EGFR-targeting therapy as an evolving concept: learning from nimotuzumab clinical development
Introduction
Basic research on the epidermal growth factor receptor (EGFR) has been connected to oncology from its very beginnings, three decades ago, when important scientific discoveries laid the basis for a new therapeutic approach in cancer—molecular targeting of the EGFR. Among these discoveries were the findings that the EGFR shares a high sequence homology with the retroviral oncogenic protein v-ERBB (1) and that it shows an increased expression in human squamous cell lung cancers (2).
In parallel with these discoveries our group found that the EGFR is expressed in human breast tumors, showing an inverse correlation with the expression of the estrogen receptor (3). These studies prompted us to extend the concept of tumor hormone-dependence to growth factors such as the EGF (3), which in turn led to the idea of blocking EGF binding to its receptor using antagonistic antibodies, in order to inhibit tumor growth. Following this therapeutic approach, two different projects were initiated by our group in the subsequent years. One of them led to the development of the antibody nimotuzumab (4-6), while the second project resulted in CimaVax, a recently registered (in Cuba) EGF-based cancer vaccine (7).
Today, the available repertoire of EGFR-targeted agents comprises several monoclonal antibodies (mAbs) and small tyrosine kinase inhibitors (STKIs), some of them already registered and others under clinical investigation (8-10). Nonetheless, there still is a long way to go to optimize the clinical benefit from EGFR-targeted therapies. In this article we briefly discuss on current paradigms guiding the use of EGFR-targeting agents in the clinic, and on new emergent concepts. The discussion is largely based on experiences from the clinical development of nimotuzumab, which has shown a quite particular clinical profile.
A clinical look at EGFR-targeted therapies
Clinical results from phase III clinical trials have been modest
EGFR-targeted therapies have been extensively evaluated in the clinic for different tumor localizations and using different EGFR-targeting products, either registered or still in clinical development (8-10). The clinical benefit achieved with these products in advanced cancer patients, however, has been limited in terms of median overall survival. Taking cetuximab (a monoclonal antibody) and erlotinib (a tyrosine kinase inhibitor) as examples of successful anti-EGFR drugs, we see that in spite of marketing approvals, their impact in terms of clinical benefit has been in general limited when evaluated for the intent-to-treat populations in different clinical trials.
For cetuximab, although encouraging results were obtained in locoregionally advanced head and neck cancer [median survival time (MST): 49 months for patients treated with cetuximab and radiotherapy (RT), vs. 29.3 months with RT alone] (11), the survival advantage was dramatically reduced in patients with recurrent or metastatic carcinomas of the head and neck that were treated with cetuximab and chemotherapy (CTP) (MST: 10.1 vs. 7.4 months) (12). In KRAS wild-type metastatic colorectal cancer, cetuximab plus CTP resulted in a modest MST improvement (23.5 vs. 20.0 months) (13). Similarly modest results were obtained in another clinical study in patients with colorectal cancer (14,15). In advanced non-small cell lung cancer (NSCLC), the small increase in overall survival precluded marketing approval (16). In pancreatic cancer, the encouraging results from preclinical and early clinical studies with cetuximab were not confirmed in a phase III trial (17). With erlotinib, the clinical trials conducted in NSCLC patients (18-20) and in pancreatic cancer (21) also evidenced a limited impact.
Is clinical efficacy necessarily bound to toxicity?
Clinical researchers are more and more pointing out the need of revising the clinical trial endpoints that are being used for EGFR-targeted agents, since neither toxicity nor tumor shrinkage have resulted adequate surrogates to evaluate their clinical efficacy (22,23). But in spite of the increasing understanding on this problem, clinical studies are still being guided by the classical cytotoxic paradigm that correlates clinical efficacy with objective clinical responses. Moreover, clinical efficacy has been linked to skin rash toxicity. This applies both to EGFR-targeting mAbs, like cetuximab (15) and panitumumab (24), and to STKIs. Overall, more than a dozen phase II and III clinical trials using EGFR inhibitors have shown an association between rash incidence, severity and survival (25). This association implies also a drawback since cumulative toxicity may impair the chronic use of the anti-EGFR agents and their combination with other therapies (26). A relevant question here is whether the clinical efficacy of EGFR-targeting drugs (in terms of overall survival) is unavoidably bound to toxicity, or not necessarily. The clinical experience with the monoclonal antibody nimotuzumab suggests that there are alternatives to the classical cytotoxic paradigm and that clinical efficacy may be accompanied with a low toxicity profile.
