and cardiac events compared to NAC. According to the exploratory subgroup analysis in GEICAM 2006-03 (12), there was no significant difference between NAE and NAC in terms of clinical response for postmenopausal patients (NAC: 57% vs. NAE: 52%). On the other hands, NAC was more effective than NAE in terms of clinical response in premenopausal patients (NAC: 75% vs. NAE: 44%, P=0.027). However, for premenopausal women, chemotherapy can exhibit both the direct cytotoxic effects of chemotherapy and the indirect effects of endocrine therapy due to chemotherapy-induced amenorrhea. Therefore, it is reasonable that NAC had a higher tumor response than NAE in the premenopausal setting. It is unclear for endocrine-sensitive premenopausal patients whether an additional effect of chemotherapy on endocrine therapy is needed to improve the surgical outcome.
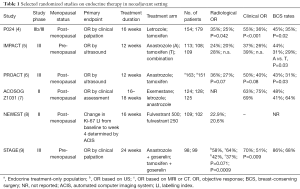
Full table

Full table
Of the cases that required mastectomy at baseline, 30% to 70% of the patients were able to undergo BCS after NAE (Table 3).
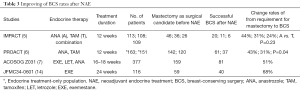
Full table
As mentioned above, NAE can improve the surgical outcome through tumor shrinkage in patients with hormone-receptor positive operable breast cancer.
Is prognostication possible based on response to NAE?
Prognostication based on treatment response and the clinicopathological characteristics of residual tumor after NAE may provide important information regarding the postoperative treatment strategy, especially in patients with a poor prognosis. Pathological complete response (pCR) after NAC is a strong prognostic factor in operable breast cancer (15). Although the prognosis of patients with non-pCR after NAC is extremely poor, additional postoperative systemic treatment such as capecitabine and trastuzumab emtansine has been shown to improve prognosis in patients with non-pCR after NAC (16,17). On the other hands, the pCR rates after NAE is quite low (<10%) (10), and pCR is not an independent prognostic factor for the patients receiving NAE. However, pCR rates after NAC in patients with luminal A-like tumor also is low (8.1%) and pCR after NAC is not a prognostic indication in those patients (15).
Akashi-Tanaka et al. reported preliminary histological findings after NAE based on the General Rules for the Clinical and Pathological Recording of Breast Cancer 2007 (18) correlated with prognosis (19). The prognosis of patients with some histological changes after NAE was better than those without histological changes after NAE (HR: 6.3, 95% CI: 1.6–23.8, P=0.0067).
Dowsett et al. showed that Ki-67 labelling index (LI) 2 weeks after NAE initiation had a better prognostic value than that before NAE (20). Ellis et al. identified four independent prognostic factors including pathological tumor size, pathological nodal status, Ki-67 LI and ER status, using surgical specimens after NAE. Based on these factors and their prognostic impact calculated by Cox proportional hazards, they developed the preoperative endocrine prognostic index (PEPI) score as a postoperative prognostic tool for patients receiving NAE for 3 to 4 months (21).
Regarding the prognosis based on the clinical efficacy of NAE, Ueno et al. demonstrated that the prognosis of patients with progressive disease (PD) following NAE was extremely poor [disease-free survival (DFS): HR: 7.7, 95% CI: 1.6–3.3; overall survival (OS): HR: 26.3 (2.4–65.5)] (22). Iwata et al. have shown similar results in 904 cases treated with letrozole for 6 months as NAE (23). Moreover, the prognosis for PD patients was extremely poor, even though all 46 (5.1%) PD patients on NAE were receiving chemotherapy after NAE or surgery. A novel treatment approach for PD patients on NAE is therefore needed. However, the prognosis based on clinical response is very limited information because of the low frequency of PD cases (<10%) treated with NAE and the inability to stratify the prognosis of PR and stable disease (SD) patients.
Taken together, the clinical response and clinicopathological findings after NAE have prognostic value, suggesting the necessity of a new approach for patients with poor prognosis.
Does early initiation of endocrine therapy improve prognosis?
There is no direct evidence that the effects of NAE and adjuvant endocrine therapy on long-term prognosis are comparable. GRETA, a randomized controlled phase III trial comparing tamoxifen with surgery and adjuvant tamoxifen in patients aged 70 years and older, showed no differences in DFS, breast cancer specific-survival (BCSS), or OS between the two groups (24,25). A similar randomized controlled study in Italy demonstrated no differences in BCSS or OS between tamoxifen alone and optimal surgery plus tamoxifen (26).
