
A narrative review of tropisetron and palonosetron for the control of chemotherapy-induced nausea and vomiting
Introduction
Chemotherapy-induced nausea and vomiting (CINV), a highly distressing and frequent complication in patients with cancer (1), can negatively impact quality of life and adherence to therapy (2-5), and may be associated with considerable healthcare costs (6).
CINV is a complex and multifactorial process mediated by multiple neurotransmitters, including serotonin, substance P, and dopamine (7). Serotonin (5-hydroxytryptamine) type 3 (5-HT3) receptor antagonists (RAs) block 5-HT3 receptors involved in regulating nausea and vomiting in the acute (0–24 hours after chemotherapy) setting. Thought to act via the central nervous system and the vagus and splanchnic nerves in the gastrointestinal tract (8), 5-HT/5-HT3 receptor signaling may also influence delayed (24–120 hours after chemotherapy) nausea and vomiting, possibly by sensitizing the vagus nerve to chemicals such as substance P (9-11).
5-HT3 RAs, which may be described as old and new generation, form the cornerstone of antiemetic regimens recommended by international guidelines (12-14). Currently used older 5-HT3 RAs include azasetron (15); dolasetron, granisetron, and ondansetron; tropisetron (16); and ramosetron (17). At the recommended dose, these agents show similar efficacy and safety (8,18-20), with cost being the main differentiator. Despite their effectiveness in controlling CINV in the acute phase, they are not as effective in the delayed phase (21-23), prompting the development of a new 5-HT3 RA, palonosetron.
Both palonosetron and tropisetron are used as first-line agents to prevent CINV in China, although anecdotal evidence suggests that tropisetron may be favored, despite the lack of evidence to support its superior efficacy. Tropisetron, one of the first 5-HT3 RAs to be developed (24), has shown promising antiemetic properties in pilot studies (25,26), with acute CINV control rates of approximately 70% (26). Palonosetron is a pharmacologically and clinically distinct new-generation 5-HT3 RA (27,28) that various meta-analyses have shown to be more effective than older 5-HT3 RAs (29-32). It exhibits a higher binding affinity for 5-HT3 receptors and synergistically interacts with the neurokinin 1 (NK1) receptor signaling pathway (27,33), which may partially account for palonosetron’s effectiveness in the delayed phase. Palonosetron comes in two formulations, oral (0.50 mg) and intravenous (IV; 0.25 mg).
This review aimed to summarize the clinical data on tropisetron IV and Aloxi® (palonosetron HCl) IV, in the first-line setting in patients with CINV, and evaluate the 5-HT3 RA benefit to patients in terms of preventing nausea and/or vomiting.
Methods
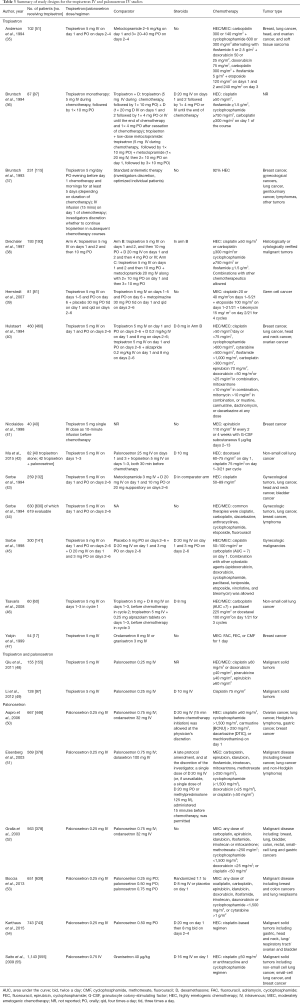
A literature search of EMBASE and PubMed was performed to identify publications reporting the results of tropisetron IV (generic forms or Navoban®) for the treatment of CINV in patients with various cancers. The search strings are detailed in Table 1; no publication date limits were applied. Table 2 details inclusion and exclusion criteria used to screen the publications.

Full table

Full table
For palonosetron, only pivotal clinical studies evaluating the IV formulation of Aloxi® were included, because of the array of publications that have previously reviewed the use of palonosetron.
The doses considered in this review are tropisetron 5 mg IV and palonosetron 0.25 mg IV, both with and without dexamethasone (at variable doses).
Results
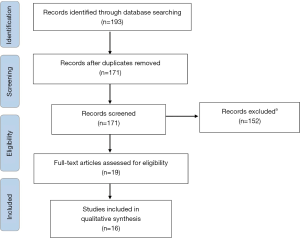
Overall, 193 publications on tropisetron were retrieved (Figure 1), comprising 131 records and two congress abstracts from EMBASE and 60 records from PubMed. After removal of 22 duplicates, 171 records were screened. Of these, 19 records fulfilled the inclusion criteria and were extracted for full analysis: a further four were discounted, and a previously identified study of interest was added (34), making a total of 16 included studies.
For palonosetron, a total of six papers describing pivotal studies on the use of Aloxi® IV in controlling CINV were identified and included; see Table 3 for study designs.
Full table
Tropisetron
Efficacy—tropisetron-only data
Definitions of the extent of nausea/vomiting control differed across publications; therefore, only complete control rates for nausea and/or vomiting were considered in this review. For the majority of papers, complete control of vomiting was described as no vomiting or retching within a 24-hour period, and complete control of nausea was defined as no episodes of nausea within 24 hours, where one episode was any period of 1 hour in which nausea occurred.
Most studies were conducted in Europe, with Navoban® or generic tropisetron used equally across studies. Nine studies were undertaken in the highly emetogenic chemotherapy (HEC) setting (34,36-38,42,43) [where 93% of the patients received HEC (39,41,45)], two in the moderately emetogenic chemotherapy (MEC) setting (46,47), and three in a mixed HEC/MEC setting (35,40,44). Dexamethasone, at varying doses, was included in six studies (36,38,40,42,45,46), although in the Hulstaert et al. study (40), it was only administered in the second cycle of treatment (data not reported). The comparator arms of each study are detailed in Table 3.
