Current status of hepatocellular carcinoma in Japan
Trends in liver cancer patients in Japan
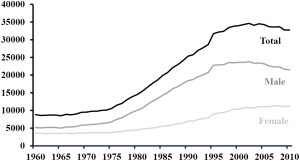
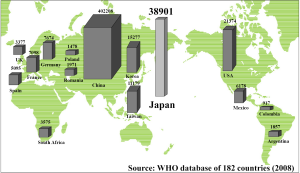
The number of deaths from primary liver cancer in Japan in the year 2011 stood at 31,875, and primary liver cancer ranked fourth among the causes of death from cancer, after lung cancer, stomach cancer and colorectal cancer (1). According to the 182-country database of the World Health Organization in 2008, the number of deaths from liver cancer in Japan ranked second in the world, after only China (2) (Figure 1). Examination of the male-female ratio of the deaths from liver cancer revealed that there were 20,972 males to 10,903 females, indicating approximately twice as many deaths among males than among females with liver cancer (1). It appears that after reaching a peak, the number of deaths has tended to slowly decline in recent years (Figure 2). The main reason for this decline is considered to be the decrease in the number of patients newly infected with hepatitis viruses because of implementation of screening of blood products for hepatitis B viral (HBV) and hepatitis C viral (HCV) infection (3,4). Another reason is the development of improved therapies for viral eradiation of HCV and HBV, such as peg-interferon plus ribavirin, or entecavir therapy (5,6). Against this backdrop of decrease in the number of deaths from primary liver cancer in Japan, it is predicted that the total number of deaths will have decreased to 24,600 in the year 2025.
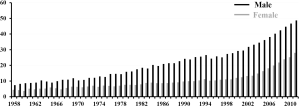
In 2011, the number of deaths from primary liver cancer of patients who were 80 years of age or older accounted for 35.1%, approximately one out of every 3, of all deaths from primary liver cancer. Stratification according to the gender showed that 28.0% of all male deaths and 48.7% of all female deaths involved patients who were 80 years of age or over. Thus, the female hepatocellular carcinoma (HCC) patients were older, and approximately half of the female patients were 80 years of age or older at the time of death. This tendency appears to be increasing year by year (1) (Figure 3).
HCC accounts for 94.0% of all primary liver cancers. In Japan, HCC is characterized by the development of the disease against a background of chronic hepatitis or liver cirrhosis caused by persistent HCV or HBV infection in a majority of the patients. According to the survey of the 20,753 patients in the Report of the 18th follow-up survey of primary liver cancer 2004-2005 of the Liver Cancer Study Group of Japan (7), 67.7% of the patients were HCV antibody-positive, and 15.0% were hepatitis B surface antigen-positive.
Screening for HCC in Japan
As described above, HCC often develops in patients with viral hepatitis, such as HBV or HCV. Therefore, periodic screening by ultrasonography or computed tomography with serum α-fetoprotein measurement has been recommended in patients with HBV or HCV who are at a high risk of development of HCC, for early detection of HCC. Owing to the implementation of periodic screening of patients at high risk in Japan, HCC tumors were 2 cm or less in diameter at diagnosis in 33.5% of cases and 2.1-5.0 cm in diameter in 45.5% of the cases. Moreover, in 57.7% of the cases, the tumors were solitary at diagnosis, and the HCCs were often diagnosed at a relatively early stage (7). According to the Japanese Evidence-based Clinical Practice Guidelines for Hepatocellular Carcinoma (8,9), tumor marker measurements and an ultrasound examination once every 3-4 months as a regular screening method, and if necessary, dynamic CT/MRI every 6-12 months, is recommended for patients with liver cirrhosis B or C. For patients with chronic hepatitis B, chronic hepatitis C, and liver cirrhosis caused without HBV or HCV, the Guidelines recommend tumor marker measurements and an ultrasound examination once every 6 months, and dynamic CT/MRI as needed. α-Fetoprotein (AFP), protein induced by vitamin K absence or antagonists-II (PIVKA-II), and the lens culinaris agglutinin-reactive fraction of alpha-fetoprotein (AFP-L3) are mainly used as the tumor markers. Ultrasound contrast agents are also often used to make a definitive diagnosis of HCC or for easy screening of liver tumors by ultrasonography. Gadolinium ethoxybenzyl diethylenetriamine pentaacetic acid (Gd-EOB-DTPA)-enhanced MRI, which allows evaluation of the blood flow in liver tumors and hepatocyte function, is used to make a more accurate diagnosis and for differential diagnosis. Thus, the advances in diagnostic imaging techniques enable the diagnosis of HCC to be confirmed in many patients even without a tumor biopsy. The Japanese Evidence-based Clinical Practice Guidelines for Hepatocellular Carcinoma (8,9) state that tumor biopsy is indicated only when it is impossible to make a definitive diagnosis by dynamic CT/MRI. Therefore, the indications of tumor biopsy should be decided cautiously, and the procedure should be avoided as far as possible.