As mentioned above, Ki-67 LI 2 weeks after initiation of NAC is important predictor of long-term survival. The POEIC trial was conducted to ascertain whether treatment with a non-steroidal AI 2 weeks before surgery improves long-term prognosis compared to patients who received no systemic treatment before surgery (27). The POETIC study registered 4,486 ER-positive patients. There were no significant differences between the group that received preoperative AI and the group that did not, in terms of the primary endpoint, time to recurrence (TTR) (% TTR event free at 5 years in the perioperative AI group was 90.9% vs. the no perioperative AI in which it was 90.3%; HR: 0.91, 95% CI: 0.74–1.12, log-rank P=0.37) (28).
Although no advantage of NAE over adjuvant endocrine therapy has been demonstrated in terms of long-term prognosis, NAE does not impair prognosis.
Can NAE be used for early evaluation of new drugs for early clinical application?
NAC is already using pCR as an indicator for early evaluation of new drugs for early clinical applications. In NAE, complete cell-cycle arrest (CCCA) was defined as a Ki-67 LI less than or equal to 2.7% at 2 weeks after NAE initiation as an indicator for early evaluation of new agents. Table 4 shows the results of the combination of endocrine treatment and molecular-targeted drugs as NAE (29-36). In most cases, the combination of endocrine therapy and molecular-targeted therapy significantly increased CCCA rates compared to endocrine therapy alone (33,35,36). However, BCS rates were not improved by a combination of endocrine therapy and molecular-targeted therapy (36). There is no early clinical application of new agents based on their early evaluation using NAE.
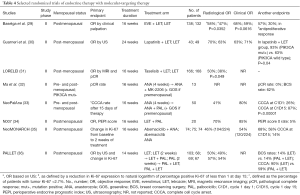
Full table
Taken together, these data show that the clinical significance of NAE at present is improvement of surgical outcome and prognostication based on tumor response and clinicopathological characteristics of residual tumor after NAE.
Optimal use of NAE
Endocrine agents
In a meta-analysis comparing clinical response and radiological response of patients treated with tamoxifen and AI (10), the rate of BCS was significantly better in the AI group. However, the pCR rate did not differ between the two groups. Therefore, AI is the first-choice drug for use as NAE.
Optimal treatment duration
Optimal treatment duration should be considered for each endpoint such as clinical response, transition to BCS and prediction of long-term outcome. Table 5 shows the response rate and BCS rate for each duration of NAE (14,37-40). In general, longer durations of NAE resulted in greater tumor response rates and BCS rates compared to shorter durations. Llombart-cussac et al. reported that the time to response and the time to maximum effect were 3.9 and 4.2 months, respectively, in 70 cases of a neoadjuvant letrozole trial. The maximum tumor response was also seen 6 to 12 months after treatment in 39% of patients (41). In a neoadjuvant exemestane trial with 116 treated, Toi et al. showed 54 cases of SD 4 months after NAE initiation. Among them, 14 (26%) had PR, 35 (63%) had SD, and 6 (11%) had PD. It was shown that the tumor response tended to be determined as the treatment duration was extended (14). If the index of the optimal administration duration is tumor response, a treatment duration of at least 6 months or so is necessary. Carpenter et al. reported that the median time to achieve a tumor response sufficient to allow BCS with NAE was 7.5 months (95% CI: 6.3–8.5 months) with 146 patients receiving letrozole preoperatively (42). If the index of the optimal administration duration is BCS, a treatment duration of at least 6 months is again necessary.

Full table
Taira et al. examined the health-related quality of life (HRQoL) for 6 months during neoadjuvant letrozole treatment in 497 patients who participated in the NEOS trial. NAE increased endocrine therapy-related side effects, but had no significant effect on global HRQoL, and also improved anxiety, depression, and emotional well-being (43). This showed that the HRQoL of patients undergoing NAE does not decrease during the NAE given to obtain tumor shrinkage, and patients’ mental condition is maintained and improved by NAE.
On the other hand, if the index of optimal administration duration is prediction of long-term prognosis, the treatment duration of NAE can be 2 weeks or 3 to 4 months after initiation of NAE. Long-term prognosis can be predicted by evaluating the Ki-67 LI 2 weeks after NAE initiation (20) or by obtaining the PEPI score of residual tumor 3 to 4 months after NAE initiation (21). When checking acquired resistance to endocrine treatment, it is necessary to confirm tumor regrowth during NAE. The progression-free survival after treatment with 1st line endocrine monotherapy is around 12 months. If the index of the optimal NAE duration is acquired resistance of endocrine therapy, NAE duration of at least 6 months or so is necessary.