Complete control of vomiting/emesis (no emesis)
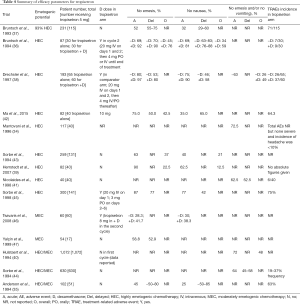
Overall, ten studies report data on the rates of no emesis (35-39,42,43,45-47) (Table 4). In the HEC setting, between 52.0–90.0% of patients in the acute phase (36-39,43), 53.0–75.0% in the delayed setting (36-38), and 22.5–45.0% in the overall phase (36,39,43) reported no emesis. When tropisetron was administered with dexamethasone, the rates of no emesis were 75.0–97.0% in the acute phase (36,38,42,45), 50.0–90.0% in the delayed setting (36,38,42,45), and 42.5–76.0% in the overall phase (36,42).
Full table
In the MEC setting, no emesis occurred in 28.3% of patients in the acute phase (46). Adding dexamethasone to tropisetron increased the rate to 41.7–58.8% in the acute phase (46,47), while 52.9% of patients reported no emesis in the delayed phase (47). Neither study reported data for the overall phase.
In the HEC/MEC setting, 45% of patients in the acute phase and 50.0–80.0% of patients in the delayed phase reported no emesis (35).
Complete control of nausea (no nausea)
In the HEC setting, six studies investigated the effect of tropisetron on controlling nausea, three of which investigated the addition of dexamethasone to tropisetron (Table 4). Tropisetron alone resulted in no-nausea rates of 32–75.0% in the acute phase (36-39,43), 29.0–83.0% in the delayed phase (36-38), and 12.5–34.0% in the overall phase (36,39,43). The addition of dexamethasone to tropisetron increased these rates to 35.0–90.0% in the acute phase (36,38,42,45), 42.0–88.0% in the delayed phase (36,38,42,45), and 59% in the overall phase (36).
Only one study in the MEC setting evaluated nausea. In total, 30% of patients reported no nausea, which increased to 38.3% when dexamethasone was added to tropisetron in the second cycle of treatment (46).
In the HEC/MEC setting, only one study reported data on nausea prevention. Approximately 23% of patients in the acute phase reported no nausea, and while absolute values were reported, a graphical representation of the data indicated that more people in the delayed phase experienced no nausea (35).
Complete control of vomiting and nausea (no emesis and no nausea)
Several studies defined complete control as no emesis and/or nausea in 24 hours. In the HEC setting, 62.5–72.5% of tropisetron-treated patients reported no acute emesis and/or nausea (34,38,41), with 100% control of emesis or nausea observed in 52.5% of patients in the delayed phase (41); 26% of patients reported no nausea and vomiting in the overall phase, increasing to 49.0% when dexamethasone was added (38). In the HEC/MEC setting, 64% of tropisetron-treated patients in the acute phase and 45.0–58.0% in the delayed phase had no emesis or nausea, respectively (44). Another study reported no-emesis or no-nausea rates of 72.0% in the acute phase and 48% over the entire 6-day study period (40).
Efficacy—tropisetron versus other 5-HT3 RAs
Several studies assessed the effectiveness of tropisetron versus other 5-HT3 RAs. Tropisetron was compared with ondansetron and granisetron in the MEC setting (47). The rates of no emesis in the acute phase were 38.8% with ondansetron, 58.8% with tropisetron, and 73.7% with granisetron; in the delayed phase, the rates were 38.8%, 52.9%, and 73.7%, respectively, demonstrating that tropisetron was not significantly better in controlling emesis compared with ondansetron or granisetron. Indeed, granisetron promoted a significantly greater major response rate [defined as the sum of complete and partial (1–4 vomiting episodes/retches in 24 hours)] in the control of delayed emesis (P=0.01), compared with tropisetron (47).
Another study compared the effects of tropisetron, ondansetron, and granisetron on complete response (defined as no nausea or vomiting, or only mild nausea in 24 hours) across multiple cycles in cisplatin-treated patients (34). In the first cycle, observed response rates in the acute phase were 72.5% with tropisetron, 82.1% with ondansetron, and 84.2% with granisetron; across multiple cycles, these values were 67.6%, 73.3%, and 72.1%, respectively. Ondansetron resulted in significantly higher numbers of patients with major efficacy [complete response plus major response (single vomiting or no vomiting but moderate to severe nausea in 24 hours)] versus tropisetron (P=0.021) (34).
Safety
The two most commonly reported adverse events (AEs) were headache (incidence range, 5–41.7%) (34,35,37-47), and constipation (incidence range, 2.5–58%) (35-47). Other AEs that were reported were: abdominal distention (42); effects on appetite and activity (46); sedation (45,46); asthenia (43,46); dizziness (39,40,43-45); tiredness (35,36,38-40,44); mild “mouth dryness” (41); diarrhea (36,38,40,43,47); other gastrointestinal symptoms (38) and sleep disturbances (38,47); paresis, anxiety, and somnolence (43); abdominal pain (40,43,45); epigastric pain (40,44); allergy and heart symptoms (44); pyrosis, hiccups, and fever (40); depression, migraine, and confusion (35); anorexia and fatigue (36); and edema (45).
Palonosetron
Efficacy—palonosetron only
Six pivotal trials evaluated the efficacy of palonosetron (Aloxi®) IV in CINV prevention (50-55) (Table 5). Three studies evaluated the 0.25- and 0.75-mg doses of palonosetron IV, and included a third arm that featured an older-generation 5-HT3 RA (50-52), while two trials compared the efficacy of the oral and IV formulations of palonosetron in the MEC (53) and HEC (54) settings. The final study evaluated the efficacy of 0.75 mg palonosetron versus granisetron in patients from Japan (where the standard dose is 0.75 mg IV) (55). Studies used the same definitions for complete response (no emesis and no rescue medication use) and complete control (no emesis, no rescue medication use, and no more than mild nausea).

