The positioning of economic principles under the changing conditions of the novel drug developmental process in cancer
Introduction
The burden of cancer to society can be defined and described as a health burden and as an economic burden. Both definitions are based on epidemiological data on incidence and mortality. In 2012, the estimated number of newly diagnosed cases of cancer was 2.7 million (1.45 million men, 1.21 million women) in the European Union (EU)-28 for example (1). Some 1.3 million people (715,000 men, 560,000 women) died from cancer in the EU-28 (1), and similar figures are expected for 2013 (2). This makes cancer the second most common cause of death in the EU after diseases of the circulatory system (3).
The increasing knowledge on cancer based on tumour biology has resulted in more individualized diagnostics and treatments. This makes the need of an increasing understanding of the economic implications of this paradigm shift. This report discusses the challenges in relation to this paradigm shift in drug development and evaluation.
Background
Burden of cancer
The most common measure of the health burden to society is disability adjusted life years (DALYs). This is a measure combining mortality and disability, developed by the World Health Organization (WHO) and the World Bank (4). One DALY represents one year of “healthy” life lost and the burden is a measurement of the gap between actual health status and an ideal situation where everyone lives into old age free of disease and disability. In 2002, cancer accounted for more than 10 million DALYs in the European countries. On average, cancer accounted for 16% of the total burden of disease, but the share varies from 11% in Estonia to almost 18% in the Netherlands (5,6). The share has been relatively stable over the last two decades (7). Cancer was third in size after mental illnesses and cardiovascular disease.
The proportions of years of life lost (YLL) and years lost due to disability (YLD) of a DALY vary considerable depending on disease group; for cancer, YLL represent over 90% of the DALYs in Europe, YLL represent 70-90% of DALYs for mental disease, cardiovascular disease and injuries, whereas for respiratory disease YLL represent less than 40% of DALYs.
The burden to society can also be measured in monetary terms as the value of production lost due to early mortality and disability due to cancer, and resources used for treatment. These resources, called direct costs, include prevention, diagnosis, treatment, rehabilitation and palliative care and other related costs. The identification, measurement and valuation of direct and indirect costs of a disease like cancer are complicated, but the methods for such studies are well developed. A growing problem with cancer increasingly being a disease in older persons is to separate the costs of cancer from the costs associated with different co-morbidities.
Assessment of outcome for health economics
For assessments of outcome in cancer, the most important variables are survival, life years gained (LYG) and quality of life (QoL). These two dimensions are sometimes combined in a composite measure, quality adjusted life years (QALY). In economic evaluations, cost per LYG and cost per QALY gained are calculated as measures of cost-effectiveness (CE). Both can be compared both between different cancers and between cancer and other diseases.
Ideally, QoL when used for construction of QALY, should be measured with a preference-based instrument such as EQ5D (8). There are also other instruments for measurement of QoL, which can be aggregated to a global score for use in constructing QALY; SF36, HUI, and 15D (9-11). The more frequent observations of QoL the better and measures should be related to different stages of the disease.
Direct medical costs
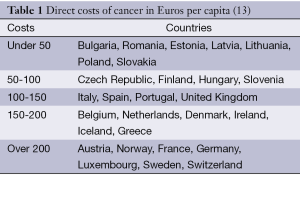
The expenditures on health care and on cancer care vary greatly within and between countries. Even though cancer causes a large economic burden to society, few countries have actually estimated how large these costs really are. It is also difficult to compare the costs across countries as the burden in terms of incidence, prevalence and mortality differs between the approximately 200 different cancer diagnoses. The provision of equipment, accessibility of drugs and the organization of the provision of treatment vary also between countries (12). Direct costs of cancer in Europe are shown in Table 1.