Nimotuzumab: diverging from the cytotoxic paradigm
In this section we briefly review the development of nimotuzumab and discuss the main lessons we have extracted from its application in the clinic and from mechanistic studies.
The development of the humanized anti-EGFR antibody known today as nimotuzumab (also as Taixinsheng, in China) started in the late 80s at the Cuban Institute of Oncology in Havana (see the timeline in Figure 1). The parent murine antibody, called R3, was generated in Balb/c mice immunized with purified human EGFR, and selected for its ability to block EGF binding to the receptor (27). Subsequently, the murine mAb was shown to have antitumor effects in vitro (28). Aiming forward to the clinic, R3 was humanized by CDR grafting as early as in 1994 (29), becoming into a human IgG1/kappa antibody that retained practically the same antigen-binding affinity of the original murine mAb (30). The humanized antibody was initially called as h-R3, and later (in 2004) given the non-proprietary name nimotuzumab (31).
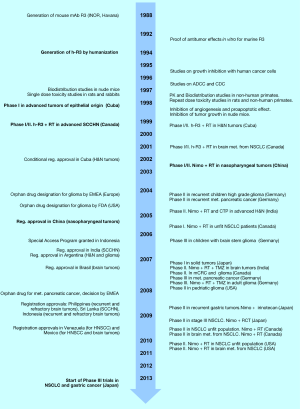
In early diagnostic clinical studies with 99Tc-radiolabelled R3, involving 148 patients with different types of cancer of epithelial origin, the antibody accumulated in primary tumors and metastases previously identified by biopsy, showing an overall sensitivity of 84% and 100% specificity (32). These and other results from preclinical studies (performed with the murine and/or the humanized mAbs) (33) opened the door to therapeutic clinical studies with nimotuzumab in patients with head and neck tumors, which started in Cuba in 1998 (34).
Since then, nimotuzumab has been extensively tested in about 30 completed clinical trials conducted in different countries, and a similar number of clinical studies are currently ongoing in a dozen countries, including several phase III and phase IV trials. A large part of the clinical development has been taking place in China, where the antibody (called as Taixinsheng) has been assayed in several phase I/II clinical trials in different types of cancer: esophageal tumors (35-38), nasopharyngeal carcinoma (39,40) and other head and neck tumors (41,42), pancreatic cancer (43), glioma (44,45) and NSCLC (46,47).
At present, nimotuzumab is indicated for the treatment of patients with nasopharyngeal cancer and other advanced head and neck tumors, adult high grade glioma, children glioma, and advanced esophageal cancer. The antibody has been registered in more than 30 developing countries, including Brazil, India and China. Altogether, about 30,000 patients have been treated with nimotuzumab worldwide.
Nimotuzumab shows a low toxicity profile, allowing long-term treatments
Clinical studies conducted in patients with head and neck tumors (48,49), NSCLC (50), pancreatic cancer (51) glioblastomas (52,53) and esophageal tumors (37,54) have shown that the combination of nimotuzumab with either RT or chemotherapy provides clinical benefits. More statistically sound results may come from the currently ongoing phase III trials.
Notably, the clinical effects obtained with nimotuzumab have been accompanied with a very low toxicity profile (55), which in turn has made possible the use of this antibody in prolonged treatments, lasting several months or even years, that have being characterized by the induction of long-term stable disease (6). A recent example of this was a randomized, double blind trial conducted in patients with high grade glioma (56). In this study nimotuzumab was administered during one year up to a maximum cumulative dose of 3,600 mg, which is probably the highest cumulative antibody dose ever administered to glioma patients. Nonetheless, no severe adverse events attributable to the antibody were observed. Although nimotuzumab did not significantly improve the rates of objective response or disease control, it did increase the progression free survival (median PFS: 15.7 vs. 6.5 months for nimotuzumab and control arms, respectively) and overall survival (median OS: 17.8 vs. 12.6 months) (56).
Nimotuzumab’s low toxicity profile has been explained at the molecular level on the basis of its medium or “intermediate” affinity (KD ~10–8 M for the Fab fragment, which represents a 10-fold lower affinity as compared with the cetuximab Fab) (30). According to this hypothesis, which was initially supported by mathematical modeling, antibodies with such medium affinities need bivalent binding for stable attachment to cells and, therefore, would accumulate mostly on those tumors that over-express the EGFR and to a lesser degree in normal epithelial tissues having lower expression levels of the receptor. Recent studies have given further support to this hypothesis. In in vitro experiments with different tumor cell lines, Garrido and coworkers (57) showed that nimotuzumab binding and inhibition of EGFR phosphorylation occurs only for tumor cell lines with medium or high levels of EGFR expression (104 receptors per cell or higher). Furthermore, while nimotuzumab Fab fragments bound only to A431 cells (having the highest EGFR expression level), the Fab of cetuximab was able to bind to tumor cells with lower EGFR expression levels.
