Management of liver cirrhosis in patients with hepatocellular carcinoma
Introduction
Cirrhosis is a common problem worldwide, accounting for significant mortality and hospital admission rate. Estimated prevalence of cirrhosis in the United States is 0.15% of the population and it is estimated that up to 1% have histological cirrhosis that is not yet clinically detected (1). Similar numbers have been reported from European countries and even higher numbers are estimated in most Asian and African countries. Main underlying etiology varies geographically; Alcohol consumption and chronic hepatitis C are the leading causes of cirrhosis in western countries. Chronic hepatitis B is highly endemic in the Asian Pacific region and appears to be the commonest cause of liver cirrhosis, with few exceptions. For example hepatitis C is common in Japan accounting for the most common cause of cirrhosis and liver cancer, while alcohol related cirrhosis is more common in China and Korea compared to other Asian countries (2).
Hepatocellular carcinoma (HCC) represents the main contributor to liver-related mortality (3) and tumor progression is the main cause of death in HCC patients (4), however a significant percentage of them die from complications relates to cirrhosis. Therefore, managing cirrhosis to delay the advent of complications as well as appropriately treating these complications early in its course both during and after treatment of the HCC is paramount to improve morbidity and mortality.
Liver cirrhosis histologically represents an advanced stage of hepatic fibrosis associated with hepatic nodules that progressively disrupts the normal hepatic architecture and transforms the liver from a low-resistance to a high-resistance organ, this process elevates the sinusoidal pressure causing impaired hepatocyte function and increases the pressure in the portal vein leading to portal hypertension (5). Portal hypertension is defined as being 6 mmHg or greater as measured by the wedged hepatic vein gradient. As the portal pressure increases so does the risk for developing complications related to cirrhosis (5). This review summarizes the current management strategies that have been shown to be effective at decreasing the morbidity and mortality associated with the development of complications from cirrhosis.
HCC in relation to cirrhosis
Most of the patients with HCC have underlying cirrhosis. Worldwide data show that prevalence of cirrhosis in persons with HCC is about 80-90% (6). Cirrhosis of all etiologies may be complicated by HCC, but persistent hepatitis B virus (HBV) or hepatitis C virus (HCV) infection account for over 80% of HCC cases worldwide (7). In Japan, the United States, Latin America, Egypt and Europe, hepatitis C is the major cause of HCC. The incidence of HCC is 2-8% per year in patients with chronic hepatitis C and established cirrhosis. While in Asia, Africa, and in some eastern European countries, chronic hepatitis B is the prime cause of HCC, far outweighing the impact of chronic hepatitis C (8).
During the evaluation of patients with HCC without a clinical diagnosis of cirrhosis, a detailed examination is important to identify symptoms and signs indicating presence of cirrhosis such as abdominal enlargement and/or swelling, insomnia or sleep pattern reversal, vascular spiders, visible collaterals and palpable liver or spleen. Laboratory findings suggesting cirrhosis include abnormalities in one or more of synthetic function (serum albumin, prothrombin time, and serum bilirubin) and/or a low platelet count (platelet count <160,000×109/L is 80% sensitive in detecting portal hypertension from cirrhosis) (9). For patients having clinical features with suggestive laboratory and imaging findings, a biopsy is not necessary to confirm presence of cirrhosis.
The development of decompensated cirrhosis is signaled by the presence of the following: jaundice, ascites, portal hypertensive gastrointestinal (GI) bleeding, and/or encephalopathy. The rate of decompensation is estimated to be 3-5% per year (10). One-year mortality in compensated cirrhosis is 1-3.4%, but with decompensation the mortality increases to 20-57% (11). The severity of hepatic decompensation clearly affects outcome and thus, the treatment choice for HCC as well as the response to treatment. Assessment of the severity of cirrhosis is usually done using the Child-Pugh classification as it reflects functional hepatic reserve. An example is surgical resection of HCC which can be safely done only in patient with well-preserved liver function (Child-Pugh A), while liver transplantation is the best option for patients with decompensation (Child-Pugh B and C cirrhosis) (12). Similarly, patients with compromised hepatic reserve such as Child-Pugh B are well known to have poorer outcome and more adverse events with targeted therapy such as sorafenib when compared to Child-Pugh A patients (13,14).
General management of cirrhosis includes
Identification and treatment of underlying etiology can slow progression or partially reverse cirrhosis both histologically and clinically. This is well seen in alcoholic liver disease, where abstinence was associated with improvement in fibrosis (15,16), normalization of portal pressure (17), and resolution (or reduction) of ascites (18). Similar results are seen in patients with compensated or even decompensated cirrhosis due to autoimmune hepatitis treated with steroids (19), HBV treated with antiviral therapy (20) and in compensated cirrhosis due to HCV treated with combination therapy (21). However, the role of antiviral therapy in patients with HCC is not clear as most official guidelines consider active HCC as a contraindication to treatment. Prevention of second insults should be achieved by avoiding hepatotoxic medications, herbal preparations and for those patients with cirrhosis that are sero-negative immunization against hepatitis A and B.
Although presently not standard practice, a number of recent studies show a beneficial effect of commonly used drugs on progression of cirrhosis and its complications. These drugs include non-selective beta blockers, statins, antibiotics and anticoagulation (22). A detailed discussion of these studies is beyond the scope of this article but these early reports suggest a beneficial effect on fibrosis progression, development of varices and bacterial translocation. However, before these concepts are applied to daily practice further studies are needed. In addition, several animal studies inhibiting the TGF-b1, renin angiotensin pathway and vascular endothelial growth factor pathways show promise in halting fibrosis progression (23).
Common complications of cirrhosis
Ascites
Cirrhosis is the most common cause of ascites (over 75%) (24) and the most common complication of cirrhosis that leads to hospital admissions (25). New onset ascites in patients with cirrhosis should be evaluated with imaging such as ultrasound with dopplers or a dynamic CT scan to document liver disease and rule out other causes of ascites. The ascitic fluid should be analyzed to document a high serum-ascites albumin gradient (SAAG) and low protein fluid. Additional testing should include a cell count with differential, cultures to rule out infection and cytology (26), which is usually negative in cirrhotic ascites even in the presence of HCC (27).
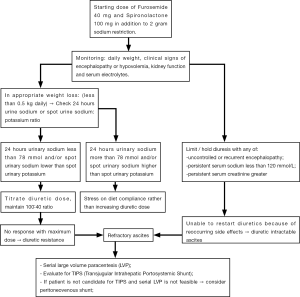
Figure 1 summarizes the treatment guidelines for ascites. First line treatments for ascites are to restrict dietary salt intake to 2 grams per day and initiate diuretic therapy. A combination of spironolactone and furosemide is recommended over starting spironolactone alone as combination therapy leads to earlier mobilization of ascites and helps maintain normokalemia (28). Usually a daily dose of 40 mg of furosemide and 100 mg of spironolactone 100 mg is started, then the dose is titrated to response every 3-5 days to a maximum of 160 mg of furosemide and 400 mg of spironolactone. A ratio of 40:100 is maintained, unless there is an abnormal potassium level (26).
A number of side effects can occur with diuretic therapy. Gynecomastia can occur with spironolactone. In cases of severe gynecomastia, other potassium sparing diuretics that can be tried are amiloride or triamterene (29). Worsening of kidney function and electrolyte disturbances is common with diuretics. As a general rule, oral diuretic therapy is preferred over intravenous loop diuretics because of good oral bioavailability and the higher potential to worsen kidney function with intravenous diuretics (30). Diuretics are often temporarily held for: uncontrolled or recurrent encephalopathy, serum sodium less than 120 mmol/L with no response to fluid restriction, or serum creatinine greater than 2.0 mg/dL (26). Daily weight monitoring is an excellent way to objectively monitor response and avoid over diuresis. While aggressive weight loss can be safely achieved in patients with significant edema, in patients with only ascites, a loss of 0.5 kg daily is preferred as more rapid mobilization of fluid increases risk of intravascular volume depletion.
Bed rest, fluid restriction in the absence of severe hyponatremia and frequent albumin infusions are not indicated in treatment of ascites due to liver cirrhosis (26). In the patient presenting with tense ascites, a single large volume paracentesis can rapidly improve symptoms and this can be followed up by titrating diuretics dose and counseling on diet modification (31). For patients who lack a response to diuretic therapy, diet adherence should discussed since it is an extremely common problem and can be evaluated with a 24 hours urinary sodium (>78 mmol) or spot urinary sodium higher than spot urinary potassium (32). When salt indiscretion is found, stress the importance of a sodium restriction diet prior to increasing the diuretic dose. Additional common causes of lack of response to diuretics are insufficient doses, NSAID use, severe hyponatremia, underlying kidney disease, local cause of ascites (such as malignancy or chronic infection specially tuberculosis or fungal infection) and true diuretic resistant ascites which is seen in advanced stages of liver disease (33).
The inability to initiate or continue diuretics because of persistent development of diuretic-induced complications is called diuretic intractable ascites. Both diuretic resistant and diuretic intractable ascites represent refractory ascites, which can be seen in 10-15% of patients (34). In refractory ascites, diuretics are discontinued and ascites managed with serial large volume paracentesis or transjugular intrahepatic portosystemic shunt (TIPS). Both strategies can be effective for ascites control (35). Surgical peritoneo-venous shunt is rarely used and limited to patient with abdominal scars (33).