Treatment policy for HCC in Japan
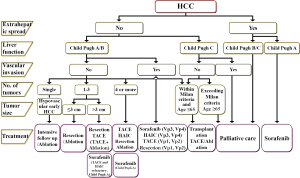
The main treatment modalities for HCC include surgical resection, liver transplantation, local ablative therapies, including radiofrequency ablation (RFA) and ethanol injection, and transcatheter arterial chemoembolization (TACE). According to the Report of the 18th follow-up survey of primary liver cancer in Japan 2004-2005 (7), the initial therapy was surgical resection in 31.7% of all treated patients, local ablative therapy in 30.6%, and TACE in 31.7%. For highly advanced HCC, such as that with vascular invasion, hepatic arterial infusion chemotherapy (HAIC) is often employed. After the introduction of sorafenib, systemic chemotherapy has also often been employed in recent years. Radiotherapy, including proton therapy and heavy-ion therapy, is sometimes employed as a treatment option. While determining the most appropriate treatment strategy for HCC, it is important to take the hepatic reserve into consideration, not just the condition of the HCC such as the number and size(s) of the tumors. The HCC treatment algorithm based on the consensus proposed by the Japan Society of Hepatology (JSH) in 2010 (10) is helpful for selecting the appropriate treatment for patients with HCC (Figure 4).
The cumulative overall survival times according to each treatment in the reports of the follow-up surveys of primary liver cancer in Japan from 1994 to 2005 are shown in Table 1 (7). The overall survival times were favorable in patients treated by surgical resection, local ablative therapy, and TACE, in that order. Also, the reported 5-year survival rates in Japan, South Korea and the United States in 2005 were 42.7%, 18.2%, and 13%, respectively, and the 5-year survival rate in Japan might be best in the world (11).
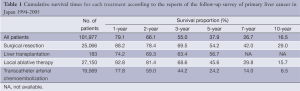
Full table
Loco-regional treatments: surgical resection, liver transplantation, local ablative therapy, and TACE
Surgical resection
Surgical resection is generally recommended for HCC patients with Child-Pugh A or B liver disease with a solitary tumor, or two or three tumors no greater than 3 cm in diameter each. However, even in patients with four or more tumors, surgical resection is sometimes performed if the tumors are considered resectable. According to the Report of the 18th follow-up survey of primary liver cancer in Japan 2004-2005 (7), the tumor diameter was under 2 cm in 17.7% of the patients, 2-5 cm in 54.9%, and 5-10 cm in 20.2%, and the tumor was solitary in 74.3% of the patients. In addition, there was portal vein invasion in 16.2% and hepatic vein invasion in 7.3% of patients.
Liver transplantation
Liver transplantation for HCC is an ideally best treatment modality, because it provides the potential for cure of HCC and underlying liver diseases. In Japan, living donor liver transplantation is predominantly applied for the treatment of HCC because of a crucial shortage of deceased donor. According to a large survey of 1,225 patients who underwent living donor liver transplantation conducted by 65 centers in Japan (12), and HCV infection was a leading cause of liver cirrhosis (60%). The survival proportions at 1, 3 and 5 years were 84.5%, 74.4% and 69.3%, respectively. Because the opportunities for liver transplantation are limited under the current circumstance of shortage of donors, the other treatment modalities were mainly considered as initial treatment in Japanese HCC patients.
Local ablative therapy
RFA is the predominant treatment among local ablative therapies. According to the Report of the 18th follow-up survey of primary liver cancer in Japan 2004-2005 (7), RFA had been performed in 72.1% of the patients, ethanol injection therapy in 18.6%, and microwave coagulation therapy in 8.5% of patients. The general indication for local ablative therapy was a tumor no greater than diameter of 3 cm and no more than 3 tumors. Patients with a single nodule accounted for 71.2%, those with a tumor diameter of 2 cm or less for 59.3%, and those with a tumor diameter of 2-3 cm for 28.5%. The therapeutic efficacy after 6 months was complete response (CR) in 80.3% and partial response (PR) in 9.9% of the patients.