The idea that an antibody with a somewhat lower affinity may have advantages in the clinic is itself a deviation from the widely accepted paradigm that the highest the affinity, the better. We are tempted to speculate that the therapeutic ratio of some of the high-affinity anti-EGFR antibodies currently in the clinic might be improved by “optimizing” (lowering) their affinity, although the expected increase in clinical benefit might be seen in particular in patients bearing EGFR-overexpressing tumors. For nimotuzumab, several studies suggest that this antibody has a better clinical effect in tumors with medium or high levels of EGFR expression (48,49,54), which in turn should have become more “EGFR-addicted”, as we discuss in the next section.
Oncogene addiction as ground for EGFR-targeted therapies
As introduced by I. Bernard Weinstein, the term “oncogene addiction” refers to the manifested dependency of some cancers on one or a few genes for maintenance of the malignant phenotype (58). It is such a dependency on a particular gene (and its associated signaling pathway) what sustains the targeted therapy approach in cancer treatment.
The clinical evidences of the EGFR oncogene addiction phenomenon are provided by those patients that show high responses to EGFR-targeted agents (59). High responses, on the other hand, have been associated with high expression levels of the receptor, as in the FLEX trial conducted in EGFR-expressing NSCLC patients, which were treated with cetuximab in combination with chemotherapy (16). Better clinical outcomes from treatment with nimotuzumab have been also associated with EGFR over-expression, as mentioned above. For erlotinib, better response and PFS rates in advanced NSCLC patients have been correlated with activating mutations in the tyrosine kinase domain of the EGFR (19,20). Activating mutations are an alternative (to EGFR over-expression) mechanism to increase downstream signaling in EGFR-addicted cells.
“Naïve” versus “adaptive” oncogene addiction
EGFR oncogene addiction may arise during tumor progression (by gene amplification and/or aberrant expression), in which case we would be in the presence of a “naïve” oncogene addiction, or it may develop as a resistant mechanism driven by chemo-therapy or RT (for example, deletion mutants and activating kinase domain mutations), being thus an “adaptive” addiction. A distinct medical positioning is required in each case regarding the use of an EGFR-targeted therapy as first or second line treatment. Naïve EGFR addiction reveals in tumors having EGFR over expression, as evidenced by the clinical responses observed in patients with locally advanced squamous cell carcinomas of the head and neck (11), NSCLC (60) and esophageal tumors (54) upon treatment with anti-EGFR agents. Adaptive EGFR addiction, on the other hand, is observed for tumors that become refractory to chemotherapeutic agents, which then respond to the combination of chemotherapy with an anti-EGFR agent, as is the case for refractory ADC tumors having low to medium EGFR expression, such as colorectal carcinomas, gastric, pancreatic and lung tumors.
Cancer stem cells and oncogene addiction
The existence of EGFR addiction in cancer stem cells is supported by an increasing number of experimental evidences [reviewed in (59)]. For instance, modulation of EGFR expression in glioblastoma multiforme (GBM)-derived tumor initiating cells enhances or reduces their tumorigenic ability (61). On the other hand, GBM CD133+ tumor initiating cells are radio-resistant and most likely are the source of tumor recurrence after radiation (62). It has been shown that the combination of an anti-EGFR antibody (either cetuximab or nimotuzumab) with RT has a cytotoxic effect on CD133+ stem cells (63), which suggests that RT reinforces EGFR oncogene addiction in neural cancer stem cells and gives further support to the combined use of anti-EGFR antibodies and RT for treating brain tumors.
The relevance of the EGFR-signaling pathway in the tumorigenic and invasion capabilities of cancer stem cells, as well as the sensitivity of these cells to EGFR-targeted treatment, has been demonstrated for cells from head and neck (64) and breast tumors. In breast cancer, for instance, it was shown that EGFR activation can induce epithelial to mesenchymal transition (EMT), favoring invasion and metastasis (65). Furthermore, inhibition of the EGFR in aggressive inflammatory breast cancer reversed the mesenchymal phenotype of cancer cells to a less aggressive and potentially more chemotherapy-sensitive epithelial phenotype (66). The capability of modulating the malignant cell phenotype has a particular translational relevance because it may imply that chronic use of EGFR-targeted therapy would have a controlling effect on EGFR-addicted metastases.