Indication for NAE
Common indications for NAE are HR-positive/HER2-negative stage II–III primary breast cancer. However, NAE should be avoided in cases with high proliferative activity or low hormone sensitivity. Toi et al. reported that if the Ki-67 LI before NAE was 30% or less, there was no PD case after 6 months NAE. On the other hand, if the Ki-67 LI was more than 30%, PD cases appeared after that (14). Ueno et al. demonstrated that when Oncotype Dx Recurrence Score (RS) category was high RS, clinical response was significantly worse than low and intermediate RS in the same study (44). Iwata et al. conducted a similar study in 295 patients with a tumor diameter of 2 cm or more who participated in the NEOS trial, and confirmed that patients with a high RS had lower tumor response than those with low or intermediate RS (45).
Development of a treatment strategy using NAE
Treatment responsiveness to NAE and clinicopathological status of residual tumor after NAE strongly predict long-term prognosis. Therefore, an attempt has been made to determine a treatment approach based on tumor responsiveness to NAE as in the POETIC study described above. In the ACOSOG Z1031B study (46), the subsequent systemic treatment was decided based on Ki-67 LI 2 to 4 weeks after neoadjuvant AI. When Ki-67 LI was 10% or less, NAE was continued, and when Ki-67 LI exceeded 10%, it was changed to NAC. The pCR rates after NAC was only 5.7%. This result was far below the pCR rates threshold of 20% in this study. The tumor response on NAC may be limited in cases of low tumor responsiveness to NAE. In the JBCRG-11CPA trial (47), patients were initially treated with neoadjuvant exemestane for 8 to 12 weeks. After that, comprehensive tumor evaluation was performed based on clinical response and measurement of Ki-67 LI. Patients with complete response (CR), partial response (PR) with Ki-67 index ≤5% after treatment, or SD with Ki-67 index ≤5% before and after treatment were defined as responders. Patients with others condition was defined as non-responder. Responders continued exemestane for the subsequent 24 weeks, while non-responders were treated with a combination of cyclophosphamide and exemestane for 24 weeks. At 36 weeks after NAE initiation, clinical response rates (responder 71% vs. non-responder 71%) and Ki-67 LI (responder 3.5% vs. non-responder 4.0%) did not differ between two groups. It has been shown that concomitant use of cyclophosphamide and endocrine treatment achieved tumor response equivalent to responders when ineffective tumor response after short-term NAE. The effect of this combination on long-term prognosis has not been reported and this result is expected in the near future.
Although there are several issues to be resolved regarding the selection of treatment using Ki-67 LI, such as intratumoral heterogeneity and analytical validity, this assay is simple and inexpensive and requires validation studies to be undertaken to lead to clinical applications.
Conclusions
NAE for HR-positive/HER2-negative stage II or III primary breast cancer with high hormone sensitivity improves the surgical outcome, and its therapeutic response is useful for predicting prognosis. Clinical trials are underway to develop more effective treatment strategies based on short-term responsiveness to NAE to improve the prognosis of HR-positive/HER2-negative breast cancer.