TACE
Because the local control rate and long-term prognosis of patients treated by TACE are generally unfavorable as compared to those of patients treated by surgical resection or local ablative therapy, TACE is usually employed for patients who would be unsuitable candidates for surgical resection or local ablative therapy, for example, those with multiple nodules. According to the Report of the 18th follow-up survey of primary liver cancer in Japan 2004-2005 (7), anticancer drugs and lipiodol were used in combination with TACE in 93.2% and 99.8% of these patients, respectively. The therapeutic response after 6 months of treatment was CR in 40.5% and PR in 27.6% of the patients.
Chemotherapy: intra-arterial chemotherapy and systemic chemotherapy
Chemotherapy is employed to treat patients who are unsuitable candidates for surgical resection, local ablative therapy or TACE, that is, patients with extrahepatic metastasis, vascular invasion or resistance to TACE. Chemotherapy consists of systemic chemotherapy and HAIC. In Japan, HAIC is mainly employed for patients with localized advanced HCC, e.g., those with vascular invasion, while systemic chemotherapy is employed for HCC patients with extrahepatic metastasis. According to the Report of the 18th follow-up survey of primary liver cancer in Japan 2004-2005 (7), HAIC accounted for the higher proportion of the patients, i.e., 85.8%.
Systemic chemotherapy
The results of two pivotal randomized controlled trials have demonstrated the evident survival benefit over placebo of the orally administered molecular-targeted agent sorafenib [a multikinase inhibitor of RAF, vascular endothelial growth factor receptor (VEGFR), platelets deprived growth factor receptor (PDGFR), etc.], and this drug has come to be regarded as a standard treatment agent for advanced HCC (13,14). In May 2009, use of sorafenib for treatment of HCC was approved for coverage by the national health insurance in Japan, and over 20,000 HCC patients have already been treated with sorafenib.
Sorafenib has some troublesome adverse effects, such as the hand-foot syndrome, hypertension, liver dysfunction, etc. Higher incidences of these adverse events have been reported in HCC patients from Asia, including Japan, than in those from Western countries (13-19) (Table 2). The reason for this difference remains unknown, although it may be related to racial differences. Therefore, it is important in clinical practice to devise methods to properly manage such adverse events so as to avoid suspension/discontinuation of treatment due to serious adverse events and enable treatment continuation for long periods of time.
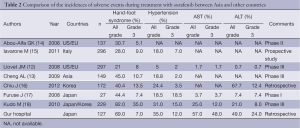
Full table
A global international prospective, non-interventional study (GIDEON trial) was performed to elucidate the safety and efficacy data of sorafenib in clinical practice worldwide (20) (Table 3). According to the interim analysis of the GIDEON trial, the background of Japanese patients treated with sorafenib was characterized by a larger number of patients who were elderly and had PS-0 as compared to the patients from other countries. The percentages of patients that had undergone surgical resection, RFA or TACE prior to the start of sorafenib treatment were also higher among patients from Japan than among patients from the other countries. Furthermore, another characteristic of the HCC patients from Japan was the longer interval between the diagnosis of HCC and commencement of sorafenib treatment (30 months), suggesting that HCC is diagnosed earlier in Japan, and that sorafenib therapy is initiated after first employing potentially effective loco-regional treatments. Regarding sorafenib therapy, the duration of administration (median) of 13 weeks in Japan was similar to that in the other countries. However, the incidence rate of serious adverse events and the proportion of patients requiring discontinuation of sorafenib due to the appearance of adverse events were higher in the HCC patients from Japan than in those from the other countries. Thus, HCC patients from Japan treated with sorafenib have often been treated heavily before the introduction of sorafenib and show a higher incidence of serious adverse events during sorafenib treatment, although their treatment outcome were almost equivalent to those in the patients from other countries.
