Reducing late effects of radiotherapy in average risk medulloblastoma
Introduction
Medulloblastoma (MB) is the most common malignant brain tumor in children and constitutes 20-25% of CNS neoplasms (1). Cerebrospinal fluid metastasis accounts for 30% of cases at diagnosis (2). This metastatic feature was the cause of treatment failure for several decades following the original description of MB in 1925 by Bailey and Cushing (3).
Since the mid-1990s, the risk classification for the relapse and treatment of MB patients has remained strictly clinical, with cases stratified into two risk groups, ‘average risk’ and ‘high risk’, based on age, extent of resection, and Chang metastasis staging (4). Accordingly, average-risk patients are those older than three years of age with non-metastatic disease and totally or near totally resected tumors (<1.5 cm of residual tumor on postoperative MRI). Patients not fulfilling these criteria are regarded as high-risk. This clinical staging can be used in predicting prognosis, but it does not differentiate high- from low-risk patients within the same clinical stage as they may have biological differences within the same tumor (5).
The overall 5-year survival rate for patients with MB is 60% with surgery, CSI, and chemotherapy but is much higher for standard-risk disease. Debilitating side effects occur in almost all patients that survive radiotherapy (RT) despite the use of protons as opposed to photons. Late side effects include cognitive impairment, psychiatric disorders, endocrine dysfunction, and skeletal growth retardation (6).
The standard treatment for average-risk MB includes postoperative CSI with a dose of 23.4 Gy, irradiation of the anatomic posterior fossa (PF) to 55.8 Gy and 12 months of combination chemotherapy according to the Children’s Oncology Group (7). Reducing the neuraxis dose in the absence of chemotherapy has had limited success (8).
Despite the use of chemotherapy and neuraxis radiation dose reductions, decline in cognitive function continues to be a significant issue (9). Conventional treatment of the entire PF irradiates 35% of the brain and 60% of either temporal lobe. As a result, investigators have attempted to reduce the effects of irradiation by using new techniques to limit the boost volume to the tumor bed (10).
Our primary goal is to cure MB as we are located in a developing country with limited resources. Intensity-modulated radiation therapy (IMRT) is not widely used due to a lack of both facilities and experience. This is the first study in Egypt to consider quality of life and cognitive function in pediatric MB by reducing the volume of RT in an attempt to limit the serious late side effects of radiation.
Patients and methods
The study included 30 MB pediatric patients who attended the pediatric oncology unit in the Kasr Al Ainy Center of Clinical Oncology (NEMROCK) at Cairo University from August 2010 to March 2012. Patients were referred after maximal resection of PF space occupying lesions. The eligibility criteria were as follows: age of 3-18 years with histopathological confirmation of average-risk MB; no evidence of dissemination based on brain and spine magnetic resonance images and cerebrospinal fluid cytology; gross total resection (no evidence of disease) or near total (>90% resection) resection on postoperative neuroimaging; residual tumor (if present) diameter <1.5 cm and Chang Stage T1-T3b; no previous RT or chemotherapy; and Eastern Cooperative Oncology Group performance (ECOG) scale score of 0-2. Parents or legal guardians provided informed written consent, and data were collected after six months of the last patient’s recruitment.
Radiotherapy
All patients received a dose of 23.4 Gy to the whole brain and spinal cord (CSI). The thirty patients were divided into two groups according to the volume of the brain receiving the boost dose of RT. Each group included 15 patients. The boost was given to the whole PF in group 1 and to the tumor bed in group 2. The given dose was 32.4 Gy and reached 55.8 Gy using 3D conformal RT techniques.
CSI was initiated within 28 days of resection. The CSI volume included the subarachnoid volume, with special attention to the inclusion of the cribriform plate and temporal fossae; the full width of the spinal subarachnoid space; and the inferior aspect of the thecal sac.
The clinical target volume (CTV) for the PF boost included the entire infratentorial region, while the planning target volume (PTV) included an additional margin of 0.5 cm to account for uncertainty in immobilization and daily patient positioning.