Full table
In the MEC setting, 63.0–81.0% of patients had a complete response during the acute phase (51-53). For the delayed and overall phases, the complete response rates were 54.0–74.1% (51-53) and 46.0–69.3% (51-53), respectively. Moreover, the Gralla study (52) reported that >70% of patients had no emetic episodes in any phase. In the Boccia study (53), the rates of no emesis and no nausea were also reported. In the acute, delayed, and overall phases, the proportion of patients with no emesis was 77.2%, 74.7%, and 67.3%, respectively, and the rates of no nausea were 57.4%, 47.5%, and 42.6%, respectively (53). This study also reported complete response rates in patients who received dexamethasone versus those who did not; these were 82.9% vs. 57.5%, 68.3% vs. 62.5%, and 65.9% vs. 52.5% in the acute, delayed, and overall phases, respectively (53).
In the HEC setting, the rates of complete response during acute, delayed, and overall phases were 59.2%, 45.3%, and 40.8%, respectively (50). The proportion of patients with no emesis was 68.5%, 56.5%, and 46.6% in the acute, delayed, and overall phases, respectively. In another study where dexamethasone was administered to all patients, 86.2% achieved a complete response in the acute phase, 74.8% in the delayed phase, and 70.2% in the overall phase. In the delayed and overall phases, 77.5% and 73.2% of patients reported no vomiting, and rates of no nausea were 75.6%, 53.4%, and 47.4%, in the acute, delayed, and overall phases, respectively (54).
Finally, a Japanese study (55) evaluated the effect of 0.75 mg palonosetron plus dexamethasone. The rates of complete response during the acute, delayed, and overall phases were 75.3%, 56.8%, and 51.5%, respectively. Complete control was observed in 73.7% of patients in the acute phase, 53.0% in the delayed phase, and 47.9% in the overall phase. Rates of no nausea were 58.7%, 37.8%, and 31.9% in the acute, delayed, and overall phases, respectively, and for no emesis these values were 77.5%, 63.2%, and 57.5%, respectively.
Efficacy—palonosetron versus older-generation 5-HT3 RAs
Four studies featured a comparator arm containing an older-generation 5-HT3 RA. Two compared the antiemetic activity of 0.25 mg palonosetron, 0.75 mg palonosetron, and 32 mg ondansetron, with one study in the MEC setting (52), and the other in the HEC setting (50). The other studies compared palonosetron with dolasetron in patients receiving MEC (51), and with granisetron in patients receiving HEC (55).
In the MEC setting, 0.25 mg palonosetron was significantly superior to ondansetron in preventing acute vomiting (lower bound of the 97.5% CI >0; P=0.009), and non-inferiority was demonstrated for both the 0.25- and 0.75-mg doses of palonosetron (52). The 0.25-mg palonosetron dose was also significantly better than ondansetron at controlling complete response in the delayed (74.1% vs. 55.1%; P<0.001) and overall (69.3% vs. 50.3%; P<0.001) phases. Significantly higher rates of patients with no emesis, no rescue medication use, and no more than mild nausea were observed with palonosetron 0.25 and 0.75 mg, compared with ondansetron during the delayed (66.7% vs. 50.3%; P=0.001) and overall (63.0% vs. 44.9%; P=0.001) phases. Palonosetron 0.25 mg was also superior to ondansetron in terms of the number of patients who experienced no emesis, used no rescue medication, and experienced no more than mild nausea on days 2, 3, and 4 (P=0.001, P=0.001, and P=0.003, respectively). At no point was palonosetron inferior to ondansetron (52).
The efficacy of 0.25 mg palonosetron, 0.75 mg palonosetron, and 100 mg dolasetron was compared in patients receiving MEC (51). Both the 0.25- and 0.75-mgdoses of palonosetron were non-inferior to dolasetron in terms of complete response in the acute phase, with numerically higher rates of complete response achieved with 0.25 mg (63.0% vs. 52.9%; P=0.049) and 0.75 mg palonosetron (57.1% vs. 52.9%; P=0.412), compared with dolasetron. In the delayed and overall phases, significantly higher complete response rates were observed for 0.25 mg palonosetron compared with dolasetron (54.0% vs. 38.7%; P=0.004, and 46.0% vs. 34.0%; P=0.21, respectively) and for 0.75 mg palonosetron (56.6% vs. 38.7%; P<0.001, and 47.1% vs. 34.0%; P=0.012, respectively). There were a significantly higher proportion of patients who experienced no emesis, used no rescue medication, and experienced no more than mild nausea for 0.25 mg palonosetron and 0.75 mg palonosetron, compared with dolasetron, during the delayed phase (48.1% and 51.9% vs. 36.1%, respectively; P=0.018 and P=0.002 for palonosetron 0.25 and 0.75 mg vs. dolasetron, respectively) and overall phases (41.8% and 42.9% versus 30.9%; P=0.027 and P=0.016, respectively). The lower dose of 0.25 mg palonosetron led to significantly fewer emetic episodes during the acute, delayed, and overall phases compared with dolasetron (P=0.0135, P=0.0183, and P=0.0036, respectively), with more patients reporting no emetic episodes during the delayed and overall phases (P=0.028 and P=0.014, respectively) (51).
Non-inferiority of palonosetron compared with ondansetron in terms of acute complete response was also demonstrated in the HEC setting (50). Numerically higher increases in the complete response rates for palonosetron 0.25 mg during the delayed (45.3% vs. 38.9%) and overall (40.8% vs. 33.0%) phases were reported. The percentage of patients who experienced no emesis, used no rescue medication, and experienced no more than mild nausea was slightly higher for palonosetron 0.25 mg compared with ondansetron in the acute phase (56.5% vs. 51.6%, respectively), although the rates were comparable in the delayed and overall phases (50).