We also know that health care spending does not immediately translate into improved health, as the USA spends about twice the amount of the Organization for Economic Co-operation and Development (OECD) average and the life expectancy is below that of OECD average (14). Jönsson and Wilking estimated the average expenditure on cancer care in Europe in 2004 to €125 per capita or 6.4% of the total health care costs (13). In 2005, the estimated share of total health care costs devoted to cancer care in Czech Republic, Hungary and Poland was about 5% (15). Related to the large differences in gross domestic product (GDP), spending on health care and cancer varies largely.
Clinical efficacy
This first part of the clinical development process will merely show that treatment may work (clinical efficacy). In advanced stages of cancer, the measure of progression-free survival (PFS) is a commonly accepted endpoint, although the PFS is not immediately transferable to prolonged overall survival (OS) (16). There may be initial interesting data on a treatment with effects on PFS, later showing a limited effect on OS. Among others, there may be biological factors behind (17). Even more problems occur when in economic evaluations improvements in survival must be estimated from data on outcome in terms of progression or other surrogate parameters (18).
There is need for further research to establish relevant outcome prediction of the link between surrogate endpoints used in trials and patient relevant outcomes as survival and QoL effects.
Other limitations with clinical controlled trials are that patients do not mirror patients treated in clinical practice; the patients in clinical trials are usually younger and with fewer co-morbidities, and/or the comparator arm may not reflect standard of care. It is often necessary to undertake follow-up studies for outcome in clinical practice. Reassessment of reimbursement status can also be carried out [coverage by evidence development and pay-for-performance (P4P)] (19).
Clinical effectiveness (CLE)
Clinical strategies differ in many ways from clinical trials and there is a need to develop knowledge of outcome in clinical practice, i.e., CLE (20).
CLE should be steered toward critical discussions related to health care costs, CE, and the comparative value of the available options for appropriate care of patients with cancer (21).
For health economic evaluations, it is important to have linked data on resource use and outcome. It is therefore necessary to collect both types of data simultaneously for individual patients. CE is a measure of efficiency or value for money, not property of a specific drug or other treatment alternative, in a defined patient in relation to a defined alternative. Relative effectiveness (RE) compares the outcome of different alternatives. It is possible to make predictions about RE from clinical trials for different target populations or treatment strategies CE, but, those predictions are uncertain, and there is a need to verify the predictions in clinical practice. Complementary studies after-market authorization should be the norm in the future (22).
In personalized cancer medicine, it is also necessary to collect a number of biomedical markers which may affect outcome. This is nothing new, neither for assessment of outcome and CE for other diseases (e.g., cardiovascular disease) nor for cancer. The assessment of outcome in cardiology was developed as a combination of information from clinical trials and from large observational studies, such as the Framingham study, started in 1948, which continues to provide data (23). Patient characteristics, such as age, sex, smoking, etc. was combined with biomarkers, such as blood pressure and lipid levels (24). In breast cancer, the characterization of patients according to stage of the cancer and the expression of genes for estrogen receptor (ER) and progesterone receptor (PR) has a long tradition, and recently, the expression of HER2 has been added. There are a rapidly increasing number of biomarkers (targets) that are used in the management of cancer treatment. With the move towards personalized medicine, there is an increased need for studies in clinical practice, as the target groups are relatively small and the costs per QALY may be high (25). Another view is that the new paradigm changes, not only the regulatory assessments, but also the broader aspects of the technology should be assessed (26). One advantage of targeted therapies is that the link between the development and the unmet medical need becomes more precise. The co-development of a test and a treatment also makes it possible to define the target population for the treatment, which is important for the translation of results from clinical trials to clinical practice; i.e., assessment of the RE. This will reduce the problem in cancer in those treatments often used outside approved indications.
The number of combined testing and treatment strategies can easily increase to a level where the result of the analysis becomes difficult to understand and to communicate to decision makers. HER2 testing and treatment with trastuzumab (Herceptin®, Roche) is a good example. The choice is not only between different tests, or the sequence of tests, but also between different cut-off levels for the test results (27).