The gross target volume (GTV) included all gross residual tumors and/or the tumor beds at the primary site, as determined using initial preoperative MRI to identify the tissues initially involved with disease. Tissue defects resulting from the surgical approaches were not included as part of the GTV. The CTV included the GTV with an added margin of 1.0 cm to cover possible subclinical microscopic disease (i.e., the CTV was limited to the confines of the bony calvaria, falx, and tentorium, as applicable, or extended up to, but not beyond, neuroanatomic structures through which tumor extension or invasion was certain not to have occurred). The PTV included a margin of 0.5 cm added to the CTV in three dimensions. The purpose of the PTV was to account for uncertainty in immobilization, image registration, and daily variability in patient positioning.
Conventional fractionation (1.8 Gy/d) was used to treat all patients. For volume-based treatment plans, the entire PTV was encompassed by the 95% isodose surface, and no more than 10% of the volume within this isodose surface received more than 110% of the prescription dose.
Patients were treated in the supine position using a mask for immobilization. The head was extended for all phases of treatment. Collimator angle and couch rotation were used to match the cranio-spinal fields, and the shifting technique was used for spinospinal fields if the spinal field could not be included in one field. Every field was verified using an electronic portal imaging device (EPID) at the first session and then on a weekly basis thereafter. All patients received RT using 6 MV photons. Intravenous general anesthesia was used for children <7 years old.
Chemotherapy
Eight cycles of Cisplatin, Lomustine (CCNU) and Vincistine were given four weeks after the end of radiation. Cisplatin was given at a dose of 75 mg/m2 IV, CCNU at 75 mg/m2/dose orally and Vincristine was given IV at a dose of 0.05 mg/Kg body weight for patients with a surface area <1. Patients with a surface area >1 received a dose of 1.4 mg/m2, with the total dose not exceeding 2 mg. Complete blood picture, creatinine (+/– creatinine clearance), and liver function tests were performed before each cycle.
- -Cisplatin and CCNU were given on D1 every sixweeks;
- -Vincristine was given on D1, 8 and 15 every six weeks;
- -Vincristine was given weekly at the same dose as during radiation therapy as a radiosensitizer.
Weekly CBC was performed during radiation therapy, MRI of the cranio-spinal region and pure tone audiometry were performed postoperatively after radiation therapy, after three and six cycles of chemotherapy, and every three months thereafter as a follow up. Objective ototoxicity was determined according to the guidelines of the Pediatric Oncology Group (POG) and was scored as follows: Grade 0, normal; Grade 1 (mild), 20 to 40 dB loss at >4 KHz; Grade 2 (moderate) ≥40 dB loss at 4 KHz; Grade 3 (severe), ≥40 dB loss at >2 KHz; and Grade 4 (profound), 40 dB loss at <2 KHz.
Dosimetric calculation of the mean doses to the cochleae, whole brain, organs at risk and the PTV in the two arms was performed. Cognitive function tests (IQ using the Stanford Binet) were performed before and after RT and every three months thereafter. Event free and overall survival was calculated from the end of radiation.
Statistical analysis
Data are presented as the mean ± standard deviation (± SD), frequencies (number of cases) and relative frequencies (percentages) as appropriate. Comparison of quantitative variables between the study groups was performed using the Mann Whitney U test for independent samples when not normally distributed. The Chi square (χ2) test was used to compare categorical data, and Fisher’s exact test was used when the expected frequency was less than 5. A probability value (P value) less than 0.05 was considered statistically significant. All statistical calculations were performed using the computer programs Microsoft Excel version 7 (Microsoft Corporation, NY, USA) and SPSS (Statistical Package for the Social Science; SPSS Inc., Chicago, IL, USA) version 13 for Microsoft Windows.
Results
The study included two prospective arms. Both included patients diagnosed with average-risk MB that were pathologically confirmed. The two arms were designed to receive craniospinal irradiation dose rates of 23.8 Gy, with a boost of 32.4 Gy to the whole PF in arm 1 and to the GTV in arm 2.
The patients were treated from August 2010 to March 2012 at the Kasr Al Ainy Oncology Department (NEMROCK) at Cairo University during this period.