Hepatic hydrothorax is seen in 5% of patients with cirrhosis and ascites (36). Hepatic hydrothorax is usually right sided. Therefore, left-sided pleural effusions in this clinical setting, warrants work up for other causes such as malignancy or infection as tuberculosis. Similar to ascites, first line treatment is diuretic therapy and sodium restriction with thoracentesis done in cases of respiratory distress. TIPS is a reasonable second line option for symptomatic plural effusion not responding to diuretics (26) but chest tube placement is contra-indicated because of the associated increased morbidity and mortality (37).
Spontaneous bacterial peritonitis (SBP)
SBP is a common and potentially fatal complication that develops in patients with cirrhosis and ascites. The overall prevalence of SBP in patients with cirrhosis and ascites admitted to the hospital is estimated to be 10-30% with the recent diagnostic techniques (38). However, the true prevalence of SBP in the setting of cirrhosis with ascites and HCC is poorly defined. A study on Korean patients with cirrhosis presenting with SBP showed that 41.5% had HCC at time of diagnosis and the presence of HCC was an independent predictive factor of higher mortality (39). Patients with SBP typically present with fever, abdominal pain and leukocytosis. However, SBP should also be suspected in unexplained encephalopathy, acute kidney injury (AKI), ileus, and a clinical picture suggestive of sepsis (hypotension, hypothermia, acidosis) (40). A definite diagnosis requires a paracentesis with ascetic fluid showing a PMN count ≥250 cells/mm3 and, although not required for diagnosis, positive ascitic fluid cultures. Bed side blood culture bottles inoculated with ascitic fluid should be obtained before antibiotics are started (41). Classic findings of SBP are ascitic fluid PMN counts ≥250 cells/mm3 and a positive ascitic fluid culture with monomicrobial infection [usually with gram negative gut (as Escherichia coli or Klebsiella) or less likely gram positive cocci (streptococcus or staphylococcus)] (42).
In patients who lack a response to appropriate antibiotics within 48 hours (as documented by rising PMNL on repeat paracentesis or less than 25% drop of the pre-treatment value), culture growing polymicrobial or “atypical” microbe that is usually associated with bowel perforation (anaerobes, enterococci or fungal) suspect secondary peritonitis (43,44). High morbidity and mortality are expected if secondary peritonitis is missed, as it requires broader antibiotic coverage compared to SBP and even urgent surgical intervention in most cases (43). Helpful tests are ascitic fluid for LDH, protein, and glucose, at least two out of three of the following are highly suspicious for secondary peritonitis (total protein greater than 1 g/dL, lactate dehydrogenase greater than the upper limit of normal for serum, and glucose less than 50 mg/dL). However, these criteria have only 50% sensitivity in secondary peritonitis without perforation (41). Therefore, a CT scan may still be needed in patients with a suspicious history or localized abdominal examination signs.
Treatment should be started based on clinical manifestations and ascitic fluid PMN count ≥250. Antibiotics are started once cultures are obtained and should not be delayed based on the availability of culture results. Importantly, a subset of patients only have a neutrocytic response with negative cultures (culture-negative neutrocytic ascites), and these patients should be treated as SBP because of the similar prognosis and mortality (45). Another group of patients may have an initial neutocytic response with a PMN <250 but cultures come back positive with one organism (mono microbial bacterascites), and the decision to treat is based on the presence of symptoms as well as a repeat ascitic fluid cell count to show if they have cleared the “colonization” or developed SBP (46).
Duration of treatment is generally 5 days in patients with a typical response (47). The preferred antibiotic therapy is an intravenous third generation cephalosporin like cefotaxime 2 grams every 8 hours or intravenous ceftriaxone 2 grams a day (48). Cultures are used to tailor the antibiotic therapy according to sensitivity and a negative culture is never an indication to stop treatment. Patients at risk of multiresistant infections should be monitored closely for their clinical response and culture results (patients with nosocomial infections, previous multiresistant infection or recent/chronic antibiotic use) (49). Quinolone resistance is a growing problem so they are no longer recommended for empiric coverage, even though some studies showed similar efficacy in well selected patients (50-52). Repeat paracentesis is not routinely indicated and is usually reserved for those patients who do not show improvement within 48 hours of therapy, especially if they are at risk for secondary peritonitis or multidrug resistant infection based on history or culture results (40). In addition to antibiotics, albumin infusion has been shown to decrease the incidence of type-1 HRS and mortality compared to antibiotics alone. The recommended dose is 1.5 g/kg body weight within 6 hours of diagnosis, followed by 1 g/kg on day 3 (53). Albumin infusions is of particular importance in patients with baseline kidney impairment (creatinine >1 mg/dL or blood urea nitrogen >30 mg) or severe liver disease (total bilirubin >4 mg/dL) (54).
Prophylaxis is another important treatment. Patients who survive an episode of SBP are at high risk for recurrence (approximately 70% cumulative risk at 1 year) (55) and lifelong antibiotic prophylaxis or until a liver transplant is indicated with oral norfloxacin 400 mg per day. If not available, other options are ciprofloxacin (750 mg once weekly, orally) or co-trimoxazole (800 mg sulfamethoxazole and 160 mg trimethoprim daily, orally) (40). Other populations in whom prophylaxis has shown to improve survival are patients with acute GI bleeding, antibiotic prophylaxis has shown to decrease incidence of SBP and also to reduce re-bleeding. In these setting, the recommended regimen is intravenous ceftriaxone for 7 days. In patients with less severe liver cirrhosis (i.e., without at least 2 of the following: ascites, severe malnutrition, encephalopathy, or bilirubin >3 mg/dL), prophylaxis can be switched to oral quinolones when oral intake is resumed (56). Prophylaxis should also be considered in patients with low ascitic fluid protein (<15 g/L). Controversial evidence exist, however, the highest evidence for benefit was shown in patients with ascitic fluid protein <15 g/L and severe liver disease (Child-Pugh score >9 points with serum bilirubin level >3 mg/dL or impaired renal function (serum creatinine level >1.2 mg/dL, blood urea nitrogen level >25 mg/dL, or serum sodium level <130 mEq/L) (57). The regimen studied was norfloxacin (400 mg/day) and it showed improved survival and decreased incidence of SBP. In addition, unnecessary long-term use of PPI is prudent since they were shown to increase the risk of SBP (58).
Patients with hepatic hydrothorax are also at risk for spontaneous bacterial pleural empyema. This complication is less common than SBP and it is associated with SBP in more than 50% of cases (59). It should be suspected in patients with a hydrothorax who develop fever, pleuritic pain, unexplained encephalopathy or other general signs of SBP. Once suspected, diagnostic thoracocentesis should be performed and a diagnosis is made with positive culture and more than 250 neutrophils/mm3 or a negative culture and more than 500 neutrophils/mm3, in the absence of lung infection. The treatment is similar to that of SBP and subsequent lifelong prophylaxis is also indicated (60).
Hepatic encephalopathy (HE)
HE is one of the common complications of chronic liver disease and it can occur with cirrhosis (Type C) and in patients with no hepatocellular dysfunction but presence of porto-systemic shunts (Type B) (61). In addition, it has been reported that HCC can rarely predispose to encephalopathy in patients without cirrhosis due to generation of ammonia from tumor breakdown and portosystemic shunting, a result of partial tumor occlusion of the hepatic veins (62).
The presentation of HE varies depending on the severity and the staging. Symptoms of HE are depression, irritability, insomnia, disturbances in the diurnal sleep pattern, lethargy, disorientation, inappropriate behavior and even coma. An underlying cause for HE should be sought, such as infection, SBP, worsening renal failure, GI bleed, electrolyte abnormalities (hypokalemia, alkalosis, hyponatremia), constipation, non-compliance and medication effect such as sedatives or narcotics. Worsening or increased frequency of encephalopathy without a clear precipitating factor indicates decompensation and can be seen in stable cirrhotic patient following progression of HCC or following treatments with liver directed therapies like TACE.
Diagnosis of HE is based on clinical grounds with a work-up that is used to prove the presence of chronic liver disease, and rule out other precipitating factors discussed above. A common finding on clinical exam is asterixis (bilateral flapping tremors). An elevated arterial ammonia level is not essential for the diagnosis (63). The role of psychometric tests is mainly for detecting mild degrees of encephalopathy (minimal HE), which may not be obvious on routine exam because of the normal mental status. However, minimal HE is important to recognize since it has an effect on long-term memory and complex intellectual tasks such as driving (64).
Treatment relies mainly on correction of underlying causes if any are found and reduction of ammonia levels. In general, sedatives should be avoided and electrolyte abnormalities corrected. Correction of hypokalemia is of particular importance since it increases renal ammonia production and the associated metabolic alkalosis may increase ammonia entry into the brain (65). For symptomatic treatment of agitation, haloperidol is a safer option compared to benzodiazepines (66).
Non-absorbable disaccharides such as lactulose are the first line treatments by acting as a laxative and also acidify the colon to limit absorption of ammonia. One approach in the acute setting is an hourly oral dose of 45 mL until patient has a bowel movement then the dose can be adjusted to have 2-3 soft stools daily. Lactulose can also be used as retention enema (300 mL in 1 L of water) and has been shown to be more effective than tap water enema (67). Another oral agent for the treatment of HE is rifaxmin that was approved by regulatory agencies for recurrent encephalopathy (68) and minimal HE. Other oral antibiotics such as metronidazole, neomycin and vancomycin have shown some effect (69). The evidence for flumazenil, acarbose and Zinc supplement is still developing. Rarely, surgical reduction or obliteration of shunts or large spontaneous porto-systemic anastomoses can be helpful (70).
Current evidence shows no added benefit for strict protein restriction compared to moderate protein intake (71). Vegetable protein may have slight benefit over animal protein in regard to nitrogen balance (72), and this can be an option in patients who are not improving with medical therapy or for patients who are noticed to have worse symptoms with protein intake.