Finally, in Japanese patients receiving HEC, 0.75 mg palonosetron was non-inferior to granisetron in terms of acute-phase complete response [75.3% vs. 73.3%, respectively; mean difference 2.9% (95% CI, −2.70% to 7.27%)]. In the delayed phase, palonosetron resulted in significantly higher complete response rates compared with granisetron (56.8% vs. 44.5%; P<0.0001) (55).
Safety
Palonosetron was well tolerated. Most AEs were mild in intensity, and the majority were assessed as not related, or unlikely to be related to the study medication (50-53). The most frequently reported were: headache (incidence range, 1.6–26.4%) (50-54); constipation (incidence range, 1.6–17.4%) (50-55); fatigue (10.9%) (51); dizziness (0.5%) (52); diarrhea (1.3%) (50); gastrointestinal disorders (3.0%); and nervous system disorders (1.6%) (54). No significant changes related to study drug were observed with respect to laboratory parameters, vital sign measurements, and electrocardiogram recordings (50-54).
Palonosetron vs. tropisetron
Two Chinese studies evaluated the efficacy of tropisetron and palonosetron (48,49). One study determined the effectiveness of these drugs in preventing emesis and nausea in the MEC (an anthracycline-based regimen) and HEC (a cisplatin-based regimen) settings (48). In patients receiving MEC, the rates of no emesis in the acute phase were 61.8% for palonosetron and 55.3% for tropisetron; in patients receiving HEC, the rates were 44.6% and 46.4%, respectively. In the delayed phase, the rates of no emesis were 63.2% for palonosetron and 47.4% for tropisetron in the MEC setting, and 39.3% and 26.8%, respectively, in the HEC setting. Considering data from the MEC and HEC settings together, no significant difference (P>0.05) was observed between the two drugs in preventing acute vomiting. This contrasted with the data observed in the delayed setting, where significantly higher rates of no emesis were observed for palonosetron versus tropisetron (53.0% vs. 38.6%; P=0.01). The overall incidence of AEs between the two drugs was similar [4.9% (palonosetron) vs. 7.4% (tropisetron); P>0.05]; the majority were mild, and there was no incidence of severe AEs. The most common were headache (2.7% vs. 2.1% for palonosetron versus tropisetron, respectively) and dizziness (2.7% vs. 2.1%, respectively).
The second study evaluated the effectiveness of palonosetron and tropisetron in the HEC setting (49). There was no significant difference in the rates of no emesis in the acute phase between palonosetron and tropisetron (79.7% vs. 75.8%, respectively; P=0.45). However, palonosetron appeared significantly more effective in controlling delayed emesis (no emesis rates: 70.3% vs. 50.8%, respectively; P<0.01). AEs were generally mild to moderate in severity and the incidence was similar for both drugs. The most commonly observed AEs were constipation (palonosetron versus tropisetron: 14.8% vs. 17.2%), distention (3.9% vs. 7.8%), headache (1.6% vs. 2.3%), fatigue (7.8% vs. 10.9%), and increased aminotransferase (2.3% each).
Discussion
In our review of the clinical evidence supporting the use of 0.5 mg tropisetron IV and 0.25 mg palonosetron IV as antiemetic agents in the HEC and MEC settings, we have discussed data from 16 publications on tropisetron and data from 6 pivotal trials of palonosetron IV. Most papers that investigated the efficacy of tropisetron measured the rates of no emesis or no nausea (both reported as no episodes within a 24-hour period), with only a few reporting on rates of no nausea and/or no vomiting. Rescue medication use was varied, with four studies not specifying whether it was used. This contrasted with palonosetron IV, where the primary efficacy parameter in each study was complete response, defined as no emetic episodes and no rescue medication. Consequently, a direct comparison of the data was not possible, so overall trends were instead considered, where sample sizes permitted.
For tropisetron, the rates of no emesis were lower in patients receiving HEC vs. MEC. For palonosetron, the rates of complete response were comparable between both settings, thus demonstrating the effectiveness of this agent in patients receiving HEC. Tropisetron was less effective at controlling nausea than emesis regardless of the phase or emetogenic potential of the chemotherapy. Lower rates of no nausea, versus rates of no emesis, were also observed with palonosetron, although the effect was not as pronounced (rates of no nausea were 57.4–75.6% in the acute phase).
These data could indirectly suggest that palonosetron may be more effective than tropisetron in controlling CINV in patients with cancer. The results of two studies examining the effectiveness of palonosetron and tropisetron (48,49) within the same trial provided direct data to support this supposition. Palonosetron was seen to be more effective than tropisetron in controlling delayed vomiting in both the MEC and HEC settings. In both studies, significantly higher rates of no emesis were seen with palonosetron in the delayed phase, compared with tropisetron, and comparable efficacy was observed in the acute phase (48,49). The results of a subgroup analysis within a recent meta-analysis of palonosetron versus the older 5-HT3 RA tropisetron also reported superiority of palonosetron in controlling CINV in the acute, delayed, and overall phases (56).
Considering the efficacy of tropisetron versus other 5-HT3 RAs, tropisetron appears less effective in controlling CINV, regardless of the phase. One study reported that granisetron was significantly better in controlling emesis in the delayed phase, compared with tropisetron (P=0.01) (47). Another study reported significantly higher complete (no emesis or nausea) and major responses (single emetic episode or no emesis but moderate to severe nausea) in the acute phase across multiple cycles for ondansetron compared with tropisetron (P=0.021) (34).