Countries could set up CLE studies together and updated data would be achieved faster. In this process, good data are needed, and it must be generated in a controlled format. The minimum amount of data required is data on tumor biology and follow-up on diagnostics, given treatments, and relevant co-morbidities. The relevant outcome variables are survival and QoL.
There are several instruments developed for resource allocation at the individual patient level. The most well-known one is resource utilization in dementia (RUD), which has been developed for Alzheimer’s disease (28). This instrument should be the first option in cancer, also a chronic disease among mainly elderly patients.
This area of cancer care is underdeveloped, and investment and organisation of data collection must be given highest priority. Patient records should be organised, making extraction of data easily. It is important for oncologists and health economists to work out what data on patient and treatment are necessary for the assessment of outcome. We need to “economize” to make sure that the most important data are collected once only and with good quality.
The cancer drug market in Europe
The European market for oncology drugs is in practice controlled by government funded health care (13). Until recently, all available oncology drugs were reimbursed by the health care systems with very few restrictions on their use. Within the EU, there is a centralized procedure for market authorization of drugs.
Certain drugs may be given a simplified or accelerated approval procedure. These are usually drugs for serious and life-threatening illnesses. Such exceptional circumstances often apply to drugs for rare cancers or cancers with high mortality.
Drugs used in ambulatory care require formal decisions on reimbursement and pricing in most countries, while drugs used in hospitals are often covered by the hospital budget (this applies to most cancer drugs).
Most of the countries in Europe have formal procedures for making national reimbursement decisions, while in the UK, there is no specific procedure before the drug may be prescribed under the reimbursement system. For countries with formal decision processes, the reimbursement decisions include price negotiations and estimates of the sales forecasts (29). In the UK, the Pharmaceutical Price Regulation Scheme (PPRS) of the Department of Health controls pharmaceutical company profits and negotiates price cuts and paybacks from companies.
In Belgium, Finland, the Netherlands, Norway, Portugal, and Sweden, the formalised decision-making process requires an economic evaluation and CE plays an important role in this process. For Denmark and Switzerland, economic evaluation and CE data are not a formalized part of the decision-making process, but the producer may submit supportive data which may facilitate a positive decision.
Price and value
The CE ratio, the cost per (quality-adjusted) LYG from new oncology drugs, is strongly dependent on the price of the drug. One important factor with CE studies is that they should be regarded as relevant to a wide group of stakeholders and the design should therefore be considered carefully; whether a prospective or retrospective design should be applied depends on the aim. The questions in CE studies should usually be broad and the endpoints be relevant for the stakeholders (30).
The high prices reflect the role of the patent system to give incentives for development of new drugs. The relevance for HTA is which price should be used for the technology assessment. The straightforward alternative is to use the price asked by the supplier of the drug. But, the price does not reflect the cost of producing the drugs, since a major part of the price is for compensation of earlier development costs. The price reduction after patent expiration of often 90% or more-tamoxifen and docetaxel are examples-illustrates the gap between production cost and price.
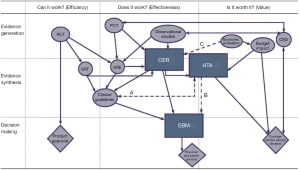
Many cancer drugs are developed for several indications. Often, the development starts with treatment of metastatic disease, and will be extended later to adjuvant treatment. It may also be possible to identify groups of patients within the same indication that have different benefits from the treatment. Since the drug comes at a single price, this may lead to differences in CE between indications, unless there is flexibility in pricing. A further problem is the different ability to pay for new cancer drugs in different countries, which makes the use of new drugs very low in some countries (13). The re-defined relationships of evidence processes are shown in Figure 1.
Economic evaluation in cancer
Health technology assessment (HTA)
HTA was developed as a policy instrument in the US in the 1970s and introduced in Europe in the 1980s. Initially, the focus was on diagnostics and procedures, but successively the target has shifted to new pharmaceuticals.