EGFR inhibition in addicted cells may induce immunogenic cell death
The antitumor effect of an anti-EGFR mAb can be mediated by T cells, as was demonstrated in a mouse model (67). In these experiments, depletion of CD4+ and CD8+ T cells abolished the anti-tumor effect of the antibody. In a subsequent study using a Lewis lung carcinoma model and the same anti-mouse EGFR antibody, it was demonstrated that this antibody, but not a STKI, promotes a CTL-activating immunogenic cell death (68). Remarkably, this immunogenic effect was independent of the antibody effector functions since removal of the Fc fragment did not prevent the induction of immunogenic apoptosis.
It has been argued that the “vaccinal effect” of anti-tumor monoclonal antibodies may be important to produce clinical benefit. In support of this hypothesis, a recent report shows that cetuximab activates NK cells and promotes dendritic cell maturation and CD8+ T-cell priming, leading to tumor antigen spreading and TH1 cytokine release. Moreover, cetuximab promoted an EGFR-specific cellular immunity (69). We believe that long-term responses require the involvement of the immune system, and in this regard, the fact that an anti-EGFR antibody may enhance the antitumor immunity gives support to the chronic use of these agents in the clinic.
Resistance mechanisms are driven by the need to satisfy oncogene addiction
The selection pressure created by treatment of an EGFR-addicted tumor with an anti-EGFR drug favors the survival of those cells that find an escape mechanism to satisfy their addiction. One of the evasion mechanisms is via mutations that impair drug binding or enhance receptor functioning. For example, the T790M mutation in the kinase domain confers resistance to gefitinib and erlotinib in lung adenocarcinomas by stabilizing the active tyrosine kinase conformation and enhancing ATP binding (70).
A second type of resistance mechanism consists in making irrelevant the function of the EGFR itself, while ensuring downstream signaling via the PI3K/AKT or RAS/RAF/MEK/ERK pathways. This is the case of KRAS mutations that make downstream signaling independent of EGFR activation. Other members of the ErbB family also may play important roles in activating compensatory signals; for example, acquired resistance to cetuximab in colorectal cancer has been linked to activation of ERBB2 signaling (71). Interestingly, although EGFR-activating mutations and KRAS mutations have been reported to be mutually exclusive in colorectal cancer, a recent study using a mouse model of pancreatic ductal adenocarcinoma shows that upregulation and activation of the EGFR is required for the KRAS-driven tumorigenesis characteristic of this type of tumor (72).
A recent study in mice revealed a novel immunological mechanism of escape to treatment with an anti-EGFR antibody, resulting from the convergence of alterations in oncogenic and immunological pathways (73). Analyses of resistant tumor cell variants showed that EGFR inhibition produced HER3 overexpression and PTEN deficiency, leading to hyperactivation of protumoral downstream signaling. Remarkably, concomitantly with these alterations MHC-I expression was downregulated as a consequence of transcriptional alterations in the IFN-γ pathway.
Connection between EGFR oncogene addiction and glucose metabolism
It has been shown that the EGFR prevents autophagic cell death by maintaining the intracellular glucose level, most likely through stabilizing interactions with the sodium/glucose co-transporter 1 (SGLT1) (74). Guaranteeing an active glucose transport is critical for tumor cell survival in the context of a tumor micro-environment characterized by hypoxia and nutrient starvation. Therefore, the role played by the EGFR in stabilizing the sodium/glucose co-transporters may reinforce the addiction to this oncogene.
Thinking of EGFR-targeting as a biological therapy
The classical approach of using EGFR-targeted agents as cytotoxic drugs has limited their actual potential. As has been widely documented, objective clinical responses often do not result in increase of survival time due to the rapid emergence of resistance, as occurs, for example, when using STKIs in NSCLC. We believe that targeted therapies have the potential to stop disease progression and transform advanced cancer into a long-term controlled chronic disease based mostly on biological regulatory mechanisms.
Inhibition of EGFR activation may trigger several different mechanisms that would contribute to stop tumor progression. One of them is the induction of immunogenic cell death that then activates an anti-tumor T cell immunity. The existence and importance of this immune response have been recently demonstrated for cetuximab (69), as mentioned above. Another mechanism is the induction of anti-angiogenic effects, as has been shown for nimotuzumab (63,75).