Clinical data
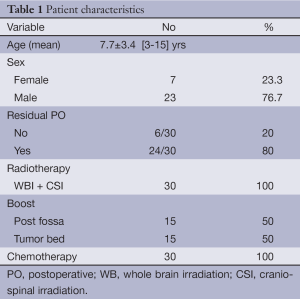
The ages of the patients ranged from 3 to 15 years, with a mean age of 7.7 (±3.426) years. Out of the 30 patients, 7 were females (23.3%), and 23 were males (76.7%). Postoperative MRI showed a residual tumor of less than 1.5 cm in diameter in 24/30 (80%) patients; the remaining 6 had no residual tumor tissue (Table 1). The mean follow-up period was 23.1±5.18 (range 4.2-21.6) months with no evidence of recurrence or disease progression in both arms. Event free survival was 100%.
Toxicity of radiotherapy
Hematological toxicity
There were no statistically significant differences in hematological toxicity between the two arms for grade 3 neutropenia and thrombocytopenia. Three patients developed grade 3 neutropenia and required admission with supportive treatment: two patients from arm 1 and one patient from arm 2 (P value: 0.3). Grade 3 thrombocytopenia occurred in one patient with a platelet count of 15,000/cm3. The patient was feverish and needed hospital admission, platelet transfusion, antibiotics and packed red blood cells. There was no significant difference in thrombocytopenia occurrence between the two arms.
Local toxicity
Patients had skin reactions, classified as grade 1 or 2 [60% (18/30) and 40% (12/30), respectively], with no grade 3 or 4 toxicity observed.
Ototoxicity
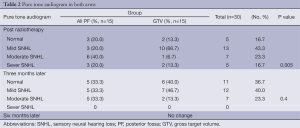
Pure tone audiograms (PTA) were performed before and after RT and every three months thereafter (Table 2). No baseline sensory neural hearing loss (SNHL) appeared after RT, and SNHL was significantly lower in patients receiving the tumor bed boost compared to those treated in the whole PF (P value: 0.005). After three months of RT, PTA results were not significantly different (P value: 0.4) and remained the same after chemotherapy.
Full table
Intellectual function
IQ tests were performed before and after RT and three months later; there was no significant difference in the results of the tests. Initially, all of the patients were in the average scale (91-99%). After RT, 40% (6/15) of arm 1 and 33.3% (5/15) of arm 2 showed a 1% decline in IQ. In arm 2, only the IQ of one patient (6.7%) declined by 2%.
Dosimetric analysis
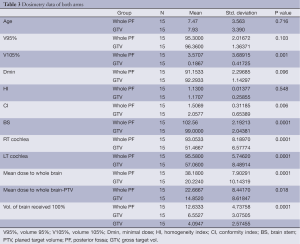
The dosimetric data are shown in Table 3. The mean of the 95% volume (V95%) from the PTV was not statistically significant between the two arms (P value: 0.103); however the difference in 105% volume (V105%) mean was highly significant (P value: 0.001). The mean homogeneity index (HI) values were not significantly different, while the difference in mean conformity index (CI) was significant (P value: 0.548 and 0.006, respectively).
Full table
Analysis of the dosimetric data showed significant differences between the two arms in mean doses to the brain stem, right (Rt) and left (Lt) cochlea; whole brain; and the volume of the brain receiving 100% of the prescribed dose (P value: 0.0001). The mean dose to the whole brain minus the PTV and the volume of the brain receiving 100% of the prescribed dose were not significantly different between the two arms (P value: 0.018 and 0.134, respectively).
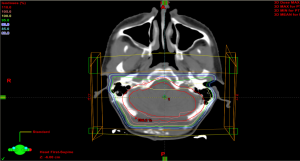
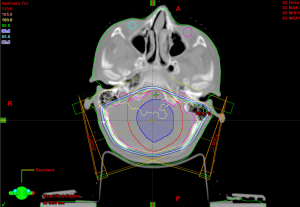
The isodose lines of the GTV +1 cm margin is shown in Figures 1,2. The arm with patients receiving the tumor bed boost showed more dose sparing for the cochleae and normal brain tissue Figure 1 than the PF boost (Figure 2).