Acknowledgments
We would like to thank NAI (www.nai.co.jp) for English language editing.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Yutaka Yamamoto and Takayuki Ueno) for the series “Neoadjuvant/Adjuvant Treatment for Early Breast Cancer” published in Chinese Clinical Oncology. The article was sent for external peer review organized by the Guest Editors and the editorial office.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/cco.2020.03.02). This series “Neoadjuvant/Adjuvant Treatment for Early Breast Cancer” was commissioned by the editorial office without any funding. YY reports personal fees from AstraZeneca, personal fees from Pfizer, personal fees from Novartis, personal fees from Nippon Kayaku, from Kyowa-Hakko-Kirin, during the conduct of the study; personal fees from Chugai, personal fees from Esai, from Lilly, from Takeda, from Sysmex, from GE Health Care Japan, from Daiichi Sankyo, outside the submitted work; and a board member of the Japanese Breast Cancer Society, a board member of the Japan Breast Cancer Research Group. YY served as the unpaid Guest Editor of the series, and serves as an unpaid editorial board member of Chinese Clinical Oncology from May 2019 to April 2021. The other authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki. Ethical approval and informed consent were not required, since this is a review paper.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Iwase H, Kurebayashi J, Tsuda H, et al. Clinicopathological analyses of triple negative breast cancer using surveillance data from the Registration Committee of the Japanese Breast Cancer Society. Breast Cancer 2010;17:118-24. [Crossref] [PubMed]
- Early Breast Cancer Trialists' Collaborative Group (EBCTCG), Davies C, Godwin J, et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet 2011;378:771-84. [Crossref] [PubMed]
- Chiba A, Hoskin TL, Heins CN, et al. Trends in neoadjuvant endocrine therapy use and impact on rates of breast conservation in hormone receptor-positive breast cancer: a National Cancer Data Base Study. Ann Surg Oncol 2017;24:418-24. [Crossref] [PubMed]
- Eiermann W, Paepke S, Appfelstaedt J, et al. Preoperative treatment of postmenopausal breast cancer patients with letrozole: a randomized double-blind multicenter study. Ann Oncol 2001;12:1527-32. [Crossref] [PubMed]
- Smith IE, Dowsett M, Ebbs SR, et al. Neoadjuvant treatment of postmenopausal breast cancer with anastrozole, tamoxifen, or both in combination: the Immediate Preoperative Anastrozole, Tamoxifen, or Combined with Tamoxifen (IMPACT) multicenter double-blind randomized trial. J Clin Oncol 2005;23:5108-16. [Crossref] [PubMed]
- Cataliotti L, Buzdar AU, Noguchi S, et al. Comparison of anastrozole versus tamoxifen as preoperative therapy in postmenopausal women with hormone receptor-positive breast cancer: the Pre-Operative "Arimidex" Compared to Tamoxifen (PROACT) trial. Cancer 2006;106:2095-103. [Crossref] [PubMed]
- Ellis MJ, Suman VJ, Hoog J, et al. Randomized phase II neoadjuvant comparison between letrozole, anastrozole, and exemestane for postmenopausal women with estrogen receptor-rich stage 2 to 3 breast cancer: clinical and biomarker outcomes and predictive value of the baseline PAM50-based intrinsic subtype--ACOSOG Z1031. J Clin Oncol 2011;29:2342-9. [Crossref] [PubMed]
- Kuter I, Gee JM, Hegg R, et al. Dose-dependent change in biomarkers during neoadjuvant endocrine therapy with fulvestrant: results from NEWEST, a randomized Phase II study. Breast Cancer Res Treat 2012;133:237-46. [Crossref] [PubMed]
- Masuda N, Sagara Y, Kinoshita T, et al. Neoadjuvant anastrozole versus tamoxifen in patients receiving goserelin for premenopausal breast cancer (STAGE): a double-blind, randomised phase 3 trial. Lancet Oncol 2012;13:345-52. [Crossref] [PubMed]
- Spring LM, Gupta A, Reynolds KL, et al. Neoadjuvant endocrine therapy for estrogen receptor-positive breast cancer: a systemic review and meta-analysis. JAMA Oncol 2016;2:1477-86. [Crossref] [PubMed]
