Status of hepatocellular carcinoma in Gulf region
Background
Hepatocellular carcinoma (HCC) is the fifth most common cancer in the world with an increasing incidence in some areas like Europe, USA and the Gulf region (1). The unique geographic distribution is likely to be determined by specific etiologic factors. There is a distinctive difference in sex and age related occurrence of disease. HCC results in about one million deaths per year. There is no clear data about the prevalence and death rate due to HCC in the Gulf region. The Arabian Peninsula (the Gulf) is a unique geographical area. Thirty five percent of the Arab World comprises Saudi Arabia, Oman, Qatar, Bahrain, United Arab Emirate (UAE), and Kuwait with population of nearly 40 million. Depending on the individual nation, the ratio of nationals and expatriates living in the area is in the range of 30-70% being expatriates (Figure 1). The information in this review is based on PubMed literature, national cancer registries and cancer incidence data from various sources (2,3).
Patients with HCC continue to have a dismal prognosis, with 1- and 3-year survival rates of 36% and 17%, respectively (4). This is in part related to more than two-thirds of tumors being diagnosed at advanced stages (5,6), as well as a substantial portion of patients with early HCC failing to receive potentially curative treatments (7). As more therapies are available for patients with HCC, treatment decisions become increasingly complex. HCC generally develops secondary to chronic liver disease and cirrhosis (8). In the Gulf region the incidence varies in males between 3.4-8.1 and in females between 1.8-3.1 cases per 100,000 per year (9,10).
Recent data on the epidemiology of HCC shows that the incidence of this liver malignancy continues to increase rapidly in most parts of the world including the Gulf Region. In contrast to the West, where HCC is less common and mainly secondary to alcoholic hepatitis and hepatitis B virus (HBV) (11,12), in many Middle Eastern countries including the Gulf Region, HCC is one of the most common cancers and usually develops secondary to hepatitis C virus (HCV) (13,14). Chronic HBV infection still is one of the main risk factor beside the HCV.
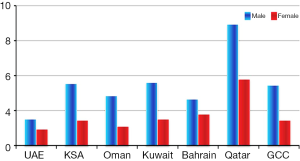
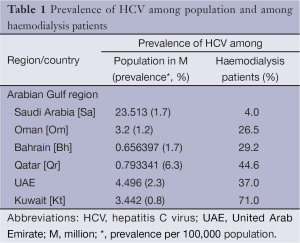
Another report from Gulf region indicates that liver cancer is the sixth most common cancer in the Gulf Cooperation Council (GCC) states. A total of 4,965 liver cancer cases (5.2% of all cancers) were reported from all GCC States in 1998-2007. The overall Age Standardized Rate (ASR) for all GCC States was 6.9 and 2.9 per 100,000 populations for males and females respectively. The liver cancer incidence was significantly higher among men compared to women in all GCC States. Qatar reported the highest incidence among men and women with ASR of 13.9 and 7.6 for males and females respectively. Kuwaiti men ranked second and Saudi men ranked third. UAE reported the lowest ASR in both genders (ASR of 3.0 and 1.9 for males and females respectively) (Figure 2) (Tables 1-3) (15,16).
Full table

Full table
Full table
Methods
Qatar has a population structure not very different from other Gulf Countries. As an example for the Gulf Region we present a retrospective analysis of all Qatari cases of HCC diagnosed in Hamad Medical Corporation as collected from patient files, Medicom data (the corporation electronic system for patient’s data) and the Qatar National Cancer Registry during the period March 2004-December 2010. One hundred fifty patients were included in the study. This study was approved by the research and ethical committee (IRB) of Hamad Medical Corporation, Doha, Qatar.
Statistical data analysis methods and quantitative variables were expressed as means. Standard deviations and frequencies (percentages) were calculated to summarize quantitative data. Medians and ranges have been reported for skewed (non-normal) data. Univariate Kaplan-Meier survival analysis was performed to estimate overall and group wise survival. Furthermore, the log-rank test was applied to determine any statistical difference in survival among various subgroups. In addition, the multivariate Cox regression method was used to assess the significant effect of various prognostic factors on outcome survival time. Statistical significant values were reported with their corresponding 95% CI values. P<0.05 was considered to indicate a statistical significant difference. All the statistical analyses were performed using statistical software package Statistical Package of Social Sciences (SPSS, version 18.0, Chicago, IL).
Results
Epidemiology
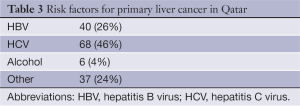
One hundred fifty HCC patients diagnosed during the period March 2004 to December 2010 at HMC were included in the study. The mean age was 58.8 [31-87] years with male: female ratio 3:1 (76% male; 24% female). There were 48 (32%) Qatari and 102 (68%) non-Qatari patients. The non-Qatari patients were of different nationalities from all over the world and those presenting with HCC mostly from Egypt and South Asia. HCV related disease was the most common cause of HCC in 68 patients (45%), HBV in 40 patients (27%) alcoholic liver disease only in 6 (4%) and the remaining 36 (24%) no underlying risk factors identified.