Discussion
In MB patients, the reduction of CSI to 23.4 Gy followed by a tumor bed boost (with a limited margin of 1 cm) using conformal RT is as effective as treating the entire PF and shows similar event free survival and overall survival times. Tumor bed boost treatment results in a significant reduction in the cochlear dose with less obvious ototoxicity in post RT PTAs. However, this difference disappeared after chemotherapy. The dose to the whole brain in patients receiving the tumor bed boost was significantly lower (28.2 versus 38.2 Gy), which we expect will affect cognitive function and quality of life in the future.
In the present study, event free survival and overall survival (OS) were 100% in both arms. There was no PF failure outside the tumor bed in GTV boost, which is consistent with Packer RJ et al., 2013 (11). This result is also consistent with Paulino et al., 2011, who reported that irradiating the tumor bed boost using IMRT showed excellent local control (12). Packer RJ et al. 2013 followed up with 379 nondisseminated MB patients between the ages of 3 and 21 years for 10 years. They were treated with 2,340 cGy of craniospinal and 5,580 cGy of PF irradiation followed by chemotherapy. The 5- and 10-year event-free survival rates were 81%±2% and 75.8%±2.3%, respectively; the OS rates were 87%±1.8% and 81.3%±2.1%, respectively (11).
It is too early to assess the effects of toxicity during RT for patients in the present study, particularly on hearing and cognitive function; more follow up is needed. The initial results and dosimetric data are consistent with the results of other studies. The PTA results one month after finishing RT were significantly different between the two arms and were consistent with the dosimetric data. The Dmean to Rt cochlea in arm 1 was 93.0533 versus 51.4667 in arm 2, which is a statistically significant difference (P=0.0001). The mean dose of the Rt cochlea was 96% (51.84 Gy) and 73% (39.42 Gy) of the full dose prescribed for PTV in arm 1 and 2, respectively. The dosimetric results of this study are consistent with the results of Merchant et al., 2008 (13).
The mean dose to the whole brain was significantly lower using the tumor bed boost [20.22% (± STD. 10.14)] than using the whole PF boost [38.2% (± STD. 7.9)] (P value: 0.0001). This is consistent with the results of Mulhern et al., 2005, who reported a 35% reduction in CSI dose (14). However, the mean dose to the whole brain minus the PTV in the present study was not statistically significant (P value: 0.018). This is consistent with results reported by Merchant et al., 2008 (13).
In our study, there was an initial significant difference in the PTA results after RT, although the difference disappeared after 3 months and did not change during the mean follow up times after 23 months with no grade 3 or 4 toxicity. This was due to the administration of cisplatin, which is ototoxic to both arms, and is consistent the results reported by Hua et al., 2008, who treated children with conformal RT without chemotherapy. In their study, hearing loss was rare and occurred at a Dmean dose below 30 Gy to the cochlea and increased at doses of 40 to 45 Gy (15). The reported dose at which hearing loss increased was 43 Gy in Paulino et al., 2010, who followed 44 patients for 41 months, with 25% (11/44) of them suffering grade 3 or 4 ototoxicity. Six of the 11 patients had unilateral Grade 3 or 4 hearing loss, which may be due to the use of IMRT, as cisplatin-related ototoxicity is usually bilateral (16). Low rates of high-grade early post-radiation ototoxicity in childhood MB were confirmed by Moeller BJ et al. 2011, which assessed proton RT and found that quality of life and cognitive function may improve as result hearing preservation in the audible speech range (6).
IQ decline was observed in 40% of the patients in both arms, but the decline was not statistically significant. This is consistent with Mulhern et al., 2005, who reported that AR (average risk) patients experienced less neurocognitive decline than HR (high risk) patients after two years. This suggests that CSI dose reduction in the AR patients preserved neurocognitive function (14). After more than five years of follow up of AR patients, Ris et al., 2013 reported a significant decline in both intellectual and academic capability over time in children who were younger at diagnosis. That study used a craniospinal dose reduction of 23.4 Gy plus adjuvant chemotherapy (17).
This study was limited by the small number of patients and short follow-up period. There was a lack of cytogenetic and biological markers predicting treatment outcome in the same group of patients.