AKI in patients with liver cirrhosis
Patients with advanced liver cirrhosis are more susceptible to AKI compared to the normal population due to the reduced effective circulating blood volume and mean arterial pressure secondary to splanchnic vasodilation leading to kidney hypoperfusion (73). Common etiologies in cirrhotic patients are hypovolemia (usually due to overdiuresis or acute GI bleeding), hepatitis virus-associated glomerulonephritis (74) and hepatorenal syndrome that is a diagnosis of exclusion observed in about 13-25% of patients (75,76).
The work-up is guided by the clinical setting, and generally includes ultrasound of the kidneys, urine electrolytes, urine analysis to assess for the presence of hematuria and proteinuria, and appropriate serological testing for antibodies against the glomerular basement membrane and for vasculitis. Occult sepsis should also be evaluated with ascitic fluid analysis for SBP. It should be noted that patients with chronic liver disease have a significantly lower baseline serum creatinine concentration than the general population and a slower rise in serum creatinine with a drop in GFR due to decreased production of creatinine (from wasted muscles and from the liver) and due to increased volume of distribution given the edema and ascites (77). Newer methods to assess renal function in cirrosis are being evaluated and includes urinary neutrophil gelatinase-associated lipocalin that was shown not only to accurately assess the degree of renal dysfunction, but also to identify the etiology (78,79).
Criteria for HRS diagnosis are based on presence of cirrhosis with ascites and a rise in serum creatinine to >1.5 mg/dL, in the absence of other causes of AKI such as absence of shock, hypovolemia, nephrotoxic drugs, abnormal renal US, proteinuria <0.5 g/day and microhaematuria (<50 red cells/high powered field) (40). Hypovolemia can be excluded by stopping diuretics for at least 2 days, volume expansion with albumin 1 g/kg/day up to a maximum of 100 g/day and documenting a CVP >3 cm water (80). Precipitating events of HRS should be identified and treated. SBP should be treated immediately with antibiotics and albumin infusion. Pentoxifylline has preventive effect in severe alcoholic hepatitis and may be in other patients with liver cirrhosis and baseline kidney dysfunction (81).
Definitive treatment for HRS is a liver transplant. Median survival of patients with untreated type 1 HRS is approximately 1 month, with survival rate substantially improving to approximately 65% after transplant (82). Pharmacologic agents including albumin infusion and vasoconstrictors are a bridge to transplant. A commonly used vasoconstrictor regimen is oral midodrine titrated to 12.5 mg 3 times per day and octreotide 100 µg/8 h subcutaneously titrated to 200 µg/8 h, to achieve an increase in mean blood pressure of 15 mmHg (83). Other options are noradrenaline continuous infusion that can be used in extremely hypotensive patients in ICU setting (84) and terlipressin which is not widely available (85). Few studies showed a beneficial effect for TIPS on kidney function in HRS type 1 and type 2, however this has not been compared to other treatment options (86) in randomized controlled trials.
Additionally, supportive management is directed towards managing electrolytes, acid base and volume status. Hemodialysis or continuous venovenous hemodialysis can be used before liver transplantation. Other alternatives to hemodialysis have been studied and showed improved survival, including molecular reabsorbent recirculating system (MARS) and another Prometheus extracorporeal liver support system, however, further studies are required (22). Kidney function is expected to recover after transplant and combined liver-kidney transplantation is generally not indicated except in patients who have been on prolonged dialysis form more than 12 weeks before transplant (44).
HRS type 2 is usually seen in the setting of refractory ascites and diuretic resistance typically evolving over months. No treatment consensus exists in this setting; however, terlipressin plus albumin are probably the most studied treatment options (87) as a bridge to liver transplant.
GI bleeding
Variceal bleeding is a common complication of cirrhosis and is associated with high mortality. Gastroesophageal varcies are present in up to 50% of patients at the time of diagnosis of cirrhosis (88) and without treatment, small varices progress to large varices at rates of 5% to 20% per year (89), which increases the risk of bleeding. Other factors to predict risk of bleeding are advanced liver disease and presence of red wale marks (90). Clearly, acute variceal bleeding is associated with high mortality; estimated 6week mortality following single bleeding is about 15% to 20% and reaches 30% in patients with Child class C (91).
Current guidelines recommend a screening endoscopy once a patient is diagnosed with cirrhosis even without decompensation or previous GI bleeding. Less invasive measures are being studied with limited accuracy such as transient elastography, platelet count/spleen diameter ratio, CT scanning for varices, and video capsule endoscopy (92,93). There is growing evidence on the role of measuring hepatic venous pressure gradient (HVPG) with a gradient more than 10 mmHg indicating clinically significant portal hypertension predicting the development of varices, decompensation and even HCC (94). In the setting of acute variceal bleed, HVPG more than 20 predicts poor outcome (95). Variceal pressure measurement was shown to be as effective as HVPG in prediction of bleeding risk and response to beta blockers with the advantage of being less invasive (96). The role of both studies among diagnosis and treatment algorithms is not yet clear.
Screening protocols include an esophagogastroduodenoscopy (EGD) once a diagnosis of cirrhosis is made. If there are no varices, no medical or endoscopic prophylaxis is recommended, and a repeat endoscopy is performed in 3 years in patients with compensated cirrhosis and earlier if hepatic decompensation occurs and then annually (97). If the screening EGD showed small varices (<5 mm), then medical prophylaxis with nonselective beta-blockers is indicated, with stronger evidence if patient has criteria for high-risk varices (Child B/C or presence of red wale marks). In patients with small varices who receive beta-blockers, a follow-up EGD is not necessary (97). In cases of large varices with the high-risk criteria, nonselective beta-blockers or endoscopic variceal ligation should be used for prophylaxis. If no high risk criteria are present, then first line should be nonselective beta-blockers and variceal ligation should be used if there is a contraindication to beta blockers (98). Generally, for patients treated with variceal ligation, it should be repeated every 1-2 weeks until varices are completely obliterated, followed by repeat endoscopy in 1-3 months then every 6-12 months to evaluate for recurrence (97).
Starting doses of NSB are propranolol 20 mg twice daily or nadolol 40 mg once daily then titrated to maximally tolerated dose or until heart rate is approximately 55 beats/min. Although not a standard therapy, low dose carvedilol was associated with lower bleeding rates and better compliance compared to variceal ligation in one study (99). Additional benefit for NSBBs and improved survival was shown (100), mainly through reducing bacterial translocation and infections like SBP and slowing progression of collaterals through anti angiogenic effect probably by affecting gene expression of endothelial growth factors (101). However, in patients with refractory ascites, recent study showed worsened survival with NSBB (102), likely due to alteration of hemodynamics (paracentesis-induced circulatory dysfunction like picture) and reduction of renal perfusion (103). Further studies are needed to clarify this, meanwhile, it is probably safer to hold NSBB in this population and use band ligation. Heavy lifting (>40 pounds) should be avoided in patients with large varices as it can predispose to bleeding through increasing intra thoracic pressure (9).
Acute variceal bleeding is an emergency and patients should be admitted with close monitoring in intensive care unit or at least intermediate care level. Intravascular volume support and blood transfusions should be started with a goal to maintain hemoglobin around 8 g/dL, as more aggressive transfusion can lead to elevation in portal pressure that can worsen variceal bleeding (104). However, other factors such as cardiopulmonary comorbidities, patient age, hemodynamic status and ongoing bleeding should also be considered. Significant coagulopathy should be corrected using fresh frozen plasma and/or platelets, although no clear guidelines are available. As mentioned before antibiotic prophylaxis is indicated even in the absence of ascites as it improves survival not only by preventing infections but also decreases risk of early re-bleeding (97,104). Pharmacologic therapy with vasoconstrictors including terlipressin, somatostatin or somatostatin analogs (octreotide) should be started once bleeding is suspected even before EGD. Once diagnosis is confirmed, it should be continued for 3-5 days. The EGD should be performed within 12 hours, after the patient is resuscitated with the aim being to confirm the diagnosis and control the bleeding using variceal ligation or sclerotherapy. Current evidence shows better control of the bleeding and less risk of re-bleeding when pharmacologic and endoscopic therapies are combined (105).
Treatment failure even with the measures mentioned above is seen in 10-20% of patients (97). Available options for these patients are temporary balloon tamponade (for <24 hours) or shunt therapy, through TIPS (or less commonly surgical shunt) in well-selected patients. Lower threshold for early TIPS (within 24-48 hours) may improve outcome in high-risk patients (those with Child class C or HVPG >20 mmHg) (106,107).
Up to 20% patients have gastric varices on endoscopy and a higher mortality is related to the fundal type of varices. Unfortunately, band ligation is usually not effective in these cases (108,109) and current evidence shows better results with endoscopic variceal obturation using cyanoacrylate. If this is not available, then TIPS should be used specially with high-risk patients who continue to bleed with pharmacologic therapy (110). Other options currently being studied are thrombin injection (111) or retrograde transvenous obliteration done by interventional radiology (112). Ectopic varices can be seen in the small bowel or rectum and are rarely discovered until they bleed. For these varices there are no clear guidelines for their management, however endoscopic therapy is usually not effective and definite treatment is either TIPS, embolization or surgical control of bleeding (113). Non-variceal portal hypertension related GI bleeding is commonly seen as portal hypertensive gastropathy (PHG), which usually manifests as chronic bleeding and anemia and can be treated with NSB, iron supplements and blood transfusion if needed. Patients, who require frequent transfusion, should be evaluated for TIPS. PHG rarely causes acute bleeding, in this case treatment is usually medical with octreotide or terlipressin with adequate response (114,115) and TIPS can be considered as a second line option (116). There is also no clear role for PPI (117). Gastric antral vascular ectasia is different from PHG, it does not usually correlate with portal hypertension but disappears after liver transplant. It is an uncommon cause of acute bleeding in cirrhotic patients and usual treatment in this case is endoscopic therapy using laser or Argon plasma photocoagulation (118). Little information exists from case reports about portal hypertensive enteropathy and colopathy but usual treatment in cases of severe bleeding is aiming towards reduction of portal pressure using somatostatin analogues or TIPS, also endoscopic therapy and surgical resection in cases of localized lesions (119).