- Semiglazov VF, Semiglazov VV, Dashyan GA, et al. Phase 2 randomized trial of primary endocrine therapy versus chemotherapy in postmenopausal patients with estrogen receptor-positive breast cancer. Cancer 2007;110:244-54. [Crossref] [PubMed]
- Alba E, Calvo L, Albanell J, et al. Chemotherapy (CT) and hormonotherapy (HT) as neoadjuvant treatment in luminal breast cancer patients: results from the GEICAM/2006-03, a multicenter, randomized, phase-II study. Ann Oncol 2012;23:3069-74. [Crossref] [PubMed]
- Palmieri C, Cleator S, Kilburn LS, et al. NEOCENT: a randomised feasibility and translational study comparing neoadjuvant endocrine therapy with chemotherapy in ER-rich postmenopausal primary breast cancer. Breast Cancer Res Treat 2014;148:581-90. [Crossref] [PubMed]
- Toi M, Saji S, Masuda N, et al. Ki67 index changes, pathological response and clinical benefits in primary breast cancer patients treated with 24 weeks of aromatase inhibition. Cancer Sci 2011;102:858-65. [Crossref] [PubMed]
- Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 2014;384:164-72. [Crossref] [PubMed]
- Masuda N, Lee SJ, Ohtani S, et al. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N Engl J Med 2017;376:2147-59. [Crossref] [PubMed]
- von Minckwitz G, Huang CS, Mano MS, et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med 2019;380:617-28. [Crossref] [PubMed]
- Kurosumi M, Akashi-Tanaka S, Akiyama F, et al. Histopathological criteria for assessment of therapeutic response in breast cancer (2007 version). Breast Cancer 2008;15:5-7. [Crossref] [PubMed]
- Akashi-Tanaka S, Omatsu M, Shimizu C, et al. Favorable outcome in patients with breast cancer in the presence of pathological response after neoadjuvant endocrine therapy. Breast 2007;16:482-8. [Crossref] [PubMed]
- Dowsett M, Smith IE, Ebbs SR, et al. Prognostic value of Ki67 expression after short-term presurgical endocrine therapy for primary breast cancer. J Natl Cancer Inst 2007;99:167-70. [Crossref] [PubMed]
- Ellis MJ, Tao Y, Luo J, et al. Outcome prediction for estrogen receptor-positive breast cancer based on postneoadjuvant endocrine therapy tumor characteristics. J Natl Cancer Inst 2008;100:1380-8. [Crossref] [PubMed]
- Ueno T, Saji S, Masuda N, et al. Clinical significance of the expression of autophagy-associated marker, beclin 1, in breast cancer patients who received neoadjuvant endocrine therapy. ESMO Open 2018;3:e000314. [Crossref] [PubMed]
- Iwata H, Masuda N, Fujisawa T, et al. NEOS: A randomized, open label, phase 3 trial of adjuvant chemotherapy for postmenopausal breast cancer patients who responded to neoadjuvant letrozole: First report of long-term outcome and prognostic value of response to neoadjuvant endocrine therapy [abstract]. In: San Antonio: Proceedings of the 2017 San Antonio Breast Cancer Symposium, 2017. Philadelphia: AACR; Cancer Res 2018;78:Abstract nr P3-13-03.
- Mustacchi G, Ceccherini R, Milani S, et al. Tamoxifen alone versus adjuvant tamoxifen for operable breast cancer of the elderly: long-term results of the phase III randomized controlled multicenter GRETA trial. Ann Oncol 2003;14:414-20. [Crossref] [PubMed]
- Mustacchi G, Scanni A, Capasso I, et al. Update of the Phase III trial 'GRETA' of surgery and tamoxifen versus tamoxifen alone for early breast cancer in elderly women. Future Oncol 2015;11:933-41. [Crossref] [PubMed]
- Bates T, Riley DL, Houghton J, et al. Breast cancer in elderly women: a Cancer Research Campaign trial comparing treatment with tamoxifen and optimal surgery with tamoxifen alone. The Elderly Breast Cancer Working Party. Br J Surg 1991;78:591-4. [Crossref] [PubMed]
- Dowsett M, Smith I, Robertson J, et al. Endocrine therapy, new biologicals, and new study designs for presurgical studies in breast cancer. J Natl Cancer Inst Monogr 2011;2011:120-3. [Crossref] [PubMed]
- Robertson JFR, Dowsett M, Bliss JM, et al. Peri-operative aromatase inhibitor treatment in determining or predicting longterm outcome in early breast cancer – The POETIC* Trial (CRUK/07/015) [abstract]. In: San Antonio: Proceedings of the 2017 San Antonio Breast Cancer Symposium, 2017. Philadelphia: AACR; Cancer Res 2018;78:Abstract nr GS1-03.