Conversely, palonosetron had significantly higher rates of complete response compared with ondansetron in the acute, delayed, and overall phases, and was significantly superior to ondansetron in preventing acute emesis (lower bound of the 97.5% CI >0; P=0.009) (52). Palonosetron was non-inferior to dolasetron in the prevention of acute emesis (51), with significantly higher response rates observed in the delayed (P=0.004) and overall (P=0.021) phases, significantly higher numbers of patients with no emesis, no rescue medication use, and no more than mild nausea in the delayed (P=0.0018) and overall phases (P=0.027), significantly fewer emetic episodes in the acute (P=0.0135), delayed (P=0.0183), and overall (P=0.0036) phases, as well as a greater proportion of patients with no emetic episodes in the delayed and overall phases for palonosetron, compared with dolasetron (51). Finally, one pivotal Japanese study reported the non-inferiority of palonosetron to granisetron in controlling acute emesis, with significantly more patients reporting no emesis in the delayed phase (55). While this study used 0.75 mg of palonosetron, data from a subgroup analysis of a larger meta-analysis of palonosetron in CINV have shown that the doses appear to be equivalent in terms of efficacy (57). No statistical difference was seen between the 0.25- and 0.75-mg doses of palonosetron in controlling CINV) in the acute (P=0.50), delayed (P=0.68), and overall (P=0.38) phases.
Both tropisetron and palonosetron were generally well tolerated, with AE profiles consistent with drugs of this class (19). In line with other 5-HT3 RA studies, the most common AEs were headache, constipation, and diarrhea, all of which were mild to moderate in severity.
It is worth noting that today multinational guidelines (12,13) recommend the use of 5-HT3 RAs in combination with an NK1 RA (such as aprepitant) and dexamethasone for preventing HEC- (and MEC-) mediated CINV. The inclusion of this class of drugs reflects their purported ability to inhibit emesis by blocking the binding of substance P to the NK1 receptor in the brain stem emetic center (58).
Aprepitant was the first European Medicines Agency (EMA)- and US Food and Drug Administration (FDA)-approved NK1 RA for the prevention of CINV in the HEC setting [2003], and in the MEC setting [2005] (59-61); it was followed by fosaprepitant, its water-soluble prodrug. Various studies have demonstrated the safety and efficacy of both agents in the prevention of HEC- or MEC-mediated CINV (62-65). Two other NK1 RAs have since become commercially available: rolapitant was approved for delayed CINV prevention (66), and netupitant (administered as a convenient fixed combination with palonosetron, known as NEPA) was approved for the prevention of acute and delayed nausea and vomiting in the HEC and MEC settings (67). In addition, in August 2019, oral NEPA was approved by the Chinese National Medical Products Administration (NMPA) for the prevention of acute and delayed CINV associated with HEC or MEC settings. This approval was granted on the basis of the outcomes of a phase III study in adult Asian patients, in which a single dose of NEPA demonstrated comparable efficacy to a standard 3-day regimen of aprepitant plus granisetron (68). The IV formulation of NEPA was recently approved by FDA and is under evaluation by EMA. While the addition of rolapitant to a standard antiemetic regimen has proven effective [reviewed in Heo and Deeks, 2017 (69)], evidence suggests there is no consistent improvement in nausea protection (70,71). In contrast, the administration of oral NEPA and dexamethasone results in significant improvement in delayed and overall nausea control compared with oral palonosetron alone and dexamethasone in patients receiving cisplatin or anthracycline-cyclophosphamide (72,73). Finally, the addition of an NK1 RA has proven to be more effective in controlling CINV in HEC and MEC settings, compared to the standard 5-HT3 RA plus dexamethasone combination.
In conclusion, this review has shown that the newer 5-HT3 RA, palonosetron, is an effective first-line agent in preventing CINV in patients receiving MEC or HEC, and its efficacy can be further increased in combination with an NK1 RA. The high levels of emetic control observed in the acute, delayed, and overall phases twinned with its safety profile suggest that palonosetron is a very feasible prophylactic agent with a potentially improved therapeutic profile compared with tropisetron for controlling CINV in acute and delayed phases.
Acknowledgments
Editorial and medical writing assistance was provided by Joanne Franklin, PhD, CMPP, of Aptitude Health, The Hague, The Netherlands.
Funding: Editorial and medical writing assistance was funded by Helsinn Healthcare SA, Lugano, Switzerland.
Footnote
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/cco.2019.11.02). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hesketh PJ. Penny wise, dollar foolish approach to antiemetic use may compromise patient care. J Oncol Pract 2009;5:221-2. [Crossref] [PubMed]
- Bloechl-Daum B, Deuson RR, Mavros P, et al. Delayed nausea and vomiting continue to reduce patients' quality of life after highly and moderately emetogenic chemotherapy despite antiemetic treatment. J Clin Oncol 2006;24:4472-8. [Crossref] [PubMed]
- Cohen L, de Moor CA, Eisenberg P, et al. Chemotherapy-induced nausea and vomiting: incidence and impact on patient quality of life at community oncology settings. Support Care Cancer 2007;15:497-503. [Crossref] [PubMed]
- Glaus A, Knipping C, Morant R, et al. Chemotherapy-induced nausea and vomiting in routine practice: a European perspective. Support Care Cancer 2004;12:708-15. [Crossref] [PubMed]
- Jordan K, Jahn F, Aapro M. Recent developments in the prevention of chemotherapy-induced nausea and vomiting (CINV): a comprehensive review. Ann Oncol 2015;26:1081-90. [Crossref] [PubMed]
- Sommariva S, Pongiglione B, Tarricone R. Impact of chemotherapy-induced nausea and vomiting on health-related quality of life and resource utilization: a systematic review. Crit Rev Oncol Hematol 2016;99:13-36. [Crossref] [PubMed]
- Hesketh PJ. Chemotherapy-induced nausea and vomiting. N Engl J Med 2008;358:2482-94. [Crossref] [PubMed]
- Navari RM. Pathogenesis-based treatment of chemotherapy-induced nausea and vomiting: two new agents. J Support Oncol 2003;1:89-103. [PubMed]
- Jordan K, Sippel C, Schmoll HJ. Guidelines for antiemetic treatment of chemotherapy-induced nausea and vomiting: Past, present, and future recommendations. Oncologist 2007;12:1143-50. [Crossref] [PubMed]
- Miner WD, Sanger GJ. Inhibition of cisplatin-induced vomiting by selective 5-hydroxytryptamine M-receptor antagonism. Br J Pharmacol 1986;88:497-9. [Crossref] [PubMed]
- del Giglio A, Soares HP, Caparroz C, et al. Granisetron is equivalent to ondansetron for prophylaxis of chemotherapy-induced nausea and vomiting: results of a meta-analysis of randomized controlled trials. Cancer 2000;89:2301-8. [Crossref] [PubMed]
- National Comprehensive Cancer Network. 2018. NCCN Clinical Practice Guidelines in Oncology: Antiemesis. Version 1. 2018. Available online: http://www.nccn.org. Accessed 01 Oct 2018.