Hyponatremia
Hyponatremia in liver cirrhosis is a common slowly developing condition and seen in up to 50% of patents. However, severe hyponatremia characterized by a sodium level less than 125 is observed in about 5% (120). Severity of hyponatremia correlates with worse outcome before and even after liver transplant (121). Dilutional hyponatremia due to excess antidiuretic hormone (ADH) is the most common etiology, however other reversible causes should be ruled out as diarrhea, diuretic side effects and excess hypotonic fluids.
Treatment is usually not indicated unless there is severe hyponatremia less than 120 meq/L, symptomatic hyponatremia or before liver transplant. First treatment modality is free water restriction. Current AASLD guidelines recommend restriction for levels less than 125, to 1-1.5 liters per day. Generally, fluid intake should be less than urine output (26). One way to predict response to adequate fluid restriction is the urine to plasma electrolytes ratio (measured as urine sodium + potassium/plasma sodium). A ratio <0.5 predicts good response to free water restriction as it indicates the excretion of electrolyte free water in the urine (122).
Tolvaptan is the only oral Vasopressin 2 receptor antagonist available that is associated with a significant rise in serum sodium and improvement of mental status (123) as it induces selective water diuresis without affecting sodium and potassium excretion. However, a recent study showed significant hepatotoxicity with high doses of tolvaptan (124) prompting a safety alert on its use in patients with underlying liver disease by regulatory agencies until more information is available.
Hypertonic saline use can lead to permanent neurologic symptoms due to demyelination in cases of rapid correction, therefore, use in liver patients should be restricted to severe hyponatremia with neurologic symptoms (as seizures) or if patient is close to transplantation (within hours) to avoid rapid correction with fluids given during surgery or with correction of liver function during transplant (125).
Cardiopulmonary complications of liver cirrhosis
Hepatopulmonary syndrome (HPS)
HPS should be suspected in any patient with cirrhosis regardless degree of decompensation who develops dyspnea, specially exertional (as exercise increases the shunted fraction), platypnea and orthodexia (126,127). This respiratory complication is seen in 10-20% of cirrhotic patients and the median survival is 2 years from the time of diagnosis (128) and death is usually due to other complications of cirrhosis. Patients with HPS and severe hypoxia have increased mortality even after transplant.
Diagnosis is established with the triad of chronic liver disease, hypoxia (<96% on pulse oximetry or elevated Alveolar-arterial gradient) and evidence of intra pulmonary shunts (129) and other common causes of hypoxia have been ruled out. Initial diagnostic test is the contrast echocardiogram, where contrast is seen in the left side of the heart within 3-6 heart beats, compared to less than 3 beats with intra-cardiac shunts (130). Lung perfusion scans using 99m Technetium macroaggregated albumin can demonstrate intrapulmonary shunts through passage of more than 6% of the radioactive substance to the brain (130). However, this study does not differentiate between intrapulmonary and intracardiac shunts, so contrast echocardiogram is still needed.
Main treatment is liver transplant and listing using MELD exception points should be applied (130). Oxygen therapy improves exertional dyspnea and quality of life (131). In patients who fail to respond to 100% oxygen, pulmonary angiogram should be considered as it can differentiate type II HPS (intrapulmonary AV fistulae) from type I (precapillary pulmonary artery dilation), in type II, coil embolization done by interventional radiology can be helpful (132). Other treatment modalities that have been studied with controversial results include nitric oxide synthase inhibitors (as methylene blue), garlic and TIPS (130).
Portopulmonary hypertension (POPH)
POPH is seen in up to 10% of cirrhotic patients, particularly in more decompensated patients and those with refractory ascites (133). The outcome is very poor with a median survival of 6 months without liver transplant.
Screening should be performed in patients who present with unexplained dyspnea, fatigue or signs of right sided heart failure and as a part of the liver transplant evaluation as extremely high pressure >50 mmHg, carries a high operative risk (134). Screening for POPH should be started with echocardiogram, which typically shows elevated right ventricular systolic pressure (RVSP) as well as pulmonary acceleration time (diagnostic if greater than 100 msec) and can rule out other cardiac causes of pulmonary hypertension such as valvular heart disease (130). Positive echocardiogram findings should be followed by right heart catheter as it is more accurate in documenting pulmonary artery pressure (135) and also allows evaluation of response to vasodilator administration.
Treatment includes oxygen supplementation in case of hypoxia and oral endothelin receptor antagonist such as bosentan that has shown to improve exercise tolerance and hemodynamics (136). Prostacyclin analogue, esoprostenol can also be used; however it requires continuous infusion (137). Sildenafil is used in other causes of pulmonary hypertension but in POPH it can worsen portal hypertension (130). Beta blockers are associated with worsening exercise capacity and pulmonary hemodynamics so they should be stopped in patients with POPH and variceal ligation should be used (138).
Cirrhotic cardiomyopathy represents the systolic and diastolic dysfunction and electrophysiological abnormalities (mainly a prolonged QT interval) that is seen in up to one third of cirrhotic patients (139) and it can be demonstrated on echocardiogram (specially using tissue Doppler) (140). However, clinical detection usually occurs only after a stressful condition as TIPS or after surgery as liver transplant. Unfortunately, no official guidelines on diagnosis or treatment are available, however, beta blockers (141) and aldosterone antagonists seem to be beneficial and fluid status should be managed with diuretics as needed. Liver transplant may revert some of the cardiac abnormalities (130). Cardiac glycosides as digoxin do not seem to be beneficial.
Arterial hypertension can still be seen in patients with cirrhosis especially patients with fatty liver disease because of the association with metabolic syndrome. Aggressive treatment in decompensated cirrhosis should be avoided because low mean arterial pressure (<82) has been shown to independently predict worse survival in this group of patients (142). Additionally, patients start to be hypotensive once decompensation develops so close monitoring of the blood pressure by both the physician and the patient are required to determine the need to adjust or stop the anti-hypertensive medications. ACE-inhibitors should be avoided or used with caution in the cirrhotic hypertensive patient as they can lead to worsening hemodynamics and renal failure (143).
Other common problems of cirrhosis
In addition to management of complications of cirrhosis, other common issues are frequently encountered; targeting of these symptoms can improve quality of life.
Muscle cramps are common in cirrhosis (about 2/3 of patients) compared to general population, especially in advanced stages with hypoalbuminemia and water retention, and are associated with poor quality of life (144). Electrolyte imbalance should be looked for and corrected if present. However, according to one study occurrence of cramps was independent of abnormal serum electrolytes or diuretic consumption, suggesting an undiscovered underlying mechanism (145). One suggested treatment is quinine sulfate that is not approved as a drug for treatment of cramps any more but can be found in some brands of tonic water, and can help control cramps in cirrhotic patients (9).
Itching can be a problem in cirrhosis regardless the etiology and even cirrhotic patients without jaundice can complain of marked pruritus. Local dermatologic conditions should be ruled out, and then drug therapy in the form of cholestyramine can be tried. Other medications that have been studied and can be used as second line treatments include serteraline, naltrexone and rifampicin (146). Some of these medications are safer than bile acid binding agents because of less drug interactions.
Dyspepsia and nausea are common symptoms in cirrhosis affecting nutrition and quality of life. Usually due to an underlying organic cause, for which a clinical evaluation should be done looking for gastritis, gastric ulcer, gall stones, GERD or gastroparesis. For patients with functional dyspepsia (about ¼ of patients) (147), treatment is usually symptomatic with a serotonin 5-HT3 receptor antagonist such as ondansetron (148), if failed, careful use of metoclopramide can be tried (9).
Pain control
Cirrhotic patients are subjected to higher risk with pain medications compared to general population. Nonsteroidal anti-inflammatory drugs (NSAIDs) should generally be avoided because of the associated GI toxicity (in a patient that may be coagulopathic with varices) and also reduction of renal function and diuretic response by inhibiting vasodilating prostaglandins release. Selective COX-2 inhibitors may have fewer side effects, but further studies are required to confirm this findings and evaluate long term side effects including cardiac ones (149).
Acetaminophen is not contraindicated in liver patients but should be used with caution. The suggested safe daily limit is 2-4 grams in the patient with cirrhosis and in case of active alcohol drinking, the daily limit should be 2 grams or even less, because of glutathione depletion (150). Most available studies evaluated only short-term use. Further studies evaluating long term use are needed.
Whenever possible, opioids should be avoided in decompensated cirrhotic patients (or limited to severe pain as malignancies) because of the altered elimination (hepatic and renal) and prolonged half-life precipitating encephalopathy through accumulating CNS suppression effect and/or associated constipation. Additionally, there is a potential for addiction especially in alcoholic patients and can affect patients listing for transplant. Hypotension is another side effect of narcotics that can exacerbate systemic hypotension seen in cirrhosis. Fentanyl seems to have less hypotensive effect, likely because of the unchanged pharmacokinetics in cirrhosis (151) and the lack of the histamine release seen with other narcotics (152). Tramadol appears relatively safe as it works through other mechanisms beside opioid receptors and it should be the first line narcotic used (153). Other narcotics as oxycodone, morphine and hydromorphone can be cautiously used if pain is not controlled but dose reduction and less frequent administration are recommended (154).