- Baselga J, Semiglazov V, van Dam P, et al. Phase II randomized study of neoadjuvant everolimus plus letrozole compared with placebo plus letrozole in patients with estrogen receptor-positive breast cancer. J Clin Oncol 2009;27:2630-7. [Crossref] [PubMed]
- Guarneri V, Generali DG, Frassoldati A, et al. Double-blind, placebo-controlled, multicenter, randomized, phase IIb neoadjuvant study of letrozole-lapatinib in postmenopausal hormone receptor-positive, human epidermal growth factor receptor 2-negative, operable breast cancer. J Clin Oncol 2014;32:1050-7. [Crossref] [PubMed]
- Saura C, Hlauschek D, Oliveira M, et al. Neoadjuvant letrozole plus taselisib versus letrozole plus placebo in postmenopausal women with oestrogen receptor-positive, HER2-negative, early-stage breast cancer (LORELEI): a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol 2019;20:1226-38. [Crossref] [PubMed]
- Ma CX, Suman V, Goetz MP, et al. A phase II trial of neoadjuvant MK-2206, an AKT inhibitor, with anastrozole in clinical stage II or III PIK3CA-mutant ER-positive and HER2-negative breast cancer. Clin Cancer Res 2017;23:6823-32. [Crossref] [PubMed]
- Ma CX, Gao F, Luo J, et al. NeoPalAna: neoadjuvant palbociclib, a cyclin-dependent kinase 4/6 inhibitor, and anastrozole for clinical stage 2 or 3 estrogen receptor-positive breast cancer. Clin Cancer Res 2017;23:4055-65. [Crossref] [PubMed]
- Chow LWC, Morita S, Chow CY, et al. Neoadjuvant palbociclib on ER+ breast cancer (N007): clinical response and EndoPredict's value. Endocrine-Related Cancer 2018;25:123-30. [Crossref] [PubMed]
- Hurvitz SA, Martin M, Press MF, et al. Potent cell-cycle inhibition and upregulation of immune response with abemaciclib and anastrozole in neoMONARCH, phase II neoadjuvant study in HR+/HER2- breast cancer. Clin Cancer Res 2020;26:566-80. [Crossref] [PubMed]
- Johnston S, Puhalla S, Wheatley D, et al. Randomized phase II study evaluating palbociclib in addition to letrozole as neoadjuvant therapy in estrogen receptor-positive early breast cancer: PALLET trial. J Clin Oncol 2019;37:178-89. [Crossref] [PubMed]
- Dixon JM, Renshaw L, Macaskill EJ, et al. Increase in response rate by prolonged treatment with neoadjuvant letrozole. Breast Cancer Res Treat 2009;113:145-51. [Crossref] [PubMed]
- Krainick-Strobel UE, Lichtenegger W, Wallwiener D, et al. Neoadjuvant letrozole in postmenopausal estrogen and/or progesterone receptor positive breast cancer: a phase IIb/III trial to investigate optimal duration of preoperative endocrine therapy. BMC Cancer 2008;8:62. [Crossref] [PubMed]
- Fontein DB, Charehbili A, Nortier JW, et al. Efficacy of six month neoadjuvant endocrine therapy in postmenopausal, hormone receptor-positive breast cancer patients--a phase II trial. Eur J Cancer 2014;50:2190-200. [Crossref] [PubMed]
- Hojo T, Kinoshita T, Imoto S, et al. Use of the neo-adjuvant exemestane in post-menopausal estrogen receptor-positive breast cancer: a randomized phase II trial (PTEX46) to investigate the optimal duration of preoperative endocrine therapy. Breast 2013;22:263-7. [Crossref] [PubMed]
- Llombart-Cussac A, Guerrero Á, Galán A, et al. Phase II trial with letrozole to maximum response as primary systemic therapy in postmenopausal patients with ER/PgR[+] operable breast cancer. Clin Transl Oncol 2012;14:125-31. [Crossref] [PubMed]
- Carpenter R, Doughty JC, Cordiner C, et al. Optimum duration of neoadjuvant letrozole to permit breast conserving surgery. Breast Cancer Res Treat 2014;144:569-76. [Crossref] [PubMed]
- Taira N, Iwata H, Hasegawa Y, et al. Health-related quality of life and psychological distress during neoadjuvant endocrine therapy with letrozole to determine endocrine responsiveness in postmenopausal breast cancer. Breast Cancer Res Treat 2014;145:155-64. [Crossref] [PubMed]
- Ueno T, Masuda N, Yamanaka T, et al. Evaluating the 21-gene assay Recurrence Score® as a predictor of clinical response to 24 weeks of neoadjuvant exemestane in estrogen receptor-positive breast cancer. Int J Clin Oncol 2014;19:607-13. [Crossref] [PubMed]
- Iwata H, Masuda N, Yamamoto Y, et al. Validation of the 21-gene test as a predictor of clinical response to neoadjuvant hormonal therapy for ER+, HER2-negative breast cancer: the TransNEOS study. Breast Cancer Res Treat 2019;173:123-33. [Crossref] [PubMed]
- Ellis MJ, Suman VJ, Hoog J, et al. Ki67 proliferation index as a tool for chemotherapy decisions during and after neoadjuvant aromatase inhibitor treatment of breast cancer: results from the American College of Surgeons Oncology Group Z1031 Trial (Alliance). J Clin Oncol 2017;35:1061-9. [Crossref] [PubMed]
- Sato N, Masuda N, Morimoto T, et al. Neoadjuvant endocrine therapy with exemestane followed by response-guided combination therapy with low-dose cyclophosphamide in postmenopausal patients with estrogen receptor-positive breast cancer: a multicenter, open-label, phase II study. Cancer Med 2018;7:3044-56. [Crossref] [PubMed]