In the present study we conclude that irradiation of the tumor bed after 23.4 Gy of craniospinal irradiation in average-risk MB results in disease control comparable to that of irradiating the entire PF. The dosimetric sparing effect for the cochleae and normal tissue is evident in patients receiving tumor bed boosts. The treatment’s possible improvement of hearing loss and preservation of cognitive function, growth and development requires more study.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Jakacki RI. Treatment strategies for high-risk medulloblastoma and supratentorial primitive neuroectodermal tumors. Review of the literature. J Neurosurg 2005;102:44-52. [PubMed]
- Fouladi M, Gajjar A, Boyett JM, et al. Comparison of CSF cytology and spinal magnetic resonance imaging in the detection of leptomeningeal disease in pediatric medulloblastoma or primitive neuroectodermal tumor. J Clin Oncol 1999;17:3234-7. [PubMed]
- Bailey P, Cushing H. Medulloblastoma cerebelli: a common type of midcerebellar glioma of childhood. Arch Neurol Psychiatry 1925;14:192-224.
- Zeltzer PM, Boyett JM, Finlay JL, et al. Metastasis stage, adjuvant treatment, and residual tumor are prognostic factors for medulloblastoma in children: conclusions from the Children’s Cancer Group 921 randomized phase III study. J Clin Oncol 1999;17:832-45. [PubMed]
- Eberhart CG, Burger PC. Anaplasia and grading in medulloblastomas. Brain Pathol 2003;13:376-85. [PubMed]
- Moeller BJ, Chintagumpala M, Philip JJ, et al. Low early ototoxicity rates for pediatric medulloblastoma patients treated with proton radiotherapy. Radiat Oncol 2011;6:58. [PubMed]
- Packer RJ, Gajjar A, Vezina G, et al. Phase III study of craniospinal radiation therapy followed by adjuvant chemotherapy for newly diagnosed average-risk medulloblastoma. J Clin Oncol 2006;24:4202-8. [PubMed]
- Thomas PR, Deutsch M, Kepner JL, et al. Low-stage medulloblastoma: final analysis of trial comparing standard-dose with reduced-dose neuraxis irradiation. J Clin Oncol 2000;18:3004-11. [PubMed]
- Holland AA. Neuropsychological comparison of pediatric medulloblastoma and pilocytic astrocytoma: existing knowledge and future directions. New School Psychology Bulletin 2013;10:1-20.
- Merchant TE, Pritchard DL, Vargo JA, et al. Radiation therapy for the treatment of childhood medulloblastoma: the rationale for current techniques, strategies, and dose-volume considerations. Electromedica 2001;69:69-71.
- Packer RJ, Zhou T, Holmes E, et al. Survival and secondary tumors in children with medulloblastoma receiving radiotherapy and adjuvant chemotherapy: results of Children’s Oncology Group trial A9961. Neuro Oncol 2013;15:97-103. [PubMed]
- Paulino AC, Mazloom A, Teh BS, et al. Local control after craniospinal irradiation, intensity-modulated radiotherapy boost, and chemotherapy in childhood medulloblastoma. Cancer 2011;117:635-41. [PubMed]
- Merchant TE, Kun LE, Krasin MJ, et al. Multi-institution prospective trial of reduced-dose craniospinal irradiation (23.4 Gy) followed by conformal posterior fossa (36 Gy) and primary site irradiation (55.8 Gy) and dose-intensive chemotherapy for average-risk medulloblastoma. Int J Radiat Oncol Biol Phys 2008;70:782-7. [PubMed]
- Mulhern RK, Palmer SL, Merchant TE, et al. Neurocognitive consequences of risk-adapted therapy for childhood medulloblastoma. J Clin Oncol 2005;23:5511-9. [PubMed]
- Hua C, Bass JK, Khan R, et al. Hearing loss after radiotherapy for pediatric brain tumors: effect of cochlear dose. Int J Radiat Oncol Biol Phys 2008;72:892-9. [PubMed]
- Paulino AC, Lobo M, Teh BS, et al. Ototoxicity after intensity-modulated radiation therapy and cisplatin-based chemotherapy in children with medulloblastoma. Int J Radiat Oncol Biol Phys 2010;78:1445-50. [PubMed]
- Ris MD, Walsh K, Wallace D, et al. Intellectual and academic outcome following two chemotherapy regimens and radiotherapy for average-risk medulloblastoma: COG A9961. Pediatr Blood Cancer 2013;60:1350-7. [PubMed]