HTA has over time become more closely linked to specific decisions about reimbursement and allocation of resources in health care (32). Earlier HTA studies reviewed the evidence and published the findings with a very weak link to clinical and administrative decisions. The establishment of the National Institute for Health and Care Excellence (NICE) in 1999 is a good example of the shift in focus for HTA; HTA assessment linked to guidance for resource allocation and strong focus on new (oncology) drugs, and a key role for CE as a decision criterion (33).
HTA is defined as an assessment of all relevant aspects of a technology, CLE, safety, economic, social and ethical aspects (34). The economic aspects of HTAs were in the beginning a minor part and if included at all, very often reduced to simple calculations of treatment costs. But, when HTA studies started to be more closely linked to reimbursement decisions, the role of formal and explicit economic evaluations increased in importance. With NICE, economic evaluations became the centre point, including information on effectiveness, safety and costs, integrated in a model used for assessment of value for money for a new technology, compared to relevant alternatives in a defined indication. Such studies do not only determine price and access, but also position the new technology on the market through restrictions on reimbursement within the licenced indication (35).
An important shortcoming in HTA assessments is that they are based on data from clinical studies, as no data on CLE are available. Thus, HTA studies require updates as the knowledge of the technology increases over time. Actually, NICE spend 80% of its recourses on updates (36).
Consequences of HTA for regulatory approval
HTA and economic evaluation have been described as a “fourth” hurdle for drug approval. But, this is a too simplified view of HTA. HTA does not take assessment for market authorization as a given input. In most cases, data are re-analysed and used for different purposes compared to the market authorization, which assess the drug, only in medical aspects. Focus may be on different comparators, endpoints and subgroups of patients, and the available clinical trial data may not always be suitable. Over time, we will see an impact on which studies undertaken during development, for which patient groups and with which endpoints. For several years now, pharmaceutical companies investing in development of new drugs are engaged in early HTA or joint HTA/regulatory advice (37), trying to find out how clinical trials should be designed in order to satisfy not only criteria for effectiveness and safety, but also wider criteria set up by HTA and reimbursement agencies.
Are new oncology drugs different?
HTA is used as a policy instrument for informed decisions about resource use between competing demands. The aim is to create most value for money, directing spending on technologies, which give most benefits for the actual and potential patients (who also pay for the services through taxes). The principles for how such studies are carried out should not differ between diseases or type of technology. There is also a strong resistance by researchers to develop disease and technology specific methods and criteria for HTA and economic evaluation (38). But in practice, we may see both adaptations of methods and criteria to the specific characteristics of diseases and technologies. It is for example more common to have a social perspective on costs in assessment of public health interventions than for pharmaceuticals and surgical procedures (39). The cost per QALY is an established benchmark for CE, despite controversies about its value base and which benchmark value to use. It has been more difficult, similar to what we see for regulatory decisions about market authorization, to introduce explicit quantitative methods for comparing other factors that are important for the decision maker (40).
The most striking differences between oncology drugs and other technologies are the different criteria used by NICE. New technology drugs (many cancer drugs) are accepted at a significantly higher cost per QALY (GBP 50,000) compared to GBP 38,000 for other drugs (41).
NICE has rationalized this difference in the guidelines, recently published. A life-extending treatment aimed at small groups of patients, with a life expectancy of less than 24 months, should be accepted at a higher cost per QALY (42). Even if this is not explicitly directed towards oncology drugs, the aim may be to find a more general definition and avoid a reference to oncology drugs.
Short life expectancy is a characteristic of many patients treated with new oncology drugs. The QALY take into account life expectancy, as well as QoL. Since QoL is often low in the late stages of cancer, the gain in survival is reduced by the QoL weight used. It has thus been discussed if the QALY fully capture the benefits of new cancer drugs (43,44).