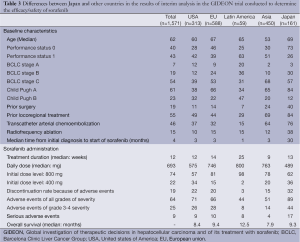
Full table
HAIC
Because the anticancer agents are directly injected into the hepatic arteries, HAIC is associated with increased local concentrations of the anticancer agents in the tumor and reduced systemic distribution of the drugs. Therefore, HAIC may be expected to have a stronger antitumor effect and lower incidence of systemic adverse reactions, as compared to systemic chemotherapy. Among the numerous chemotherapeutic regimens employed for HAIC, cisplatin (21-24), 5-fluorouracil (5-FU) plus cisplatin (25-28), and 5-FU plus interferon (29-33) are the most frequently used in Japan, and high response rates and favorable long-term outcomes have been reported (Table 4). Thus, HAIC is an effective treatment, however, no large-scale prospective randomized controlled trials have been conducted until date. Because no randomized controlled trials have demonstrated any survival advantage of HAIC, no consensus has been reached as to the standard treatment for advanced HCC. Sorafenib has been approved as a treatment for similar subjects with advanced HCC in Japan. Nonetheless, HAIC is still often performed in Japan, because a favorable tumor-shrinking effect and long-term survival of the patients are often observed in patients with highly advanced HCC in response to HAIC. To elucidate the usefulness of HAIC, several studies of sorafenib and HAIC, including randomized controlled trials of sorafenib plus intra-arterial cisplatin and sorafenib alone (UMIN000005703), of sorafenib plus intra-arterial 5-FU + cisplatin and sorafenib alone (NCT01214343), and of intra-arterial 5-FU + interferon therapy and sorafenib alone (UMIN00000240) are currently underway. In the future, demonstration of the survival advantage of HAIC and recognition of HAIC as one of the standard treatments for patients with advanced HCC are expected.
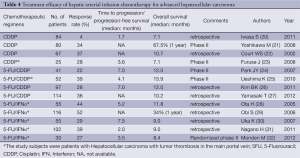
Full table
Agents against HCC under development in Japan
Various chemotherapeutic agents such as sunitinib, brivanib, linifanib, tivantinib, bevacizumab, cabozantinib, etc., are under development worldwide for the treatment of advanced HCC. In Japan, phase III trials of lenvatinib (34), orantinib (35), S-1 (36) and peretinoin (37), all originally developed in Japan and expected to be effective against HCC, are currently underway.
Lenvatinib
Lenvatinib (34) is a tyrosine kinase inhibitor of VEGFR2, RET, etc., and a phase II trial of the drug as first-line treatment and second-line treatment was conducted in 46 patients with advanced HCC. Favorable treatment outcomes were reported, with a response rate [assessed according to the Response Evaluation Criteria in Solid Tumors (RECIST) criteria] of 23.9%, median time to progression of 9.4 months, and median survival time of 18.3 months. A global phase III trial comparing lenvatinib and sorafenib in the first-line setting is currently ongoing (NCT01761266).
Orantinib
Orantinib is a tyrosine kinase inhibitor of PDGFR, VEGFR-2, etc (35). A randomized controlled phase II trial comparing orantinib with observation in the adjuvant setting was conducted in 101 patients who had undergone TACE. The median time to progression was 5.2 months in the orantinib arm as compared to 4.0 months in the observation arm, and a favorable trend with a hazard ratio of 0.699 [95% confidence interval (CI): 0.450-1.088, P=0.054] was reported in the orantinib group. A placebo-controlled phase III trial of orantinib is currently underway in Japan, South Korea and Taiwan, to elucidate its usefulness in combination with TACE (NCT01465464).
S-1
S-1 is an oral anticancer agent composed of a mixture of tegafur and two modulators, gimeracil and oteracil, that was developed with the aim of intensifying the antitumor effect of 5-FU by increasing the serum concentration of the drug and mitigating its gastrointestinal toxicity (36). A phase II trial of the drug was conducted in 23 patients with advanced HCC against a background of Child-Pugh A or B liver disease, and favorable treatment outcomes were reported, with a response rate of 21.7% (5/23), median time to progression of 3.7 months, and median survival time of 16.6 months. A placebo-controlled phase III trial of S-1 (JapicCTI-090920) is currently ongoing in Japan in patients with advanced HCC refractory to sorafenib.
Peretinoin
Peretinoin is an oral acyclic retinoid vitamin-A derivative targeted at the retinoid nuclear receptor. A placebo-controlled phase II/III trial of peretinoin 300 mg and peretinoin 600 mg was conducted on 401 HCC patients who had undergone surgical resection or RFA (37). The trial demonstrated a significant difference in the 2-year recurrence-free survival rate between the peretinoin 600 mg group, but not peretinoin 300 mg group, and the placebo group (hazard ratio 0.27, 95% CI: 0.07-0.96), and a new placebo-controlled phase III trial of peretinoin 600 mg is underway in Japan (NCT01640808).
Thus, phase III trials of several anticancer agents originally developed in Japan are underway at present, and positive results are expected in the near future.