Diagnosis
Diagnosis was confirmed by histopathology in 56 patients (37%) and by AASLD criteria for the remaining 94 (63%). The criteria were typical radiological features of HCC with arterial enhancement and venous wash from lesions more than 2 cm in diameter in cirrhotic liver or other radiologically evident lesion with high alpha-fetoprotein (AFP) levels (more than 400 ng/mL).
Liver function and stage
Child-Pugh assessment was A in (33%), B in (37%) and C in (30%), using Barcelona Clinic for Liver Cancer (BCLC) which depend on the liver functions status, patients performance and the TNM stage of the tumor. One quarter of the patients were in early stages, another quarter intermediate stage and nearly half of them were in advanced stages of disease.
Treatment
Surgery
Surgery, whether resection or liver transplantation, was the treatment option in a small group of patients 18 (12%). Most of them were done abroad but have follow up at our center. Recently a center for liver transplantation was established in Qatar as another treatment option for HCC patients in our country. Up until now two patients had successful cadaveric liver transplants. There is a liver transplant center established in Saudi Arabia with more extensive experience in liver transplantation, a close collaboration partner for Qatar.
Local therapy
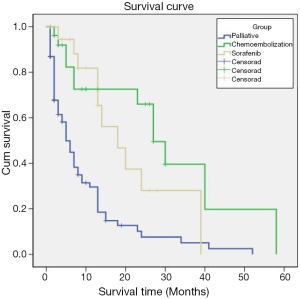
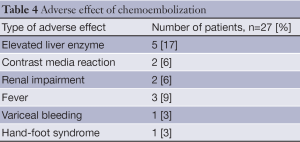
Ablation with radiofrequency or percutaneous ethanol injection was the treatment of choice in a smaller group of of 6 (5%) patients and chemoembolization was possible in 27 (17%) patients. The treatment was generally well tolerated with some side effects as summarized in Table 4. Median survival time for patients who received local therapy was 27 months (Figure 3).
Full table
Systemic therapy
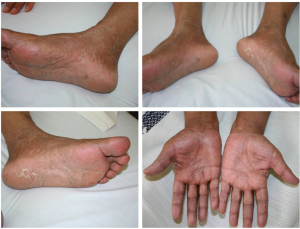
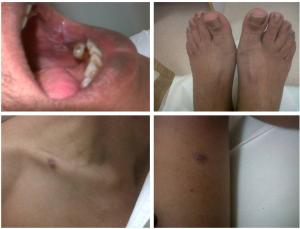
Systemic targeted therapy with sorafenib was offered in 19 (13%) patients and palliative care in 80 (53%). The most common toxicity observed with sorafenib therapy was fatigue. Others were skin rash (Figure 4) and hand foot syndrome, one patient developed grade 3 hand-foot syndrome reaction (Figure 5). The survival for group received sorafenib was 18 months (Figure 3). There was no statistically significant effect detected on survival time for factors like age, gender, or bilirubin level. Though not statistically significant, it was observed that patients who had AFP level higher than 150 ng/mL are likely to have less survival than those having AFP level less than 150 ng/mL/[hazard ratio, 1.34; 95% CI (0.86-2.11); P=0.2]. The only statistical significance was observed with Child Pugh staging and survival time. Patients having a Child Pugh score of C were likely to have significantly less survival than those patients with Child Pugh score as A [hazard ratio, 3.35; 95% CI (1.7-6.6); P<0.0001]. As expected, though having not statistically significant, patients with a Child Pugh score B were likely to have a survival disadvantage over patients with Child Pugh A [hazard ratio, 1.49; 95% CI (0.79-2.1); P=0.22]. It is clear that Child Pugh A has the best survival time as they have beast liver function.
Palliative therapy
The median survival for the patients who received palliative treatment was five months (Figure 3). Eighty patients (53%) received only palliative care, pain and other symptom control, at a newly established unit of palliative care at the national center for care and research in Qatar.
Discussion
The Gulf region is a unique geographical area, it includes six countries; Bahrain, Qatar, Saudi Arabia, Oman and United Arab Emirates (UAE). There are heterogeneous patterns for the prevalence of HCC in the Gulf region. The main predisposing factor is viral hepatitis, both HCV and HBV. It is more common in males (ratio M: F, 3:1). Diagnosis is usually in advanced stage, and the treatment outcome meets international standards when compared to other parts of the world (15,18-21).
From the data presented herein, it is deduced that a 3-fold increase in the age-adjusted rates for HCC, up to seven cases per 100,000 people, is possibly correlated to the increasing incidence of HCV infections in the last three decades. Moving forward, the reduction of the incidence of HCV infections, which has currently plateaued, would ultimately contribute to a reduced incidence of HCC due to HCV (22). This could be accomplished through preventive and educational strategies. However, a liver injury and repair model characterized by HCV damaged liver cells, developing dysplasia and ultimately HCC, is estimated to occur only over 10-30 years (23). As there are more people infected with HCV coming to Qatar we may expect a rising incidence.