The capability of targeting cancer stem cells and modulate their malignant cell phenotype is a third mechanism, which is particularly relevant because it may induce a long-term stable disease and have a controlling effect on metastases. The clinical experience using nimotuzumab in long-term treatments of advanced cancer patients gives certain support to this hypothesis. And last, but not least, the recently uncovered interconnection between the EGFR and glucose metabolism suggests that EGFR-targeted agents may control cell growth also by impairing the interaction between the EGFR and the glucose receptor SGLT1.
The interconnection of several cellular pathways in the oncogene addiction phenomenon opens a wide spectrum of possibilities for combinatorial therapies. In this regard, a better comprehension of the resistance mechanisms that emerge from the use of EGFR-targeted agents will provide important clues for the rational design of these therapies.
Concluding remarks
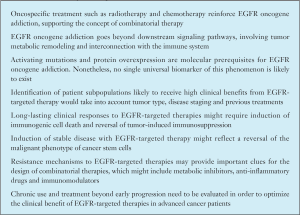
In our understanding, EGFR-targeted therapy is still an evolving concept. It is moving apart from the cytotoxic paradigm to become a biological therapy, with implications far beyond the control of tumor cell proliferation. In Box 1 we summarize a few of the main ideas we have discussed here, together with our vision on the future progress of EGFR-targeted therapies. The design of EGFR-targeting therapies should take into account the convergence of different mechanisms and cellular pathways in the oncogene addiction phenomenon, and should be based on the concept of “personalized medicine”. Clinical trials need to incorporate more translational research, so the existing gap between basic research and clinical investigation has to be filled. One important milestone in the development of EGFR-targeted therapies is gaining a better understanding of the connection between the EGFR pathway and anti-tumor immunity.
Full table
Acknowledgements
We are grateful to Dr. Normando Iznaga for his valuable help in compiling the timeline of nimotuzumab clinical development.
Disclosure: The authors declare no conflict of interest.
References
- Downward J, Yarden Y, Mayes E, et al. Close similarity of epidermal growth factor receptor and v-erb-B oncogene protein sequences. Nature 1984;307:521-7. [PubMed]
- Hendler FJ, Ozanne BW. Human squamous cell lung cancers express increased epidermal growth factor receptors. J Clin Invest 1984;74:647-51. [PubMed]
- Pérez R, Pascual M, Macías A, et al. Epidermal growth factor receptors in human breast cancer. Breast Cancer Res Treat 1984;4:189-93. [PubMed]
- Ramakrishnan MS, Eswaraiah A, Crombet T, et al. Nimotuzumab, a promising therapeutic monoclonal for treatment of tumors of epithelial origin. MAbs 2009;1:41-8. [PubMed]
- Bode U, Massimino M, Bach F, et al. Nimotuzumab treatment of malignant gliomas. Expert Opin Biol Ther 2012;12:1649-59. [PubMed]
- Perez R, Moreno E, Garrido G, et al. EGFR-targeting as a biological therapy: understanding nimotuzumab’s clinical effects. Cancers (Basel) 2011;3:2014-31. [PubMed]
- Rodriguez G, Gonzalez G, Crombet T, et al. Therapeutic vaccination with an EGF-based vaccine in lung cancer: a step in the transition to a chronic disease. Expert Rev Respir Med 2011;5:337-42. [PubMed]
- Cohen RB. Current challenges and clinical investigations of epidermal growth factor receptor (EGFR)- and ErbB family-targeted agents in the treatment of head and neck squamous cell carcinoma (HNSCC). Cancer Treat Rev 2014;40:567-77. [PubMed]
- Hirsch FR, Jänne PA, Eberhardt WE, et al. Epidermal growth factor receptor inhibition in lung cancer: status 2012. J Thorac Oncol 2013;8:373-84. [PubMed]
- Masuda H, Zhang D, Bartholomeusz C, et al. Role of epidermal growth factor receptor in breast cancer. Breast Cancer Res Treat 2012;136:331-45. [PubMed]
- Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med 2006;354:567-78. [PubMed]
- Vermorken JB, Herbst RS, Leon X, et al. Overview of the efficacy of cetuximab in recurrent and/or metastatic squamous cell carcinoma of the head and neck in patients who previously failed platinum-based therapies. Cancer 2008;112:2710-9. [PubMed]
- Van Cutsem E, Köhne CH, Hitre E, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med 2009;360:1408-17. [PubMed]
- Cunningham D, Humblet Y, Siena S, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med 2004;351:337-45. [PubMed]
- Jonker DJ, O’Callaghan CJ, Karapetis CS, et al. Cetuximab for the treatment of colorectal cancer. N Engl J Med 2007;357:2040-8. [PubMed]
- Pirker R, Pereira JR, Szczesna A, et al. Cetuximab plus chemotherapy in patients with advanced non-small-cell lung cancer (FLEX): an open-label randomised phase III trial. Lancet 2009;373:1525-31. [PubMed]
- Faloppi L, Andrikou K, Cascinu S. Cetuximab: still an option in the treatment of pancreatic cancer? Expert Opin Biol Ther 2013;13:791-801. [PubMed]
- Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med 2005;353:123-32. [PubMed]
- Cappuzzo F, Ciuleanu T, Stelmakh L, et al. Erlotinib as maintenance treatment in advanced non-small-cell lung cancer: a multicentre, randomised, placebo-controlled phase 3 study. Lancet Oncol 2010;11:521-9. [PubMed]
- Pérol M, Chouaid C, Pérol D, et al. Randomized, phase III study of gemcitabine or erlotinib maintenance therapy versus observation, with predefined second-line treatment, after cisplatin-gemcitabine induction chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol 2012;30:3516-24. [PubMed]
- Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 2007;25:1960-6. [PubMed]
- Di Cosimo S, Baselga J. Pharmacodynamic endpoints in primary breast cancer. Ann Oncol 2007;18 Suppl 9:ix21-3. [PubMed]
- Hales RK, Banchereau J, Ribas A, et al. Assessing oncologic benefit in clinical trials of immunotherapy agents. Ann Oncol 2010;21:1944-51. [PubMed]
- Van Cutsem E, Peeters M, Siena S, et al. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol 2007;25:1658-64. [PubMed]
- Bebb G, Boland W, Melosky B. Don’t jump to rash conclusions. Cancer Biol Ther 2011;11:639-41. [PubMed]
- Lacouture ME. Mechanisms of cutaneous toxicities to EGFR inhibitors. Nat Rev Cancer 2006;6:803-12. [PubMed]
- Fernández A, Pérez R, Macías A, et al. Generación y caracterización primaria de anticuerpos monoclonales contra el receptor del factor de crecimiento epidérmico. Interferón y Biotecnología 1989;6:289-98.
- Fernandez A, Spitzer E, Perez R, et al. A new monoclonal antibody for detection of EGF-receptors in western blots and paraffin-embedded tissue sections. J Cell Biochem 1992;49:157-65. [PubMed]
- Mateo C, Moreno E, Amour K, et al. Humanization of a mouse monoclonal antibody that blocks the epidermal growth factor receptor: recovery of antagonistic activity. Immunotechnology 1997;3:71-81. [PubMed]
- Talavera A, Friemann R, Gómez-Puerta S, et al. Nimotuzumab, an antitumor antibody that targets the epidermal growth factor receptor, blocks ligand binding while permitting the active receptor conformation. Cancer Res 2009;69:5851-9. [PubMed]
- Proposed International Nonproprietary Names: List 94. WHO Drug Information 2005;19:333.
- Ramos-Suzarte M, Rodríguez N, Oliva JP, et al. 99mTc-labeled antihuman epidermal growth factor receptor antibody in patients with tumors of epithelial origin: Part III. Clinical trials safety and diagnostic efficacy. J Nucl Med 1999;40:768-75. [PubMed]
- Iznaga-Escobar N, Perez R. Ior egf/r3 Antineoplastic Monoclonal Antibody. Murine IgG2a Monoclonal Antibody Against the Epidermal Growth Factor Receptor (EGF-R). Drugs Fut 1999;24:1301-5.