Another aspect of oncology drugs is the uncertainty about outcome due to a pressure to take new drugs fast to the market. For many oncology drugs aimed at small groups of patients, it is difficult to undertake conclusive studies within a short time frame. It is also common that new as well as old oncology drugs are used outside the approved indications with limited evidence on outcome. Due to this uncertainty, and the binary outcomes of life and death, we may be willing to pay a little extra for the chance of higher benefit than expected (45). The principle is often doing too much instead of too little, particularly if side effects are limited.
Targeted therapies and personalized medicine
Targeted cancer therapeutics are drugs or other substances that block the growth and spread of cancer by interfering with specific molecules involved in tumor growth and progression. Personalized medicine uses information about a person’s genes, proteins, and environment to prevent, diagnose, and treat disease (46). The co-development of a test and a treatment is a new and important strategy in the development of new cancer drugs—a so-called companion diagnostics development. The consequences for HTA of this new strategy have not yet been worked out. One view is that the basic principles of HTA still apply and that this is not different from diagnostics and treatments in other diseases. Another view is that this new paradigm changes, not only the regulatory assessments, but also the broader aspects of the technology assessed (26).
One advantage of targeted therapies is that the link between development and the unmet medical need it addresses becomes clearer and more precise, which is important for the translation of results from clinical trials to clinical practice; i.e., assessment of the RE (focus of HTA). Since HTAs start from the concept of the unmet medical need, this is an important step forward. This will reduce the problem for HTA in cancer (treatments are often used outside approved indications).
The joint assessment of a diagnostics and a treatment has additional methodological complexities compared to analysing both separately, and the data requirements are increased. The number of combined testing and treatment strategies can easily increase to a level where the result of the analysis becomes difficult to understand and communicate to decision makers. HER2-testing and treatment with trastuzumab (in breast cancer) as well as KRAS testing in relation to the use of anti-EGFR drugs (cetuximab and panitumumab) are good examples. The choice is not only between different tests, or the sequence of tests, but also between different cut-off levels for the test results (47). In addition, a technology assessment must take into account the translation of test and treatment results from the clinical trials into clinical practice. When a new combined technology is introduced, the health care system must have the competence and resources for implementation that maintain the high quality in the whole process of patient care. Personalized medicine is an established concept for drug development, but health care systems are not yet designed to translate the new technologies into cost-effective clinical practice.
Methodological aspects in economic evaluation
An economic evaluation, aimed at providing information about the CE of a new technology, is an increasingly important part of HTA. There are several steps from translating the results from clinical trials into an assessment of CE. One obvious problem is that it is seldom possible to base an economic evaluation directly on the results from a clinical trial. Most clinical trials provide information on gains in median survival, while an economic evaluation uses the mean survival for the calculations (48). Even more problems occur when improvements in survival in economic evaluations must be estimated from data on outcome in terms of disease progression or other surrogate parameters (18).
The preferred outcome measure in economic evaluation is QALY. This requires data on the patient’s QoL during treatment and follow-up. While such data, since long time, have been part of clinical trials in oncology, the economic evaluation requires the data collected with appropriate instruments and preferably on several occasions.
Economic evaluations also include information on costs. While it is helpful to have resource utilization data collected in clinical trials, there is a need to collect data on local costs to make results relevant for decision makers. It is also necessary to make predictions of how costs may change after the end of the clinical trial, related to outcome.
For all the reasons above, it is standard practice that CE is calculated in sophisticated statistical models, which includes predictions about both costs and outcome of different testing and treatment strategies. While regulatory decision about market access is based mainly on data derived from clinical trials, the economic evaluation is an estimate of CE under different assumptions. Even if the degree of uncertainty in CE studies can be calculated, the validity may be challenged. It is thus often necessary to undertake follow-up studies in clinical practice to evaluate to what extent the estimates could be confirmed in clinical practice. Increasingly such follow-up studies are linked to reassessment of reimbursement status or even payment [coverage by evidence development and P4P (19)].
What is the alternative (to HTA)?