Other ways to control pain should be addressed like paracentesis for tense ascites and switching diuretics for symptomatic gynecomastia. Neuropathic pain can be managed with other medications. Theoretically, the safe tricyclic antidepressants (TCA) are nortriptyline and desipramine because they are less potent with less sedative effects, less hypotension and less intestinal slowing effect (155). Carbamazepine should be avoided due to high incidence of hepatotoxicity but other antiepileptic drugs as gabapentin can be used with careful dose reduction with abnormal kidney functions or with development of side effects as nausea or sedation. Pregabalin is a more expensive option with fewer side effects (154).
As a general rule unnecessary use of medication should be avoided in cirrhosis because of potential hepatotoxic or nephrotoxic effects and because of possible drug interaction. Examples are unnecessary antibiotics for likely viral respiratory infection, over the counter herbal “liver stimulants” and chronic oral vitamin K (9).
Nutrition
Muscle wasting is a common problem in cirrhosis due to appetite suppression that is either central or due to ascites and intestinal wall edema (156). It is estimated that up to 50% of patients have energy and protein malnutrition according to one Japanese study (157). Generally recommended calorie intake for cirrhotic patients is 40 kcal/kg/day in energy and 1.2-1.5 kcal/kg/day in proteins (158) with spreading the daily intake into 4-6 meals including a late evening snack of less than 200 kcal rich in branched chain amino acids (159). Further adjustment according to the patient’s co morbidities and current nutritional status is required. For example, in case of impaired glucose tolerance, recommended daily calorie intake is 25-30 kcal/kg ideal body weight, with focusing on dietary fibers and complex rather than simple carbohydrates (160,161) and oral branched chain amino acid supplement as it was shown to improve insulin sensitivity (162).
Unnecessary diet restrictions should be avoided. This includes sodium restriction in compensated patients without evidence of fluid retention as this can worsen malnutrition by making food less palatable. Similarly free water restriction is not recommended unless serum sodium is markedly low. Unnecessary protein restriction should be avoided, as mentioned in the encephalopathy section (71).
Certain life style modifications are shown to improve cirrhosis progression. In addition to alcohol cessation, quitting smoking (163) and avoiding cannabis use (164) were also associated with less fibrosis progression in patients with chronic viral hepatitis. On the other hand, coffee was shown to have anti-oxidant helping with reducing fibrosis risk (165) increasing chance of sustained virological response (SVR) to antiviral treatment (166), and dark chocolate probably affects endothelial function helping portal hypertension (167).
Conclusions
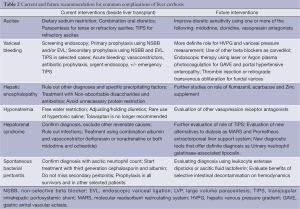
The management of cirrhosis is an important element in treating patients with HCC given the associated morbidity and mortality. Despite advances in the management of patients with cirrhosis and availability of high-quality evidence as well as comprehensive clinical guidelines, there remains poor implementation of recommended care for patient with cirrhosis in clinical practice. Recognizing the subtle signs of cirrhosis and the decompensation events are essential to the successful management of cirrhosis. General measures (Table 1) should be applied to all patients with cirrhosis to prevent further damage and loss of the residual liver function, which in addition to worsening survival, also limits the available treatment options for HCC and affects response and safety of therapeutic interventions. Hepatotoxic medications should be avoided and whenever possible, underlying etiology of the liver disease should be treated. Early detection of manifestations of decompensation events including ascites, encephalopathy, varices, SBP and hepatorenal syndrome and their management together with other important complications of cirrhosis (pruritis, cramps, malnutrition) can substantially minimize morbidity and improve outcomes (Table 2).

Full table
Full table
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Schuppan D, Afdhal NH. Liver cirrhosis. Lancet 2008;371:838-51. [PubMed]
- Qua CS, Goh KL. Liver cirrhosis in Malaysia: peculiar epidemiology in a multiracial Asian country. J Gastroenterol Hepatol 2011;26:1333-7. [PubMed]
- Fattovich G, Pantalena M, Zagni I, et al. Effect of hepatitis B and C virus infections on the natural history of compensated cirrhosis: a cohort study of 297 patients. Am J Gastroenterol 2002;97:2886-95. [PubMed]
- Mukerji AN, Patel V, Jain A. Improving survival in decompensated cirrhosis. Int J Hepatol 2012;2012:318627.
- Kuiper JJ, de Man RA, van Buuren HR. Review article: Management of ascites and associated complications in patients with cirrhosis. Aliment Pharmacol Ther 2007;26 Suppl 2:183-93. [PubMed]
- Simonetti RG, Camma C, Fiorello F, et al. Hepatocellular carcinoma. A worldwide problem and the major risk factors. Dig Dis Sci 1991;36:962-72. [PubMed]
- Bosch FX, Ribes J, Borras J. Epidemiology of primary liver cancer. Semin Liver Dis 1999;19:271-85. [PubMed]
- Ferenci P, Fried M, Labrecque D, et al. World gastroenterology organisation guideline. Hepatocellular carcinoma (HCC): a global perspective. JGLD 2010;19:311-7. [PubMed]
- Runyon BA. A primer on detecting cirrhosis and caring for these patients without causing harm. Int J Hepatol 2011;2011:801983.
- Fleming KM, Aithal GP, Card TR, et al. The rate of decompensation and clinical progression of disease in people with cirrhosis: a cohort study. Aliment Pharmacol Ther 2010;32:1343-50. [PubMed]
- D’Amico G, Morabito A, Pagliaro L, et al. Survival and prognostic indicators in compensated and decompensated cirrhosis. Dig Dis Sci 1986;31:468-75. [PubMed]
- Davis GL, Dempster J, Meler JD, et al. Hepatocellular carcinoma: management of an increasingly common problem. Proc (Bayl Univ Med Cent) 2008;21:266-80. [PubMed]
- Abou-Alfa GK, Amadori D, Santoro A, et al. Safety and efficacy of sorafenib in patients with hepatocellular carcinoma (HCC) and Child-Pugh A versus B cirrhosis. Gastrointest Cancer Re 2011;4:40-4. [PubMed]
- Marrero JA, Lencioni R, Kudo M, et al. Global investigation of therapeutic decisions in hepatocellular carcinoma and of its treatment with sorafenib (GIDEON) second interim analysis in more than 1,500 patients: clinical findings in patients with liver dysfunction. J Clin Oncol 2011;29:256s.