- Roila F, Molassiotis A, Herrstedt J, et al. 2016 MASCC and ESMO guideline update for the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting and of nausea and vomiting in advanced cancer patients. Ann Oncol 2016;27:v119-33. [Crossref] [PubMed]
- Hesketh PJ, Kris MG, Basch E, et al. Antiemetics: American Society of Clinical Oncology Clinical Practice Guideline update. J Clin Oncol 2017;35:3240-61. [Crossref] [PubMed]
- Kimura E, Niimi E, Watanabe A, et al. Study on clinical effect of a continuous intravenous infusion of azasetron against nausea and vomiting induced by anticancer drugs including CDDP. Gan To Kagaku Ryoho 1996;23:477-81. [PubMed]
- Simpson K, Spencer CM, McClellan KJ. Tropisetron: an update of its use in the prevention of chemotherapy-induced nausea and vomiting. Drugs 2000;59:1297-315. [Crossref] [PubMed]
- Taguchi T, Tsukamoto F, Watanabe T, et al. Usefulness of ramosetron hydrochloride on nausea and vomiting in CMF or CEF therapy for breast cancer. Gan To Kagaku Ryoho 1999;26:1163-70. [PubMed]
- Miner WD, Sanger GJ, Turner DH. Evidence that 5-hydroxytryptamine3 receptors mediate cytotoxic drug and radiation-evoked emesis. Br J Cancer 1987;56:159-62. [Crossref] [PubMed]
- Hesketh PJ. Comparative review of 5-HT3 receptor antagonists in the treatment of acute chemotherapy-induced nausea and vomiting. Cancer Invest 2000;18:163-73. [Crossref] [PubMed]
- Navari RM, Koeller JM. Electrocardiographic and cardiovascular effects of the 5-hydroxytryptamine-3 receptor antagonists. Ann Pharmacother 2003;37:1276-86. [Crossref] [PubMed]
- Roila F, Warr D, Clark-Snow RA, et al. Delayed emesis: moderately emetogenic chemotherapy. Support Care Cancer 2005;13:104-8. [Crossref] [PubMed]
- Geling O, Eichler H. Should 5-Hydroxytryptamine-3 receptor antagonists be administered beyond 24 hours after chemotherapy to prevent delayed emesis? Systematic re-evaluation of clinical evidence and drug cost implications. J Clin Oncol 2005;23:1289-94. [Crossref] [PubMed]
- Hickok JT, Roscoe JA, Morrow GR, et al. 5-HT3 receptor antagonists versus prochlorperazine for control of delayed nausea caused by doxorubicin: a URCC CCOP randomized controlled trial. Lancet Oncol 2005;6:765-72. [Crossref] [PubMed]
- Donatsch P, Engel G, Richardson BP, et al. A highly selective and potent antagonist at peripheral neuronal 5-hydroxy tryptamine receptors. Br J Pharmacol 2016;81:34P.
- Leibundgut U, Lancranjan I. First results with ICS 205-930 (5-HT3x receptor antagonist) in prevention of chemotherapy-induced emesis. Lancet 1987;1:1198. [Crossref] [PubMed]
- Zhang P, Sun Y, Zhang H. A randomized trial of tropisetron in the prophylaxis of nausea and vomiting induced by chemotherapy. Zhonghua Zhong Liu Za Zhi 1996;18:154-6. [PubMed]
- Rojas C, Raje M, Tsukamoto T, Slusher BS. Molecular mechanisms of 5-HT(3) and NK(1) receptor antagonists in prevention of emesis. Eur J Pharmacol 2014;722:26-37. [Crossref] [PubMed]
- Navari RM, Aapro M. Antiemetic Prophylaxis for Chemotherapy-Induced Nausea and Vomiting. N Engl J Med 2016;374:1356-67. [Crossref] [PubMed]
- Botrel TE, Clark OA, Clark L, et al. Efficacy of palonosetron (PAL) compared to other serotonin inhibitors (5-HT3R) in preventing chemotherapy-induced nausea and vomiting (CINV) in patients receiving moderately or highly emetogenic (MoHE) treatment: systematic review and meta-analysis. Support Care Cancer 2011;19:823-32. [Crossref] [PubMed]
- Popovic M, Warr DG, Deangelis C, et al. Efficacy and safety of palonosetron for the prophylaxis of chemotherapy-induced nausea and vomiting (CINV): a systematic review and meta-analysis of randomized controlled trials. Support Care Cancer 2014;22:1685-97. [Crossref] [PubMed]
- Chow R, Warr DG, Navari RM, et al. Should palonosetron be a preferred 5-HT3 receptor antagonist for chemotherapy-induced nausea and vomiting? An updated systematic review and meta-analysis. Support Care Cancer 2018;26:2519-49. [Crossref] [PubMed]
- Schwartzberg L, Barbour SY, Morrow GR, et al. Pooled analysis of phase III clinical studies of palonosetron versus ondansetron, dolasetron, and granisetron in the prevention of chemotherapy-induced nausea and vomiting (CINV). Support Care Cancer 2014;22:469-77. [Crossref] [PubMed]
- Stathis M, Pietra C, Rojas C, et al. Inhibition of substance P-mediated responses in NG108-15 cells by netupitant and palonosetron exhibit synergistic effects. Eur J Pharmacol 2012;689:25-30. [Crossref] [PubMed]
- Mantovani G, Macciò A, Bianchi A, et al. Comparison of granisetron, ondansetron, and tropisetron in the prophylaxis of acute nausea and vomiting induced by cisplatin for the treatment of head and neck cancer: a randomized controlled trial. Cancer 1996;77:941-8. [Crossref] [PubMed]
- Anderson H, Thatcher N, Howell A, et al. Tropisetron compared with a metoclopramide-based regimen in the prevention of chemotherapy-induced nausea and vomiting. Eur J Cancer 1994;30A:610-5. [Crossref] [PubMed]
- Bruntsch U, Drechsler S, Eggert J, et al. Prevention of chemotherapy-induced nausea and vomiting by tropisetron (Navoban) alone or in combination with other antiemetic agents. Semin Oncol 1994;21:7-11. [PubMed]