Over the last three decades, HTA has evolved as one of the most important new health policy instruments. The increase in the number of national HTA agencies as well as the creation of institutions at the European level for HTA are examples of this (34). The transition from assessment of well-established non-pharmaceutical technologies to assessment of new oncology drugs is also an example of the use of this new policy instrument. But, it may also reflect a lack of good alternatives. Innovation and new technologies are the driving forces behind the improvements in efficiency and quality of health care systems but also a constant pressure on public budgets. Traditional cost containment policies, focusing on budgets, prices and control of input of resources, do not work. The focus in health care policy has shifted from input to output; i.e., improvements in outcome and quality within. HTA is a policy instrument that can offer help for the difficult decision about trade-offs between different resources and outcomes. The only realistic alternative is to take a step back on public finance of health care towards increasing use of co-payments and thus letting the individual patient take a greater responsibility for the choices.
This is not a realistic scenario for cancer and new oncology drugs based on the seriousness of the diseases and on the very high treatment costs. Even moderate co-payments of 20-30% will make access to new treatments a matter of ability to pay rather than a real choice between relevant alternative treatments.
The primary policy option should be to make HTA a more efficient instrument for the necessary priorities, both in the short run and in the long run, through incentives for innovation. The two most obvious things to do would be increased international collaboration in collection and analysis of data related to a specific oncology drug, and more studies of the costs and outcome of the actual use of oncology drugs in clinical practice. That will form the basis for specific RE studies, which are directly set up to answer key questions for technology assessment. More systematic studies of HTA as a policy instrument in oncology, looking into the link between technology assessments, decisions and outcomes in different jurisdictions over time, are also important to help in building knowledge how to optimize the use of HTA as a health policy instrument. This new landscape for the evaluation of the value of new cancer drugs is summarized in Figure 1.
Concluding remarks
Cancer is a major health care problem, especially in developed countries (like Europe), but is rapidly becoming a major part of health care also in developing countries. New cancer drugs have increased cure rates in most cancers. New cancer drugs have also increased the time with disease and symptom control in most cancers. This revolution in cancer care has come at a dramatic increase in the cost of cancer care. It is therefore of importance that new innovations are properly evaluated and health care resources are distributed fair and rationally. There is huge uncertainty about outcome of most new cancer drugs at regulatory approval in relation to efficacy and tolerance, but even more about the overall value in relation to costs. A proper HTA evaluation is therefore a key component. Our position, as pointed out in this paper, is that new cancer drugs should be evaluated by the same standards and principles as other pharmaceuticals, with an emphasis on LYG and special efforts on post-approval, population-based studies on outcome (CLE).
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, et al. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries in 2012. Eur J Cancer 2013;49:1374-403. [PubMed]
- Malvezzi M, Bertuccio P, Levi F, et al. European cancer mortality predictions for the year 2013. Ann Oncol 2013;24:792-800. [PubMed]
- Eurostat. Causes of death - Standardised death rate (per 100,000 inhabitants) (Annual Data) [hlth_cd_asdr]. Available online: http://epp.eurostat.ec.europa.eu/portal/page/portal/product_details/publication?p_product_code=KS-30-08-357 (accessed Oct 24, 2013).