Conclusions
Before the introduction of sorafenib, the three major treatments used for the treatment of HCC in Japan were surgical resection, local ablative therapy and TACE. HAIC was used for patients in whom these potentially effective treatments were not indicated. However, the situation has changed greatly after the advent of sorafenib. It is now necessary to clarify the role of HAIC for the treatment of advanced HCC, especially from the standpoint of the availability of sorafenib. In addition, it is also necessary to aggressively address the development of other effective chemotherapeutic agents after sorafenib. Japan is the country with the second largest number of HCC patients in the world, and it will be important to strive to further improve the treatment outcome of HCC in collaboration with Western and other Asian countries.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Matsuda A, Matsuda T, Shibata A, et al. Cancer incidence and incidence rates in Japan in 2007: a study of 21 population-based cancer registries for the Monitoring of Cancer Incidence in Japan (MCIJ) project. Jpn J Clin Oncol 2013;43:328-36. [PubMed]
- Ferlay J, Shin HR, Bray F, et al. GLOBOCAN 2008 v2.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 10. Lyon, France: International Agency for Research on Cancer; 2010. Available online: , accessed on day/month/year
- Kiyosawa K, Umemura T, Ichijo T, et al. Hepatocellular carcinoma: recent trends in Japan. Gastroenterology 2004;127:S17-26. [PubMed]
- Chung H, Ueda T, Kudo M. Changing trends in hepatitis C infection over the past 50 years in Japan. Intervirology 2010;53:39-43. [PubMed]
- Yoshida H, Shiratori Y, Moriyama M, et al. Interferon therapy reduces the risk for hepatocellular carcinoma: national surveillance program of cirrhotic and noncirrhotic patients with chronic hepatitis C in Japan. IHIT Study Group. Inhibition of hepatocarcinogenesis by interferon therapy. Ann Intern Med 1999;131:174-81. [PubMed]
- Liaw YF, Sung JJ, Chow WC, et al. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med 2004;351:1521-31. [PubMed]
- Ikai I, Kudo M, Arii S, et al. Report of the 18th follow-up survey of primary liver cancer in Japan. Hepatol Res 2010;40:1043-59.
- The Japan Society of Hepatology. Evidence-based clinical practice guidelines for hepatocellular carcinoma, revised version (in Japanese). Tokyo: Kanehara, 2009.
- Makuuchi M, Kokudo N, Arii S, et al. Development of evidence-based clinical guidelines for the diagnosis and treatment of hepatocellular carcinoma in Japan. Hepatol Res 2008;38:37-51. [PubMed]
- Kudo M, Izumi N, Kokudo N, et al. Management of hepatocellular carcinoma in Japan: consensus-based clinical practice guidelines proposed by the Japan Society of Hepatology (JSH) 2010 updated version. Dig Dis 2011;29:339-64. [PubMed]
- Kudo M, Izumi N, Kokudo N, et al. Improved survival in patients with HCC over 30 years in Japan: analysis of nationwide prospective registry of 148,161 patients. J Clin Oncol 2011;29:abstr 4054.
- Liver transplantation in Japan-registry by the Japanese Liver Transplantation Society-(in Japanese)-registry by the Japanese Liver Transplantation Society-. Ishoku 2011;46:524-36.
- Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378-90. [PubMed]
- Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 2009;10:25-34. [PubMed]
- Abou-Alfa GK, Schwartz L, Ricci S, et al. Phase II study of sorafenib in patients with advanced hepatocellular carcinoma. J Clin Oncol 2006;24:4293-300. [PubMed]
- Iavarone M, Cabibbo G, Piscaglia F, et al. Field-practice study of sorafenib therapy for hepatocellular carcinoma: a prospective multicenter study in Italy. Hepatology 2011;54:2055-63. [PubMed]
- Chiu J, Tang YF, Yao TJ, et al. The use of single-agent sorafenib in the treatment of advanced hepatocellular carcinoma patients with underlying Child-Pugh B liver cirrhosis: a retrospective analysis of efficacy, safety, and survival benefits. Cancer 2012;118:5293-301. [PubMed]
- Furuse J, Ishii H, Nakachi K, et al. Phase I study of sorafenib in Japanese patients with hepatocellular carcinoma. Cancer Sci 2008;99:159-65. [PubMed]
- Kudo M, Imanaka K, Chida N, et al. Phase III study of sorafenib after transarterial chemoembolisation in Japanese and Korean patients with unresectable hepatocellular carcinoma. Eur J Cancer 2011;47:2117-27. [PubMed]
- Kudo M, Ye SL, Venook A, et al. Second interim analysis of GIDEON (Global Investigation of therapeutic DEcisions in unresectable HCC and Of its treatment with sorafeNib): multiregional variation in patient characteristics, previous treatment history, and sorafenib use. The 62nd Annual Meeting of AASLD, 2011:abstr 2183.