- Crombet T, Torres O, Neninger E, et al. Phase I clinical evaluation of a neutralizing monoclonal antibody against epidermal growth factor receptor. Cancer Biother Radiopharm 2001;16:93-102. [PubMed]
- Ling Y, Chen J, Tao M, et al. A pilot study of nimotuzumab combined with cisplatin and 5-FU in patients with advanced esophageal squamous cell carcinoma. J Thorac Dis 2012;4:58-62. [PubMed]
- Zhao KL, Hu XC, Wu XH, et al. A phase I dose escalation study of Nimotuzumab in combination with concurrent chemoradiation for patients with locally advanced squamous cell carcinoma of esophagus. Invest New Drugs 2012;30:1585-90. [PubMed]
- Liang J. Nimotuzumab combined with radiotherapy for esophageal cancer: preliminary study of a Phase II clinical trial. Onco Targets Ther 2013;6:1589-96. [PubMed]
- Ma NY, Cai XW, Fu XL, et al. Safety and efficacy of nimotuzumab in combination with radiotherapy for patients with squamous cell carcinoma of the esophagus. Int J Clin Oncol 2013. [Epub ahead of print]. [PubMed]
- Huang XD, Yi JL, Gao L, et al. Multi-center phase II clinical trial of humanized anti-epidermal factor receptor monoclonal antibody h-R3 combined with radiotherapy for locoregionally advanced nasopharyngeal carcinoma. Zhonghua Zhong Liu Za Zhi 2007;29:197-201. [PubMed]
- Yan S, Jiang X, Yang J, et al. Radiotherapy for nasopharyngeal carcinoma and combined capecitabine and nimotuzumab treatment for lung metastases in a liver transplantation recipient: a case experience of sustained complete response. Cancer Biother Radiopharm 2012;27:519-23. [PubMed]
- Zhao XY, Guo Y, Zhu YX, et al. Clinical analysis of nimotuzumab plus cisplatin and fluorouracil regimen as induction treatment in resectable head and neck squamous cell carcinoma. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 2012;47:536-9. [PubMed]
- Meng J, Gu QP, Meng QF, et al. Efficacy of nimotuzumab combined with docetaxel-cisplatin-fluorouracil regimen in treatment of advanced oral carcinoma. Cell Biochem Biophys 2014;68:181-4. [PubMed]
- Su D, Jiao SC, Wang LJ, et al. Efficacy of nimotuzumab plus gemcitabine usage as first-line treatment in patients with advanced pancreatic cancer. Tumour Biol 2013. [Epub ahead of print]. [PubMed]
- Yang QY, Shen D, Sai K, et al. Nimotuzumab in combination with chemotherapy for patients with malignant gliomas. Zhonghua Zhong Liu Za Zhi 2011;33:232-5. [PubMed]
- Hong J, Peng Y, Liao Y, et al. Nimotuzumab prolongs survival in patients with malignant gliomas: A phase I/II clinical study of concomitant radiochemotherapy with or without nimotuzumab. Exp Ther Med 2012;4:151-7. [PubMed]
- Li LF, Wang HQ, Liu XM, et al. Nimotuzumab in combination with chemotherapy in patients with advanced non-small cell lung cancer. Zhonghua Zhong Liu Za Zhi 2011;33:626-8. [PubMed]
- Qi DL, Wang HQ, Li Y, et al. Efficacy and adverse effets of nimotuzumab plus paclitaxel liposome and carboplatin in the treatment for advanced non-small cell lung cancer. Zhonghua Zhong Liu Za Zhi 2012;34:152-5. [PubMed]
- Basavaraj C, Sierra P, Shivu J, et al. Nimotuzumab with chemoradiation confers a survival advantage in treatment-naïve head and neck tumors over expressing EGFR. Cancer Biol Ther 2010;10:673-81. [PubMed]
- Rodríguez MO, Rivero TC, del Castillo Bahi R, et al. Nimotuzumab plus radiotherapy for unresectable squamous-cell carcinoma of the head and neck. Cancer Biol Ther 2010;9:343-9. [PubMed]
- Boland W, Bebb G. The emerging role of nimotuzumab in the treatment of non-small cell lung cancer. Biologics 2010;4:289-98. [PubMed]
- Strumberg D, Schultheis B, Scheulen ME, et al. Phase II study of nimotuzumab, a humanized monoclonal anti-epidermal growth factor receptor (EGFR) antibody, in patients with locally advanced or metastatic pancreatic cancer. Invest New Drugs 2012;30:1138-43. [PubMed]
- Massimino M, Bode U, Biassoni V, et al. Nimotuzumab for pediatric diffuse intrinsic pontine gliomas. Expert Opin Biol Ther 2011;11:247-56. [PubMed]
- Cabanas R, Saurez G, Rios M, et al. Treatment of children with high grade glioma with nimotuzumab: a 5-year institutional experience. MAbs 2013;5:202-7. [PubMed]