- WHO. Quantifying the Burden of Disease from mortality and morbidity. 1990; Available online: http://www.who.int/healthinfo/global_burden_disease/metrics_daly/en/
- Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010;127:2893-917. [PubMed]
- de Pouvourville G. Risk-sharing agreements for innovative drugs: a new solution to old problems? Eur J Health Econ 2006;7:155-7. [PubMed]
- Murray CJ, Vos T, Lozano R, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2197-223. [PubMed]
- EuroQol. EQ5D. 2013; Available online: http://www.euroqol.org/
- SF36. 2013; Available online: http://www.sf-36.org/
- HUI. Preference-based (utility) measures of health-related quality of life. 2013; Available online: http://www.healthutilities.com/
- 15D-instrument. 15D the health-related quality of life (HRQoL) instrument 2013; Available online: http://www.15d-instrument.net/15d
- Wilking N, Högberg D, Kasteng F, et al. Benchmarking Report of Lung Cancer Care in selected Countries 2008; Available online: http://www.comparatorreports.se/Lung_cancer_benchmarking_July_080910.pdf
- Jönsson B, Wilking N. A global comparison regarding patient access to cancer drugs. Ann Oncol 2007;18:iii1-77. [PubMed]
- OECD. Health at a glance. 2011; Available online: http://www.oecd.org/health/health-systems/49105858.pdf
- Bonsanquet N, Attridge J, Sikora K. Can the new EU countries catch up in cancer care? 2005; Available online: http://www.lse.ac.uk/LSEHealthAndSocialCare/pdf/eurohealth/vol11No1.pdf
- Alimujiang S, Zhang T, Han ZG, et al. Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor Versus Placebo as Maintenance Therapy for Advanced Non- small-cell Lung Cancer: A Meta-analysis of Randomized Controlled Trials. Asian Pac J Cancer Prev 2013;14:2413-9. [PubMed]
- Liu Y, Starr MD, Bulusu A, et al. Correlation of angiogenic biomarker signatures with clinical outcomes in metastatic colorectal cancer patients receiving capecitabine, oxaliplatin, and bevacizumab. Cancer Med 2013;2:234-42. [PubMed]
- Latimer N. Technical Support Document 14: Undertaking survival analysis for economic evaluations alongside clinical trials - extrapolation with patient-level data. 2011; Available online: http://www.nicedsu.org.uk
- Walker S, Sculpher M, Claxton K, et al. Coverage with evidence development, only in research, risk sharing, or patient access scheme? A framework for coverage decisions. Value Health 2012;15:570-9. [PubMed]
- Frueh FW. Real-world clinical effectiveness, regulatory transparency and payer coverage: three ingredients for translating pharmacogenomics into clinical practice. Pharmacogenomics 2010;11:657-60. [PubMed]
- Lyman GH. Comparative effectiveness research in oncology. Oncologist 2013;18:752-9. [PubMed]
- Carlson JJ, Sullivan SD, Garrison LP, et al. Linking payment to health outcomes: a taxonomy and examination of performance-based reimbursement schemes between healthcare payers and manufacturers. Health Policy 2010;96:179-90. [PubMed]
- Sayin MR, Cetiner MA, Karabag T, et al. Framingham risk score and severity of coronary artery disease. Herz 2013;21:21. [PubMed]
- Framingham. Framingham Heart Study. 1948; Available online: http://www.framinghamheartstudy.org/about-fhs/history.php
- Weinstein MC, Skinner JA. Comparative effectiveness and health care spending--implications for reform. N Engl J Med 2010;362:460-5. [PubMed]
- Gaultney JG, Sanhueza E, Janssen JJ, et al. Application of cost-effectiveness analysis to demonstrate the potential value of companion diagnostics in chronic myeloid leukemia. Pharmacogenomics 2011;12:411-21. [PubMed]
- Lidgren M, Jonsson B, Rehnberg C. Cost-effectiveness of HER2 testing and 1-year adjuvant trastuzumab therapy for early breast cancer. Ann Oncol 2008;19:487-95. [PubMed]
- Wimo A, Gustavsson A, Jonsson L, et al. Application of Resource Utilization in Dementia (RUD) instrument in a global setting. Alzheimers Dement 2013;9:429-35.e17.
- EUROMET 2004. The Influence of Economic Evaluation Studies on Health Care Decision-Making - A European survey. Amsterdam: IOS Press, 2005.