- Alexander JF, Lischner MW, Galambos JT. Natural history of alcoholic hepatitis. II. The long-term prognosis. Am J Gastroenterol 1971;56:515-25. [PubMed]
- Niemelä O, Risteli J, Blake JE, et al. Markers of fibrogenesis and basement membrane formation in alcoholic liver disease. Relation to severity, presence of hepatitis, and alcohol intake. Gastroenterology 1990;98:1612-9. [PubMed]
- Reynolds TB, Geller HM, Kuzma OT, et al. Spontaneous decrease in portal pressure with clinical improvement in cirrhosis. N Engl J Med 1960;263:734-9. [PubMed]
- Runyon BA. Historical aspects of treatment of patients with cirrhosis and ascites. Semin Liver Dis 1997;17:163-73. [PubMed]
- Cotler SJ, Jakate S, Jensen DM. Resolution of cirrhosis in autoimmune hepatitis with corticosteroid therapy. J Clin Gastroenterol 2001;32:428-30. [PubMed]
- Yao FY, Bass NM. Lamivudine treatment in patients with severely decompensated cirrhosis due to replicating hepatitis B infection. J Hepatol 2000;33:301-7. [PubMed]
- Mallet V, Gilgenkrantz H, Serpaggi J, et al. Brief communication: the relationship of regression of cirrhosis to outcome in chronic hepatitis C. Ann Intern Med 2008;149:399-403. [PubMed]
- Tsochatzis EA, Bosch J, Burroughs AK. New therapeutic paradigm for patients with cirrhosis. Hepatology 2012;56:1983-92. [PubMed]
- Sohrabpour AA, Mohamadnejad M, Malekzadeh R. Review article: the reversibility of cirrhosis. Aliment Pharmacol Ther 2012;36:824-32. [PubMed]
- Moore KP, Wong F, Gines P, et al. The management of ascites in cirrhosis: report on the consensus conference of the International Ascites Club. Hepatology 2003;38:258-66. [PubMed]
- Lucena MI, Andrade RJ, Tognoni G, et al. Multicenter hospital study on prescribing patterns for prophylaxis and treatment of complications of cirrhosis. Eur J Clin Pharmacol 2002;58:435-40. [PubMed]
- Runyon BA. Introduction to the revised American Association for the Study of Liver Diseases practice guideline management of adult patients with ascites due to cirrhosis 2012. Hepatology 2013;57:1651-3. [PubMed]
- Thrall MJ, Giampoli EJ. Routine review of ascites fluid from patients with cirrhosis or hepatocellular carcinoma is a low-yield procedure: an observational study. Cytojournal 2009;6:16. [PubMed]
- Angeli P, Fasolato S, Mazza E, et al. Combined versus sequential diuretic treatment of ascites in non-azotaemic patients with cirrhosis: results of an open randomised clinical trial. Gut 2010;59:98-104. [PubMed]
- Angeli P, Dalla Pria M, De Bei E, et al. Randomized clinical study of the efficacy of amiloride and potassium canrenoate in nonazotemic cirrhotic patients with ascites. Hepatology 1994;19:72-9. [PubMed]
- Daskalopoulos G, Laffi G, Morgan T, et al. Immediate effects of furosemide on renal hemodynamics in chronic liver disease with ascites. Gastroenterology 1987;92:1859-63. [PubMed]
- Ginés P, Arroyo V, Quintero E, et al. Comparison of paracentesis and diuretics in the treatment of cirrhotics with tense ascites. Results of a randomized study. Gastroenterology 1987;93:234-41. [PubMed]
- El-Bokl MA, Senousy BE, El-Karmouty KZ, et al. Spot urinary sodium for assessing dietary sodium restriction in cirrhotic ascites. World J Gastroenterol 2009;15:3631-5. [PubMed]
- Senousy BE, Draganov PV. Evaluation and management of patients with refractory ascites. World J Gastroenterol 2009;15:67-80. [PubMed]
- Sanyal AJ, Genning C, Reddy KR, et al. The North American Study for the treatment of refractory ascites. Gastroenterology 2003;124:634-41. [PubMed]
- Salerno F, Merli M, Riggio O, et al. Randomized controlled study of TIPS versus paracentesis plus albumin in cirrhosis with severe ascites. Hepatology 2004;40:629-35. [PubMed]
- Strauss RM, Boyer TD. Hepatic hydrothorax. Semin Liver Dis 1997;17:227-32. [PubMed]
- Orman ES, Lok AS. Outcomes of patients with chest tube insertion for hepatic hydrothorax. Hepatol Int 2009;3:582-6. [PubMed]
- Parsi MA, Atreja A, Zein NN. Spontaneous bacterial peritonitis: recent data on incidence and treatment. Cleve Clin J Med 2004;71:569-76. [PubMed]
- Tsung PC, Ryu SH, Cha IH, et al. Predictive factors that influence the survival rates in liver cirrhosis patients with spontaneous bacterial peritonitis. Clin Mol Hepatol 2013;19:131-9. [PubMed]
- Runyon BA. Management of adult patients with ascites due to cirrhosis: an update. Hepatology 2009;49:2087-107. [PubMed]
- Akriviadis EA, Runyon BA. Utility of an algorithm in differentiating spontaneous from secondary bacterial peritonitis. Gastroenterology 1990;98:127-33. [PubMed]
- Fernández J, Navasa M, Gomez J, et al. Bacterial infections in cirrhosis: epidemiological changes with invasive procedures and norfloxacin prophylaxis. Hepatology 2002;35:140-8. [PubMed]
- Soriano G, Castellote J, Alvarez C, et al. Secondary bacterial peritonitis in cirrhosis: a retrospective study of clinical and analytical characteristics, diagnosis and management. J Hepatol 2010;52:39-44. [PubMed]
- European Association for the Study of the Liver. EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol 2010;53:397-417. [PubMed]
- Runyon BA, Hoefs JC. Culture-negative neutrocytic ascites: a variant of spontaneous bacterial peritonitis. Hepatology 1984;4:1209-11. [PubMed]
- Runyon BA. Monomicrobial nonneutrocytic bacterascites: a variant of spontaneous bacterial peritonitis. Hepatology 1990;12:710-5. [PubMed]
- Runyon BA, McHutchison JG, Antillon MR, et al. Short-course versus long-course antibiotic treatment of spontaneous bacterial peritonitis. A randomized controlled study of 100 patients. Gastroenterology 1991;100:1737-42. [PubMed]
- Rimola A, Salmeron JM, Clemente G, et al. Two different dosages of cefotaxime in the treatment of spontaneous bacterial peritonitis in cirrhosis: results of a prospective, randomized, multicenter study. Hepatology 1995;21:674-9. [PubMed]
- Fernández J, Acevedo J, Castro M, et al. Prevalence and risk factors of infections by multiresistant bacteria in cirrhosis: a prospective study. Hepatology 2012;55:1551-61. [PubMed]
- Navasa M, Follo A, Llovet JM, et al. Randomized, comparative study of oral ofloxacin versus intravenous cefotaxime in spontaneous bacterial peritonitis. Gastroenterology 1996;111:1011-7. [PubMed]
- Tuncer I, Topcu N, Durmus A, et al. Oral ciprofloxacin versus intravenous cefotaxime and ceftriaxone in the treatment of spontaneous bacterial peritonitis. Hepatogastroenterology 2003;50:1426-30. [PubMed]
- Angeli P, Guarda S, Fasolato S, et al. Switch therapy with ciprofloxacin vs. intravenous ceftazidime in the treatment of spontaneous bacterial peritonitis in patients with cirrhosis: similar efficacy at lower cost. Aliment Pharmacol Ther 2006;23:75-84. [PubMed]
- Sort P, Navasa M, Arroyo V, et al. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. N Engl J Med 1999;341:403-9. [PubMed]
- Sigal SH, Stanca CM, Fernandez J, et al. Restricted use of albumin for spontaneous bacterial peritonitis. Gut 2007;56:597-9. [PubMed]
- Garcia-Tsao G. Current management of the complications of cirrhosis and portal hypertension: variceal hemorrhage, ascites, and spontaneous bacterial peritonitis. Gastroenterology 2001;120:726-48. [PubMed]
- Fernández J, Ruiz del Arbol L, Gomez C, et al. Norfloxacin vs ceftriaxone in the prophylaxis of infections in patients with advanced cirrhosis and hemorrhage. Gastroenterology 2006;131:1049-56. [PubMed]
- Fernández J, Navasa M, Planas R, et al. Primary prophylaxis of spontaneous bacterial peritonitis delays hepatorenal syndrome and improves survival in cirrhosis. Gastroenterology 2007;133:818-24. [PubMed]
- Goel GA, Deshpande A, Lopez R, et al. Increased rate of spontaneous bacterial peritonitis among cirrhotic patients receiving pharmacologic acid suppression. Clin Gastroenterol Hepatol 2012;10:422-7. [PubMed]
- Chen TA, Lo GH, Lai KH. Risk factors for spontaneous bacterial empyema in cirrhotic patients with hydrothorax. J Chin Med Assoc 2003;66:579-86. [PubMed]
- Allam NA. Spontaneous bacterial empyema in liver cirrhosis: an underdiagnosed pleural complication. Saudi J Gastroenterol 2008;14:43-5. [PubMed]
- Ferenci P, Lockwood A, Mullen K, et al. Hepatic encephalopathy--definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology 2002;35:716-21. [PubMed]
- Jeffers LJ, Dubow RA, Zieve L, et al. Hepatic encephalopathy and orotic aciduria associated with hepatocellular carcinoma in a noncirrhotic liver. Hepatology 1988;8:78-81. [PubMed]
- Ong JP, Aggarwal A, Krieger D, et al. Correlation between ammonia levels and the severity of hepatic encephalopathy. Am J Med 2003;114:188-93. [PubMed]
- Amodio P, Del Piccolo F, Marchetti P, et al. Clinical features and survivial of cirrhotic patients with subclinical cognitive alterations detected by the number connection test and computerized psychometric tests. Hepatology 1999;29:1662-7. [PubMed]
- Gabduzda GJ, Hall PW 3rd. Relation of potassium depletion to renal ammonium metabolism and hepatic coma. Medicine (Baltimore) 1966;45:481-90. [PubMed]
- Prabhakar S, Bhatia R. Management of agitation and convulsions in hepatic encephalopathy. Indian J Gastroenterol 2003;22 Suppl 2:S54-8. [PubMed]
- Uribe M, Campollo O, Vargas F, et al. Acidifying enemas (lactitol and lactose) vs. nonacidifying enemas (tap water) to treat acute portal-systemic encephalopathy: a double-blind, randomized clinical trial. Hepatology 1987;7:639-43. [PubMed]
- Bass NM, Mullen KD, Sanyal A, et al. Rifaximin treatment in hepatic encephalopathy. N Engl J Med 2010;362:1071-81. [PubMed]
- Morgan MH, Read AE, Speller DC. Treatment of hepatic encephalopathy with metronidazole. Gut 1982;23:1-7. [PubMed]
- Sawano T, Kawashima T, Takase Y, et al. Hepatic coma recovered after interventional obliteration for ileocecal-inferior vena cava shunt--report of one case. Wiad Lek 1997;50 Suppl 1 Pt 1:296-7. [PubMed]
- Córdoba J, Lopez-Hellin J, Planas M, et al. Normal protein diet for episodic hepatic encephalopathy: results of a randomized study. J Hepatol 2004;41:38-43. [PubMed]
- Bianchi GP, Marchesini G, Fabbri A, et al. Vegetable versus animal protein diet in cirrhotic patients with chronic encephalopathy. A randomized cross-over comparison. J Intern Med 1993;233:385-92. [PubMed]
- Slack A, Yeoman A, Wendon J. Renal dysfunction in chronic liver disease. Crit Care 2010;14:214. [PubMed]
- Minemura M, Tajiri K, Shimizu Y. Systemic abnormalities in liver disease. World J Gastroenterol 2009;15:2960-74. [PubMed]
- Garcia-Tsao G, Parikh CR, Viola A. Acute kidney injury in cirrhosis. Hepatology 2008;48:2064-77. [PubMed]
- Martin-Llahi M, Guevara M, Torre A, et al. Prognostic importance of the cause of renal failure in patients with cirrhosis. Gastroenterology 2011;140:488-496.e4.