- Ramos-Suzarte M, Lorenzo-Luaces P, Lazo NG, et al. Treatment of malignant, non-resectable, epithelial origin esophageal tumours with the humanized anti-epidermal growth factor antibody nimotuzumab combined with radiation therapy and chemotherapy. Cancer Biol Ther 2012;13:600-5. [PubMed]
- Boland WK, Bebb G. Nimotuzumab: a novel anti-EGFR monoclonal antibody that retains anti-EGFR activity while minimizing skin toxicity. Expert Opin Biol Ther 2009;9:1199-206. [PubMed]
- Solomón MT, Selva JC, Figueredo J, et al. Radiotherapy plus nimotuzumab or placebo in the treatment of high grade glioma patients: results from a randomized, double blind trial. BMC Cancer 2013;13:299. [PubMed]
- Garrido G, Tikhomirov IA, Rabasa A, et al. Bivalent binding by intermediate affinity of nimotuzumab: a contribution to explain antibody clinical profile. Cancer Biol Ther 2011;11:373-82. [PubMed]
- Weinstein IB. Cancer. Addiction to oncogenes--the Achilles heal of cancer. Science 2002;297:63-4. [PubMed]
- Perez R, Crombet T, de Leon J, et al. A view on EGFR-targeted therapies from the oncogene-addiction perspective. Front Pharmacol 2013;4:53. [PubMed]
- Pirker R, Pereira JR, von Pawel J, et al. EGFR expression as a predictor of survival for first-line chemotherapy plus cetuximab in patients with advanced non-small-cell lung cancer: analysis of data from the phase 3 FLEX study. Lancet Oncol 2012;13:33-42. [PubMed]
- Mazzoleni S, Politi LS, Pala M, et al. Epidermal growth factor receptor expression identifies functionally and molecularly distinct tumor-initiating cells in human glioblastoma multiforme and is required for gliomagenesis. Cancer Res 2010;70:7500-13. [PubMed]
- Bao S, Wu Q, McLendon RE, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 2006;444:756-60. [PubMed]
- Diaz Miqueli A, Rolff J, Lemm M, et al. Radiosensitisation of U87MG brain tumours by anti-epidermal growth factor receptor monoclonal antibodies. Br J Cancer 2009;100:950-8. [PubMed]
- Abhold EL, Kiang A, Rahimy E, et al. EGFR kinase promotes acquisition of stem cell-like properties: a potential therapeutic target in head and neck squamous cell carcinoma stem cells. PLoS One 2012;7:e32459. [PubMed]
- Del Vecchio CA, Jensen KC, Nitta RT, et al. Epidermal growth factor receptor variant III contributes to cancer stem cell phenotypes in invasive breast carcinoma. Cancer Res 2012;72:2657-71. [PubMed]
- Zhang D, LaFortune TA, Krishnamurthy S, et al. Epidermal growth factor receptor tyrosine kinase inhibitor reverses mesenchymal to epithelial phenotype and inhibits metastasis in inflammatory breast cancer. Clin Cancer Res 2009;15:6639-48. [PubMed]
- Garrido G, Lorenzano P, Sánchez B, et al. T cells are crucial for the anti-metastatic effect of anti-epidermal growth factor receptor antibodies. Cancer Immunol Immunother 2007;56:1701-10. [PubMed]
- Garrido G, Rabasa A, Sánchez B, et al. Induction of immunogenic apoptosis by blockade of epidermal growth factor receptor activation with a specific antibody. J Immunol 2011;187:4954-66. [PubMed]
- Srivastava RM, Lee SC, Andrade Filho PA, et al. Cetuximab-activated natural killer and dendritic cells collaborate to trigger tumor antigen-specific T-cell immunity in head and neck cancer patients. Clin Cancer Res 2013;19:1858-72. [PubMed]
- Yoshikawa S, Kukimoto-Niino M, Parker L, et al. Structural basis for the altered drug sensitivities of non-small cell lung cancer-associated mutants of human epidermal growth factor receptor. Oncogene 2013;32:27-38. [PubMed]
- Yonesaka K, Zejnullahu K, Okamoto I, et al. Activation of ERBB2 signaling causes resistance to the EGFR-directed therapeutic antibody cetuximab. Sci Transl Med 2011;3:99ra86. [PubMed]
- Ardito CM, Grüner BM, Takeuchi KK, et al. EGF receptor is required for KRAS-induced pancreatic tumorigenesis. Cancer Cell 2012;22:304-17. [PubMed]
- Garrido G, Rabasa A, Garrido C, et al. Preclinical modeling of EGFR-specific antibody resistance: oncogenic and immune-associated escape mechanisms. Oncogene 2013. [Epub ahead of print]. [PubMed]
- Weihua Z, Tsan R, Huang WC, et al. Survival of cancer cells is maintained by EGFR independent of its kinase activity. Cancer Cell 2008;13:385-93. [PubMed]
- Crombet-Ramos T, Rak J, Pérez R, et al. Antiproliferative, antiangiogenic and proapoptotic activity of h-R3: A humanized anti-EGFR antibody. Int J Cancer 2002;101:567-75. [PubMed]