- Bruntsch U, Rüfenacht E, Parker I, et al. Tropisetron in the prevention of chemotherapy-induced nausea and vomiting in patients responding poorly to previous conventional antiemetic therapy. Ann Oncol 1993;4 Suppl 3:25-9. [Crossref] [PubMed]
- Drechsler S, Bruntsch U, Eggert J, et al. Comparison of three tropisetron-containing antiemetic regimens in the prophylaxis of acute and delayed chemotherapy-induced emesis and nausea. Support Care Cancer 1997;5:387-95. [Crossref] [PubMed]
- Herrstedt J, Sigsgaard TC, Nielsen HA, et al. Randomized, double-blind trial comparing the antiemetic effect of tropisetron plus metopimazine with tropisetron plus placebo in patients receiving multiple cycles of multiple-day cisplatin-based chemotherapy. Support Care Cancer 2007;15:417-26. [Crossref] [PubMed]
- Hulstaert F, Van Belle S, Bleiberg H, et al. Optimal combination therapy with tropisetron in 445 patients with incomplete control of chemotherapy-induced nausea and vomiting. J Clin Oncol 1994;12:2439-46. [Crossref] [PubMed]
- Nicolaides C, Giannakakis T, Skarlos D, et al. Tropisetron in the prevention of acute nausea and vomiting in patients treated with high dose epirubicin. J Exp Clin Cancer Res 1998;17:71-5. [PubMed]
- Ma Y, Su L, Liu L, et al. Phase II clinical trial of palonosetron combined with tropisetron in preventing chemotherapy-induced nausea and vomiting. Int J Clin Exp Med 2015;8:7989-94. [PubMed]
- Sorbe BG, Högberg T, Glimelius B, et al. A randomized, multicenter study comparing the efficacy and tolerability of tropisetron, a new 5-HT3 receptor antagonist, with a metoclopramide-containing antiemetic cocktail in the prevention of cisplatin-induced emesis. Cancer 1994;73:445-54. [Crossref] [PubMed]
- Sorbe B, Andersson H, Schmidt M, et al. Tropisetron (Navoban) in the prevention of chemotherapy-induced nausea and vomiting – the Nordic experience. Support Care Cancer 1994;2:393-9. [Crossref] [PubMed]
- Sorbe BG, Berglind AM, Andersson H, et al. A study evaluating the efficacy and tolerability of tropisetron in combination with dexamethasone in the prevention of delayed platinum-induced nausea and emesis. Cancer 1998;83:1022-32. [Crossref] [PubMed]
- Tsavaris N, Kosmas C, Kopterides P, et al. Efficacy of tropisetron in patients with advanced non-small-cell lung cancer receiving adjuvant chemotherapy with carboplatin and taxanes. Eur J Cancer Care (Engl) 2008;17:167-73. [Crossref] [PubMed]
- Yalçin S, Tekuzman G, Baltali E, et al. Serotonin receptor antagonists in prophylaxis of acute and delayed emesis induced by moderately emetogenic, single-day chemotherapy: a randomized study. Am J Clin Oncol 1999;22:94-6. [Crossref] [PubMed]
- Qiu LH, Wang HQ, Yu Z, et al. Efficacy and safety of palonosetron versus tropisetron in the prevention of highly emetogenic chemotherapy-induced acute and delayed vomiting in Chinese cancer patients. Zhonghua Yi Xue Za Zhi 2011;91:2555-7. [PubMed]
- Li RC, Zheng LJ, Qiu H. Comparison of the effect of palonosetron versus tropisetron in prevention of vomiting in patients receiving high dose cisplatin-based chemotherapy. Zhonghua Zhong Liu Za Zhi 2012;34:228-31. [PubMed]
- Aapro MS, Grunberg SM, Manikhas GM, et al. A phase III, double-blind, randomized trial of palonosetron compared with ondansetron in preventing chemotherapy-induced nausea and vomiting following highly emetogenic chemotherapy. Ann Oncol 2006;17:1441-9. [Crossref] [PubMed]
- Eisenberg P, Figueroa-Vadillo J, Zamora R, et al. Improved prevention of moderately emetogenic chemotherapy-induced nausea and vomiting with palonosetron, a pharmacologically novel 5-HT3 receptor antagonist: results of a phase III, single-dose trial versus dolasetron. Cancer 2003;98:2473-82. [Crossref] [PubMed]
- Gralla R, Lichinitser M, Van Der Vegt S, et al. Palonosetron improves prevention of chemotherapy-induced nausea and vomiting following moderately emetogenic chemotherapy: results of a double-blind randomized phase III trial comparing single doses of palonosetron with ondansetron. Ann Oncol 2003;14:1570-7. [Crossref] [PubMed]
- Boccia R, Grunberg S, Franco-Gonzales E, et al. Efficacy of oral palonosetron compared to intravenous palonosetron for the prevention of chemotherapy-induced nausea and vomiting associated with moderately emetogenic chemotherapy: a phase 3 trial. Support Care Cancer 2013;21:1453-60. [Crossref] [PubMed]
- Karthaus M, Tibor C, Lorusso V, et al. Efficacy and safety of oral palonosetron compared with IV palonosetron administered with dexamethasone for the prevention of chemotherapy-induced nausea and vomiting (CINV) in patients with solid tumors receiving cisplatin-based highly emetogenic chemotherapy (HEC). Support Care Cancer 2015;23:2917-23. [Crossref] [PubMed]
- Saito M, Aogi K, Sekine I, et al. Palonosetron plus dexamethasone versus granisetron plus dexamethasone for prevention of nausea and vomiting during chemotherapy: a double-blind, double-dummy, randomised, comparative phase III trial. Lancet Oncol 2009;10:115-24. [Crossref] [PubMed]
- Long L, Zhan H, Long B, et al. Efficacy and safety of palonosetron hydrochloride injection for the prevention of chemotherapy-induced nausea and vomiting associated with moderately or highly emetogenic chemotherapy: a meta-analysis. Chin J Bases and Clin Gen Surg 2017;24:1.