- Takabatake T, Ohta H, Ishida Y, et al. Low serum creatinine levels in severe hepatic disease. Arch Intern Med 1988;148:1313-5. [PubMed]
- Verna EC, Brown RS, Farrand E, et al. Urinary neutrophil gelatinase-associated lipocalin predicts mortality and identifies acute kidney injury in cirrhosis. Dig Dis Sci 2012;57:2362-70. [PubMed]
- Fagundes C, Pepin MN, Guevara M, et al. Urinary neutrophil gelatinase-associated lipocalin as biomarker in the differential diagnosis of impairment of kidney function in cirrhosis. J Hepatol 2012;57:267-73. [PubMed]
- Péron JM, Bureau C, Gonzalez L, et al. Treatment of hepatorenal syndrome as defined by the international ascites club by albumin and furosemide infusion according to the central venous pressure: a prospective pilot study. Am J Gastroenterol 2005;100:2702-7. [PubMed]
- Tyagi P, Sharma P, Sharma BC, et al. Prevention of hepatorenal syndrome in patients with cirrhosis and ascites: a pilot randomized control trial between pentoxifylline and placebo. Eur J Gastroenterol Hepatol 2011;23:210-7. [PubMed]
- Gonwa TA, Morris CA, Goldstein RM, et al. Long-term survival and renal function following liver transplantation in patients with and without hepatorenal syndrome--experience in 300 patients. Transplantation 1991;51:428-30. [PubMed]
- Esrailian E, Pantangco ER, Kyulo NL, et al. Octreotide/Midodrine therapy significantly improves renal function and 30-day survival in patients with type 1 hepatorenal syndrome. Dig Dis Sci 2007;52:742-8. [PubMed]
- Gluud LL, Christensen K, Christensen E, et al. Systematic review of randomized trials on vasoconstrictor drugs for hepatorenal syndrome. Hepatology 2010;51:576-84. [PubMed]
- Sanyal AJ, Boyer T, Garcia-Tsao G, et al. A randomized, prospective, double-blind, placebo-controlled trial of terlipressin for type 1 hepatorenal syndrome. Gastroenterology 2008;134:1360-8. [PubMed]
- Brensing KA, Textor J, Perz J, et al. Long term outcome after transjugular intrahepatic portosystemic stent-shunt in non-transplant cirrhotics with hepatorenal syndrome: a phase II study. Gut 2000;47:288-95. [PubMed]
- Alessandria C, Venon WD, Marzano A, et al. Renal failure in cirrhotic patients: role of terlipressin in clinical approach to hepatorenal syndrome type 2. Eur J Gastroenterol Hepatol 2002;14:1363-8. [PubMed]
- Garcia-Tsao G, Bosch J. Management of varices and variceal hemorrhage in cirrhosis. N Engl J Med 2010;362:823-32. [PubMed]
- Dib N, Oberti F, Cales P. Current management of the complications of portal hypertension: variceal bleeding and ascites. CMAJ 2006;174:1433-43. [PubMed]
- D’Amico G, Pagliaro L, Bosch J. Pharmacological treatment of portal hypertension: an evidence-based approach. Semin Liver Dis 1999;19:475-505. [PubMed]
- Abraldes JG, Villanueva C, Banares R, et al. Hepatic venous pressure gradient and prognosis in patients with acute variceal bleeding treated with pharmacologic and endoscopic therapy. J Hepatol 2008;48:229-36. [PubMed]
- de Franchis R. Non-invasive (and minimally invasive) diagnosis of oesophageal varices. J Hepatol 2008;49:520-7. [PubMed]
- de Franchis R, Eisen GM, Laine L, et al. Esophageal capsule endoscopy for screening and surveillance of esophageal varices in patients with portal hypertension. Hepatology 2008;47:1595-603. [PubMed]
- Ripoll C, Groszmann RJ, Garcia-Tsao G, et al. Hepatic venous pressure gradient predicts development of hepatocellular carcinoma independently of severity of cirrhosis. J Hepatol 2009;50:923-8. [PubMed]
- Moitinho E, Escorsell A, Bandi JC, et al. Prognostic value of early measurements of portal pressure in acute variceal bleeding. Gastroenterology 1999;117:626-31. [PubMed]
- Kong DR, Zhang C, Zhang L, et al. Measurement of variceal pressure with a computerized endoscopic manometry: validation and effect of propranolol therapy in cirrhotic patients. PloS One 2013;8:e56332. [PubMed]
- Garcia-Tsao G, Sanyal AJ, Grace ND, et al. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology 2007;46:922-38. [PubMed]
- Chen YI, Ghali P. Prevention and management of gastroesophageal varices in cirrhosis. Int J Hepatol 2012;2012:750150.
- Tripathi D, Ferguson JW, Kochar N, et al. Randomized controlled trial of carvedilol versus variceal band ligation for the prevention of the first variceal bleed. Hepatology 2009;50:825-33. [PubMed]
- Lo GH, Chen WC, Lin CK, et al. Improved survival in patients receiving medical therapy as compared with banding ligation for the prevention of esophageal variceal rebleeding. Hepatology 2008;48:580-7. [PubMed]
- Léauté-Labrèze C, Dumas de la Roque E, Hubiche T, et al. Propranolol for severe hemangiomas of infancy. N Engl J Med 2008;358:2649-51. [PubMed]
- Sersté T, Melot C, Francoz C, et al. Deleterious effects of beta-blockers on survival in patients with cirrhosis and refractory ascites. Hepatology 2010;52:1017-22. [PubMed]
- Sersté T, Francoz C, Durand F, et al. Beta-blockers cause paracentesis-induced circulatory dysfunction in patients with cirrhosis and refractory ascites: a cross-over study. J Hepatol 2011;55:794-9. [PubMed]
- Kravetz D, Sikuler E, Groszmann RJ. Splanchnic and systemic hemodynamics in portal hypertensive rats during hemorrhage and blood volume restitution. Gastroenterology 1986;90:1232-40. [PubMed]
- Bañares R, Albillos A, Rincon D, et al. Endoscopic treatment versus endoscopic plus pharmacologic treatment for acute variceal bleeding: a meta-analysis. Hepatology 2002;35:609-15. [PubMed]
- Monescillo A, Martinez-Lagares F, Ruiz-del-Arbol L, et al. Influence of portal hypertension and its early decompression by TIPS placement on the outcome of variceal bleeding. Hepatology 2004;40:793-801. [PubMed]
- Riggio O, Ridola L, Lucidi C, et al. Emerging issues in the use of transjugular intrahepatic portosystemic shunt (TIPS) for management of portal hypertension: time to update the guidelines? Dig Liver Dis 2010;42:462-7. [PubMed]
- Lo GH, Lai KH, Cheng JS, et al. A prospective, randomized trial of butyl cyanoacrylate injection versus band ligation in the management of bleeding gastric varices. Hepatology 2001;33:1060-4. [PubMed]
- Shiha G, El-Sayed SS. Gastric variceal ligation: a new technique. Gastrointest Endosc 1999;49:437-41. [PubMed]
- Lo GH, Liang HL, Chen WC, et al. A prospective, randomized controlled trial of transjugular intrahepatic portosystemic shunt versus cyanoacrylate injection in the prevention of gastric variceal rebleeding. Endoscopy 2007;39:679-85. [PubMed]
- Ramesh J, Limdi JK, Sharma V, et al. The use of thrombin injections in the management of bleeding gastric varices: a single-center experience. Gastrointest Endosc 2008;68:877-82. [PubMed]
- Akahoshi T, Hashizume M, Tomikawa M, et al. Long-term results of balloon-occluded retrograde transvenous obliteration for gastric variceal bleeding and risky gastric varices: a 10-year experience. J Gastroenterol Hepatol 2008;23:1702-9. [PubMed]
- Sort P, Elizalde I, Llach I, et al. Duodenal variceal bleeding treated with a transjugular intrahepatic portosystemic shunt. Endoscopy 1995;27:626-7. [PubMed]
- Kouroumalis EA, Koutroubakis IE, Manousos ON. Somatostatin for acute severe bleeding from portal hypertensive gastropathy. Eur J Gastroenterol Hepatol 1998;10:509-12. [PubMed]
- Bruha R, Marecek Z, Spicak J, et al. Double-blind randomized, comparative multicenter study of the effect of terlipressin in the treatment of acute esophageal variceal and/or hypertensive gastropathy bleeding. Hepatogastroenterology 2002;49:1161-6. [PubMed]
- Mezawa S, Homma H, Ohta H, et al. Effect of transjugular intrahepatic portosystemic shunt formation on portal hypertensive gastropathy and gastric circulation. Am J Gastroenterol 2001;96:1155-9. [PubMed]
- Zhou Y, Qiao L, Wu J, et al. Comparison of the efficacy of octreotide, vasopressin, and omeprazole in the control of acute bleeding in patients with portal hypertensive gastropathy: a controlled study. J Gastroenterol Hepatol 2002;17:973-9. [PubMed]
- Ward EM, Raimondo M, Rosser BG, et al. Prevalence and natural history of gastric antral vascular ectasia in patients undergoing orthotopic liver transplantation. J Clin Gastroenterol 2004;38:898-900. [PubMed]
- Kalafateli M, Triantos CK, Nikolopoulou V, et al. Non-variceal gastrointestinal bleeding in patients with liver cirrhosis: a review. Dig Dis Sci 2012;57:2743-54. [PubMed]
- Sigal SH. Hyponatremia in cirrhosis. J Hosp Med 2012;7 Suppl 4:S14-7. [PubMed]
- Arroyo V, Claria J, Salo J, et al. Antidiuretic hormone and the pathogenesis of water retention in cirrhosis with ascites. Semin Liver Dis 1994;14:44-58. [PubMed]
- Furst H, Hallows KR, Post J, et al. The urine/plasma electrolyte ratio: a predictive guide to water restriction. Am J Med Sci 2000;319:240-4. [PubMed]
- Boyer TD. Tolvaptan and hyponatremia in a patient with cirrhosis. Hepatology 2010;51:699-702. [PubMed]
- Torres VE, Chapman AB, Devuyst O, et al. Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med 2012;367:2407-18. [PubMed]
- Abbasoglu O, Goldstein RM, Vodapally MS, et al. Liver transplantation in hyponatremic patients with emphasis on central pontine myelinolysis. Clin Transplant 1998;12:263-9. [PubMed]
- Krowka MJ, Cortese DA. Pulmonary aspects of chronic liver disease and liver transplantation. Mayo Clinic proceedings Mayo Clin Proc 1985;60:407-18. [PubMed]
- Seward JB, Hayes DL, Smith HC, et al. Platypnea-orthodeoxia: clinical profile, diagnostic workup, management, and report of seven cases. Mayo Clin Proc 1984;59:221-31. [PubMed]
- Krowka MJ, Cortese DA. Hepatopulmonary syndrome. Current concepts in diagnostic and therapeutic considerations. Chest 1994;105:1528-37. [PubMed]
- Hoeper MM, Krowka MJ, Strassburg CP. Portopulmonary hypertension and hepatopulmonary syndrome. Lancet 2004;363:1461-8. [PubMed]
- Sawant P, Vashishtha C, Nasa M. Management of cardiopulmonary complications of cirrhosis. Int J Hepatol 2011;2011:280569.