- Ramsey SD, Sullivan SD, Reed SD, et al. Oncology Comparative Effectiveness Research: A Multistakeholder Perspective on Principles for Conduct and Reporting. Oncologist 2013;18:760-7. [PubMed]
- Luce BR, Drummond M, Jönsson B, et al. EBM, HTA, and CER: Clearing the confusion. 2010; Available online: http://onlinelibrary.wiley.com/enhanced/doi/10.1111/j.1468-0009.2010.00598.x/
- Drummond MF, Schwartz JS, Jonsson B, et al. Key principles for the improved conduct of health technology assessments for resource allocation decisions. Int J Technol Assess Health Care 2008;24:244-58; discussion 362-8. [PubMed]
- NICE. 2013; Available online: www.nice.org.uk
- What is HTA? 2013; Available online: http://www.eunethta.eu/about-us/faq#t287n73
- O’Neill P, Devlin NJ. An analysis of NICE’s ‘restricted’ (or ‘optimized’) decisions. Pharmacoeconomics 2010;28:987-93. [PubMed]
- Willis S. Health Economics in NICE Clinical Guidelines. 2011; Available online: http://www.ncin.org.uk/news_and_events/economics
- Backhouse ME, Wonder M, Hornby E, et al. Early dialogue between the developers of new technologies and pricing and reimbursement agencies: a pilot study. Value Health 2011;14:608-15. [PubMed]
- Sculpher M, Claxton K. Sins of omission and obfuscation: IQWIG’s guidelines on economic evaluation methods. Health Econ 2010;19:1132-6. [PubMed]
- Jönsson B. Ten arguments for a societal perspective in the economic evaluation of medical innovations. Eur J Health Econ 2009;10:357-9. [PubMed]
- Devlin N, Sussex J. Incorporating multiple criteria in HTA - Methods and processes. 2011; Available online: http://www.ohe.org/publications/article/incorporating-multiple-criteria-in-hta-methods-and-processes-8.cfm
- Dakin H, Devlin N, Feng Y, et al. The influence of cost-effectiveness and other factors on NICE decisions. 2011; Available online: http://www.herc.ox.ac.uk/downloads/NICE
- NICE. Appraising life-extending, end of life treatments. 2012; Accessed 4 August 2012. Available online: www.nice.org.uk/
- Garau M, Shah KK, Mason AR, et al. Using QALYs in cancer: a review of the methodological limitations. Pharmacoeconomics 2011;29:673-85. [PubMed]
- Tengs TO. Cost-effectiveness versus cost-utility analysis of interventions for cancer: does adjusting for health-related quality of life really matter? Value Health 2004;7:70-8. [PubMed]
- Gaskin DJ, Kong J, Meropol NJ, et al. Treatment choices by seriously ill patients: the Health Stock Risk Adjustment model. Med Decis Making 1998;18:84-94. [PubMed]
- NCI. Targeted Cancer Therapies. 2014; Available online: http://www.cancer.gov/cancertopics/factsheet/therapy/targeted
- Lidgren M, Wilking N, Jonsson B, et al. Cost-effectiveness of HER2 testing and trastuzumab therapy for metastatic breast cancer. Acta Oncol 2008;47:1018-28. [PubMed]
- Davies A, Briggs A, Schneider J, et al. The Ends Justify the Mean: Outcome Measures for Estimating the Value of New Cancer Therapies. 2012; Available online: http://www.google.se/url?sa=t&rct=j&q=&esrc=s&source=web&cd=2&ved=0CEIQFjAB&url=http%3A%2F%2Fwww.researchgate.net%2Fpublication%2F241110515_CN1_Mean_Versus_Median_Overall_Survival_%28OS%29_For_Describing_Value_of_New_Cancer_Therapies_A_Case_Study%2Ffile%2F504635211292756142.pdf&ei=Unr8UvmyAcq0yAOz5YHQBg&usg=AFQjCNFGon5V4LvCJOUTDRMBQ5uVa-71Rg&sig2=OnhMBaXwbrlbjnKINdSJSQ&bvm=bv.61190604,d.bGQ