All therapies are readily available in the Gulf, with few limitations though. Recently a center for liver transplantation was established in Qatar as another treatment option for HCC patients in our country. There is a liver transplant center established in Saudi Arabia with more extensive experience in liver transplantation, a close collaboration partner for Qatar. This is an excellent example for the critical need for collaboration in the region that will help better use available resources and optimize medical care.
So far, only few hospitals in the Gulf Region have established multidisciplinary hepatobiliary teams for the management of HCC patients. In Qatar a well established hepatobiliary multidisciplinary team has been up and running since 2011. Same applies for several centers in Saudi Arabia.
This study have it is limitation, it is a small sample size only 150 patients with HCC included, different modalities of treatment were applied, local, systemic and palliative measures. The survival outcomes were similar to what were reported internationally in the literature.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. [PubMed]
- Daw MA, Dau AA. Hepatitis C virus in Arab world: a state of concern. ScientificWorldJournal 2012;2012:719494.
- Alter MJ. Epidemiology of hepatitis C virus infection. World J Gastroenterol 2007;13:2436-41. [PubMed]
- Marrero JA, Fontana RJ, Barrat A, et al. Prognosis of hepatocellular carcinoma: comparison of 7 staging systems in an American cohort. Hepatology 2005;41:707-16. [PubMed]
- Stuart KE, Anand AJ, Jenkins RL. Hepatocellular carcinoma in the United States. Prognostic features, treatment outcome, and survival. Cancer 1996;77:2217-22. [PubMed]
- El-Serag HB, Siegel AB, Davila JA, et al. Treatment and outcomes of treating of hepatocellular carcinoma among Medicare recipients in the United States: a population-based study. J Hepatol 2006;44:158-66. [PubMed]
- Rasul KI, Saleh MM, Bener A. Epidemiology of cancer in Qatar, Asian Pacific Organization for Cancer Prevention (APOCP) Cancer Report 2010, 391.
- Munoz N, Bosch X. Epidemiology of hepatocellular carcinoma. In: In: Neoplasms of the Liver. Okuda K, Ishak KG. eds. Springer, Tokyo, 1989:3.
- Ribes J, Clèries R, Borràs J, et al. Time trends in incidence and mortality for chronic liver disease and liver cancer in the interval 1980-1997 in Catalonia, Spain. Eur J Gastroenterol Hepatol 2004;16:865-72. [PubMed]
- Bosch FX, Ribes J, Díaz M, et al. Primary liver Cancer: worldwide incidence and trends. Gastroenterology 2004;127:S5-S16. [PubMed]
- Khoja T. Council of Health ministries of GCC, the Gulf center for cancer registration, 10 years incidence of cancers among nationals of GCC states Sep. 2011:32-4.
- Rasul KI, Al-Azawi SH, Chandra P. Hepatocellular carcinoma in qatar. Gulf J Oncolog 2013;1:70-5. [PubMed]
- Fasani P, Sangiovanni A, De Fazio C, et al. High prevalence of multinodular hepatocellular carcinoma in patients with cirrhosis attributable to multiple risk factors. Hepatology 1999;29:1704-7. [PubMed]
- Lehman EM, Wilson ML. Epidemiology of hepatitis viruses among hepatocellular carcinoma cases and healthy People in Egypt: a systematic review and meta-analysis. Int J Cancer 2009;124:690-7. [PubMed]
- Marrero JA. Multidisciplinary management of hepatocellular carcinoma: where are we today? Semin Liver Dis 2013;33 Suppl 1:S3-10. [PubMed]
- Parkin DM, Bray F, Ferlay J, et al. Global Cancer statistics, 2002. CA Cancer J Clin 2005;55:74-108. [PubMed]
- Bruix J, Sherman M, Llovet JM, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol 2001;35:421-30. [PubMed]
- Cormier JN, Thomas KT, Chari RS, et al. Management of hepatocellular carcinoma. J Gastrointest Surg 2006;10:761-80. [PubMed]
- Bruix J, Sherman M, Practice Guidelines Committee, et al. Management of hepatocellular carcinoma. Hepatology 2005;42:1208-36. [PubMed]
- Marelli L, Stigliano R, Triantos C, et al. Treatment outcomes for hepatocellular carcinoma using chemoembolization in combination with other therapies. Cancer Treat Rev 2006;32:594-606. [PubMed]
- Forner A, Hessheimer AJ, Isabel Real M, et al. Treatment of hepatocellular carcinoma. Crit Rev Oncol Hematol 2006;60:89-98. [PubMed]
- Daniels D, Grytdal S, Wasley A, et al. Surveillance for acute viral hepatitis - United States, 2007. MMWR Surveill Summ 2009;58:1-27. [PubMed]
- Okuda K. Hepatocellular carcinoma. J Hepatol 2000;32:225-37. [PubMed]