- Likun Z, Xiang J, Yi B, et al. A systematic review and meta-analysis of intravenous palonosetron in the prevention of chemotherapy-induced nausea and vomiting in adults. Oncologist 2011;16:207-16. [Crossref] [PubMed]
- Tattersall FD, Rycroft W, Francis B, et al. Tachykinin NK1 receptor antagonists act centrally to inhibit emesis induced by the chemotherapeutic agent cisplatin in ferrets. Neuropharmacology 1996;35:1121-9. [Crossref] [PubMed]
- Merck & Co., Inc. Emend (aprepitant) capsules prescribing information. 2003.
- Merck & Co., Inc. Emend (aprepitant) capsules label. 2005.
- Merck Sharpe & Dohme Corp. Emend (aprepitant) capsules, for oral use; EMEND (aprepitant) for oral suspension; prescribing information. 2015.
- Hesketh PJ, Grunberg SM, Gralla RJ, et al. The oral neurokinin-1 antagonist aprepitant for the prevention of chemotherapy-induced nausea and vomiting: a multinational, randomized, double-blind, placebo-controlled trial in patients receiving high-dose cisplatin – the Aprepitant Protocol 052 Study Group. J Clin Oncol 2003;21:4112-9. [Crossref] [PubMed]
- Poli-Bigelli S, Rodrigues-Pereira J, Carides AD, et al. Addition of the neurokinin 1 receptor antagonist aprepitant to standard antiemetic therapy improves control of chemotherapy-induced nausea and vomiting. Results from a randomized, double-blind, placebo-controlled trial in Latin America. Cancer 2003;97:3090-8. [Crossref] [PubMed]
- Grunberg S, Chua D, Maru A, et al. Single-dose fosaprepitant for the prevention of chemotherapy-induced nausea and vomiting associated with cisplatin therapy: randomized, double-blind study protocol--EASE. J Clin Oncol 2011;29:1495-501. [Crossref] [PubMed]
- Kang HJ, Loftus S, DiCristina C, et al. Randomized, placebo-controlled, phase III study of aprepitant in preventing chemotherapy-induced nausea and vomiting in children: analysis by age group. J Clin Oncol 2016.34. abstract 10579.
- TESARO, Inc. Varubi (rolapitant) prescribing information. 2018. Available online: http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/206500s000lbl.pdf. Accessed 15 Aug 2018.
- Helsinn Birex Pharmaceuticals Ltd. Akynzeo (netupitant and palonosetron) prescribing information. 2018.
- Zhang L, Lu S, Feng J, et al. A randomized phase III study evaluating the efficacy of single-dose NEPA, a fixed antiemetic combination of netupitant and palonosetron, versus an aprepitant regimen for prevention of chemotherapy-induced nausea and vomiting (CINV) in patients receiving highly emetogenic chemotherapy (HEC). Ann Oncol 2018;29:452-8. [Crossref] [PubMed]
- Heo YA, Deeks ED. Rolapitant: A Review in Chemotherapy-Induced Nausea and Vomiting. Drugs 2017;77:1687-94. [Crossref] [PubMed]
- Rapoport BL, Chasen MR, Gridelli C, et al. Safety and efficacy of rolapitant for prevention of chemotherapy-induced nausea and vomiting after administration of cisplatin-based highly emetogenic chemotherapy in patients with cancer: two randomised, active-controlled, double-blind, phase 3 trials. Lancet Oncol 2015;16:1079-89. [Crossref] [PubMed]
- Schwartzberg LS, Modiano MR, Rapoport BL, et al. Safety and efficacy of rolapitant for prevention of chemotherapy-induced nausea and vomiting after administration of moderately emetogenic chemotherapy or anthracycline and cyclophosphamide regimens in patients with cancer: a randomised, active-controlled, double-blind, phase 3 trial. Lancet Oncol 2015;16:1071-8. [Crossref] [PubMed]
- Hesketh PJ, Rossi G, Rizzi G, et al. Efficacy and safety of NEPA, an oral combination of netupitant and palonosetron, for prevention of chemotherapy-induced nausea and vomiting following highly emetogenic chemotherapy: a randomized dose-ranging pivotal study. Ann Oncol 2014;25:1340-6. [Crossref] [PubMed]
- Aapro M, Rugo H, Rossi G, et al. A randomized phase III study evaluating the efficacy and safety of NEPA, a fixed-dose combination of netupitant and palonosetron, for prevention of chemotherapy-induced nausea and vomiting following moderately emetogenic chemotherapy. Ann Oncol 2014;25:1328-33. [Crossref] [PubMed]