- Henrion J, Schapira M, Luwaert R, et al. Hypoxic hepatitis: clinical and hemodynamic study in 142 consecutive cases. Medicine (Baltimore) 2003;82:392-406. [PubMed]
- Poterucha JJ, Krowka MJ, Dickson ER, et al. Failure of hepatopulmonary syndrome to resolve after liver transplantation and successful treatment with embolotherapy. Hepatology 1995;21:96-100. [PubMed]
- Castro M, Krowka MJ, Schroeder DR, et al. Frequency and clinical implications of increased pulmonary artery pressures in liver transplant patients. Mayo Clin Proc 1996;71:543-51. [PubMed]
- Restrepo R, Singer EF, Baram M. Hepatopulmonary syndrome and portopulmonary hypertension. Hosp Pract (1995) 2013;41:62-71. [PubMed]
- Cotton CL, Gandhi S, Vaitkus PT, et al. Role of echocardiography in detecting portopulmonary hypertension in liver transplant candidates. Liver Transpl 2002;8:1051-4. [PubMed]
- Rubin LJ, Badesch DB, Barst RJ, et al. Bosentan therapy for pulmonary arterial hypertension. N Engl J Med 2002;346:896-903. [PubMed]
- Shapiro SM, Oudiz RJ, Cao T, et al. Primary pulmonary hypertension: improved long-term effects and survival with continuous intravenous epoprostenol infusion. J Am Coll Cardiol 1997;30:343-9. [PubMed]
- Enache I, Oswald-Mammosser M, Woehl-Jaegle ML, et al. Cirrhotic cardiomyopathy and hepatopulmonary syndrome: prevalence and prognosis in a series of patients. Respir Med 2013;107:1030-6. [PubMed]
- Myers RP, Lee SS. Cirrhotic cardiomyopathy and liver transplantation. Liver Transpl 2000;S44-52. [PubMed]
- Kazankov K, Holland-Fischer P, Andersen NH, et al. Resting myocardial dysfunction in cirrhosis quantified by tissue Doppler imaging. Liver Int 2011;31:534-40. [PubMed]
- Henriksen JH, Bendtsen F, Hansen EF, et al. Acute non-selective beta-adrenergic blockade reduces prolonged frequency-adjusted Q-T interval (QTc) in patients with cirrhosis. J Hepatol 2004;40:239-46. [PubMed]
- Llach J, Gines P, Arroyo V, et al. Prognostic value of arterial pressure, endogenous vasoactive systems, and renal function in cirrhotic patients admitted to the hospital for the treatment of ascites. Gastroenterology 1988;94:482-7. [PubMed]
- Wang F, Wang BY. Progress in the diagnosis and treatment of ascites in cirrhosis: introduction of EASL clinical practice guidelines on management of ascites in cirrhosis. Zhonghua Gan Zang Bing Za Zhi 2010;18:951-4. [PubMed]
- Chatrath H, Liangpunsakul S, Ghabril M, et al. Prevalence and morbidity associated with muscle cramps in patients with cirrhosis. Am J Med 2012;125:1019-25. [PubMed]
- Abrams GA, Concato J, Fallon MB. Muscle cramps in patients with cirrhosis. Am J Gastroenterol 1996;91:1363-6. [PubMed]
- Lindor KD, Gershwin ME, Poupon R, et al. Primary biliary cirrhosis. Hepatology 2009;50:291-308. [PubMed]
- Kalaitzakis E, Simren M, Olsson R, et al. Gastrointestinal symptoms in patients with liver cirrhosis: associations with nutritional status and health-related quality of life. Scand J Gastroenterol 2006;41:1464-72. [PubMed]
- Grassi M, Albiani B, De Matteis A, et al. Prevalence of dyspepsia in liver cirrhosis: a clinical and epidemiological investigation. Minerva Med 2001;92:7-12. [PubMed]
- Bosch-Marcè M, Claria J, Titos E, et al. Selective inhibition of cyclooxygenase 2 spares renal function and prostaglandin synthesis in cirrhotic rats with ascites. Gastroenterology 1999;116:1167-75. [PubMed]
- Benson GD, Koff RS, Tolman KG. The therapeutic use of acetaminophen in patients with liver disease. Am J Ther 2005;12:133-41. [PubMed]
- Haberer JP, Schoeffler P, Couderc E, et al. Fentanyl pharmacokinetics in anaesthetized patients with cirrhosis. Br J Anaesth 1982;54:1267-70. [PubMed]
- Jacobi J, Fraser GL, Coursin DB, et al. Clinical practice guidelines for the sustained use of sedatives and analgesics in the critically ill adult. Crit Care Med 2002;30:119-41. [PubMed]
- Kotb HI, Fouad IA, Fares KM, et al. Pharmacokinetics of oral tramadol in patients with liver cancer. J Opioid Manag 2008;4:99-104. [PubMed]
- Chandok N, Watt KD. Pain management in the cirrhotic patient: the clinical challenge. Mayo Clin Proc 2010;85:451-8. [PubMed]
- Thanacoody HK, Thomas SH. Tricyclic antidepressant poisoning: cardiovascular toxicity. Toxicol Rev 2005;24:205-14. [PubMed]
- Campillo B, Richardet JP, Scherman E, et al. Evaluation of nutritional practice in hospitalized cirrhotic patients: results of a prospective study. Nutrition 2003;19:515-21. [PubMed]
- Tajika M, Kato M, Mohri H, et al. Prognostic value of energy metabolism in patients with viral liver cirrhosis. Nutrition 2002;18:229-34. [PubMed]
- Plauth M, Cabre E, Riggio O, et al. ESPEN Guidelines on Enteral Nutrition: Liver disease. Clin Nutr 2006;25:285-94. [PubMed]
- Swart GR, Zillikens MC, van Vuure JK, et al. Effect of a late evening meal on nitrogen balance in patients with cirrhosis of the liver. BMJ 1989;299:1202-3. [PubMed]
- Barkoukis H, Fiedler KM, Lerner E. A combined high-fiber, low-glycemic index diet normalizes glucose tolerance and reduces hyperglycemia and hyperinsulinemia in adults with hepatic cirrhosis. J Am Diet Assoc 2002;102:1503-7; discussion 1507-8. [PubMed]
- Jenkins DJ, Shapira N, Greenberg G, et al. Low glycemic index foods and reduced glucose, amino acid, and endocrine responses in cirrhosis. Am J Gastroenterol 1989;84:732-9. [PubMed]
- Ohno T, Tanaka Y, Sugauchi F, et al. Suppressive effect of oral administration of branched-chain amino acid granules on oxidative stress and inflammation in HCV-positive patients with liver cirrhosis. Hepatol Res 2008;38:683-8. [PubMed]
- Tsochatzis E, Papatheodoridis GV, Manolakopoulos S, et al. Smoking is associated with steatosis and severe fibrosis in chronic hepatitis C but not B. Scand J Gastroenterol 2009;44:752-9. [PubMed]
- Hézode C, Zafrani ES, Roudot-Thoraval F, et al. Daily cannabis use: a novel risk factor of steatosis severity in patients with chronic hepatitis C. Gastroenterology 2008;134:432-9. [PubMed]
- Larsson SC, Wolk A. Coffee consumption and risk of liver cancer: a meta-analysis. Gastroenterology 2007;132:1740-5. [PubMed]
- Freedman ND, Curto TM, Lindsay KL, et al. Coffee consumption is associated with response to peginterferon and ribavirin therapy in patients with chronic hepatitis C. Gastroenterology 2011;140:1961-9. [PubMed]
- De Gottardi A, Berzigotti A, Seijo S, et al. Postprandial effects of dark chocolate on portal hypertension in patients with cirrhosis: results of a phase 2, double-blind, randomized controlled trial. Am J Clin Nutr 2012;96:584-90. [PubMed]