- Iwasa S, Ikeda M, Okusaka T, et al. Transcatheter arterial infusion chemotherapy with a fine-powder formulation of cisplatin for advanced hepatocellular carcinoma refractory to transcatheter arterial chemoembolization. Jpn J Clin Oncol 2011;41:770-5. [PubMed]
- Yoshikawa M, Ono N, Yodono H, et al. Phase II study of hepatic arterial infusion of a fine-powder formulation of cisplatin for advanced hepatocellular carcinoma. Hepatol Res 2008;38:474-83. [PubMed]
- Court WS, Order SE, Siegel JA, et al. Remission and survival following monthly intraarterial cisplatinum in nonresectable hepatoma. Cancer Invest 2002;20:613-25. [PubMed]
- Furuse J, Ikeda M, Okusaka T, et al. A phase II trial of hepatic arterial infusion chemotherapy with cisplatin for advanced HCC with portal vein tumor thrombosis. J Clin Oncol 2008;26:abstr 15556.
- Park JY, Ahn SH, Yoon YJ, et al. Repetitive short-course hepatic arterial infusion chemotherapy with high-dose 5-fluorouracil and cisplatin in patients with advanced hepatocellular carcinoma. Cancer 2007;110:129-37. [PubMed]
- Ueshima K, Kudo M, Takita M, et al. Hepatic arterial infusion chemotherapy using low-dose 5-fluorouracil and cisplatin for advanced hepatocellular carcinoma. Oncology 2010;78 Suppl 1:148-53. [PubMed]
- Kim BK, Park JY, Choi HJ, et al. Long-term clinical outcomes of hepatic arterial infusion chemotherapy with cisplatin with or without 5-fluorouracil in locally advanced hepatocellular carcinoma. J Cancer Res Clin Oncol 2011;137:659-67. [PubMed]
- Yamasaki T, Sakaida I. Hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma and future treatments for the poor responders. Hepatol Res 2012;42:340-8. [PubMed]
- Ota H, Nagano H, Sakon M, et al. Treatment of hepatocellular carcinoma with major portal vein thrombosis by combined therapy with subcutaneous interferon-alpha and intra-arterial 5-fluorouracil; role of type 1 interferon receptor expression. Br J Cancer 2005;93:557-64. [PubMed]
- Obi S, Yoshida H, Toune R, et al. Combination therapy of intraarterial 5-fluorouracil and systemic interferon-alpha for advanced hepatocellular carcinoma with portal venous invasion. Cancer 2006;106:1990-7. [PubMed]
- Uka K, Aikata H, Takaki S, et al. Pretreatment predictor of response, time to progression, and survival to intraarterial 5-fluorouracil/interferon combination therapy in patients with advanced hepatocellular carcinoma. J Gastroenterol 2007;42:845-53. [PubMed]
- Nagano H, Wada H, Kobayashi S, et al. Long-term outcome of combined interferon-α and 5-fluorouracil treatment for advanced hepatocellular carcinoma with major portal vein thrombosis. Oncology 2011;80:63-9. [PubMed]
- Monden M, Sakon M, Sakata Y, et al. 5-fluorouracil arterial infusion + interferon therapy for highly advanced hepatocellular carcinoma: a multicenter, randomized, phase II study. Hepatol Res 2012;42:150-65. [PubMed]
- Okita K, Kumada K, Ikeda K, et al. Phase I/II study of E7080 (lenvatinib), a multitargeted tyrosine kinase inhibitor, in patients (pts) with advanced HCC(HCC): Initial assessment of response rate. J Clin Oncol 2012;30:abstr 320.
- Arai Y, Inaba Y, Yamamoto T, et al. A randomized phase II study of TSU-68 in patients (pts) with HCC (HCC) treated by transarterial chemoembolization (TACE). J Clin Oncol 2010;28:abstr 4030.
- Furuse J, Okusaka T, Kaneko S, et al. Phase I/II study of the pharmacokinetics, safety and efficacy of S-1 in patients with advanced hepatocellular carcinoma. Cancer Sci 2010;101:2606-11. [PubMed]
- Okita K, Matsui O, Kumada H, et al. Effect of peretinoin on recurrence of HCC (HCC): Results of a phase II/III randomized placebo-controlled trial. J Clin Oncol 2010;28:abstr 4024.