
Tailoring ablation strategies for colorectal liver metastases based upon rat sarcoma viral oncogene mutation status
Introduction
Colorectal cancer is the second most common cause of cancer-related death in both sex (1). Approximately 20% of the patients with colorectal cancer present with metastasis at the initial diagnosis, and up to 60% will develop metastasis during the course of disease. The liver plays a crucial role in the natural history of the disease as being the most common site of metastases, with progression of colorectal liver metastases (CLM) accounting for the main cause of mortality in 53% of the patients (2). Surgical eradication of the CLM is linked with improved overall survival (OS) rates and is warranted (3). Unfortunately, only 20–30% of the patients with CLM are resectable (4).
Ablation is a well-established local therapy for patients with limited CLM extension. In such population subset, the combination of ablation with systemic chemotherapy has demonstrated improved OS when compared to systemic chemotherapy alone, as showed by the latest results of the CLOCC trial (5). Accordingly, the last version of the European Society for Medical Oncology Consensus guidelines (6) acknowledges for the first time the role of ablation for patients with CLM presenting with oligometastatic disease (OMD). Moreover, ongoing trials are currently investigating the use of ablation as a first approach for the treatment of CLM (7). To correctly select patients for ablation, it is of paramount importance to identify the factors that affect its outcomes.
Rat sarcoma viral oncogene (RAS) mutational status is a key factor for the clinical management of colorectal cancer patients (8). Specifically, RAS mutation is a downstream component of the epidermal growth factor receptor (EGFR) signaling network, and it has been associated with resistance to medical treatments with EGFR antibodies. Furthermore, RAS mutation has been associated with higher incidence of disease recurrence and shorter OS following resection for CLM (9). Similarly, recent findings in the interventional radiology literature suggest interactions between RAS mutational status and image-guided ablation outcomes (10). Therefore, in this review, we aim to discuss the current literature on the influence of RAS mutational status on ablation outcomes, as well to provide a perspective on the future research on the impact of tumor molecular biology on liver ablation procedures.
Percutaneous image-guided ablation
Percutaneous image-guided ablation is routinely used as a safe and effective loco-regional therapy for the treatment of patients with CLM. Among the imaging modalities, computed tomography, ultrasound, and magnetic resonance imaging are the most commonly utilized. Its minimally invasive nature has several advantages: it can be easily repeated to treat progression; it does not impact surgical eligibility for those that can be eventually resected; it does not require prolonged chemotherapy interruption; and finally, it has minimal or no impact on patients' quality of life (11). Such facts are particularly important in view of the recurrent nature of CLM, which brings a high likelihood of requiring repeated local treatments even after successful resection or ablation.
From the early reports on the use of image-guided ablation for the treatment of patients with CLM in the late 90s (12) to the present time, technical developments and a deeper understanding of the oncologic mechanisms have led to improved therapeutic strategies and increased survival rates. During the last three decades, several ablative technologies have been proposed and reported in the literature: radiofrequency (RF), laser, cryoablation, microwave (MW), and irreversible electroporation (IRE). The two most used technologies are by far radiofrequency ablation (RFA) and, more recently, microwave ablation (MWA).
RF systems induce coagulative necrosis resulting from thermal energy delivered by an alternating electrical current through the needle probe placed within the target tumor. An electrical circuit is completed through grounding pads, normally attached to the thighs. Temperatures range between 60 to 100 °C, resulting in coagulation necrosis. The major portion of the final ablation zone is due to thermal conduction into more peripheral areas around the electrode (13). MWA uses electromagnetic waves between 900 and 2.4 GHz through the MW probe, which is called the antenna. These high-frequency electromagnetic waves cause oscillation and friction of water molecules with subsequent heat, resulting in tissue destruction by coagulation necrosis. MWA presents some theoretical advantages over RFA, such as reduced ‘heat-sink’ effect (lower heat dissipation in a vessel’s proximity) and larger ablation zones in a reduced period of time. For these reasons, MWA is thought to be more efficient in the treatment of larger lesions and for targets located near large vessels when compared to other thermal ablation modalities. However, presently, there is no clear evidence in favor of one technology over the other in the CLM ablation setting.
Factors affecting ablation outcomes
Local control of the ablated tumor is the main goal for an effective image-guided ablation. Presence of residual disease or development of tumor recurrence at the ablation zone is considered a treatment failure that the interventional radiologists should prevent. In the ablation literature, local recurrence is termed as “local tumor progression” (LTP), which is defined as the appearance of tumor foci at the edge of the ablation zone or within 1 cm from it (14). In order to differentiate LTP from residual tumor, it is necessary to have at least one contrast-enhanced follow-up study after ablation documenting adequate ablation and an absence of viable tissue in the target tumor by imaging criteria. Low LTP rates are desirable given the relationship between local disease control and disease-free survival, which is thought to ultimately influence OS (15).
Tumor size and ablation margins
Several factors have been described to be associated with local outcomes of ablation (16). Among the most relevant factors, tumor size and the minimal ablation margins are the two most widely recognized in several published series (16-20).
The impact of tumor size—normally defined as the maximum longitudinal diameter in the uni-dimensional axial plane—on ablation outcomes has been extensively investigated. The literature supports the evidence that the smaller the lesion is, the most favorable the local outcome will be. According to Veltri et al. (17), complete ablation was achieved in 66.7% of CLM <3 cm vs. 33.3% of CLM >3 cm (P<0.0001). More recently, these findings were confirmed by other investigators (18-22). Although 3 cm is the most commonly utilized tumor size cut-off for image-guided ablation eligibility in several institutions, the largest tumor size amenable to be effectively treated with thermal ablation is still a matter of debate. Indeed, image-guided ablation of CLM up to 5 cm can be acceptable in selected cases (20), and effective local tumor control rates can be achieved with adequate procedure planning and monitoring (23).
Ablation margins are another critical factor affecting ablation outcomes. Several studies have demonstrated the importance of achieving minimal ablation margins that extend beyond the tumor in a three-dimensional plane at least 5 mm. According to Wang et al. (19), 2-year local tumor progression free survival (LTPFS) rates for ablated CLM with minimal ablation margins of 0, 1–5, 6–10, and 11–15 mm were 26%, 46%, 74%, and 80%, respectively (P<0.011). In a recent report by Shady et al., CLM treated with minimal ablation margins >10 mm had no recurrence in a series including 110 patients (24). Thus, minimal ablation margin of >10 mm in all planes is considered with strong consensus as the optimal technical goal by an expert meeting position paper (16). Despite its criticality, achieving minimal ablation margins >10 mm is relatively difficult, as shown by some reports in the literature. Shady et al. reported ablation margins >10 mm were successfully obtained in only 12% (21/174) of their historical series (18), while a two-institution retrospective study showed that 28% (60/218) of the ablated CLM had ablation margins >10 mm (25). Nevertheless, it should be mentioned that these two published studies (18,25) consisted of retrospective patient series that pre-dated the most current evidence in regards optimal results associated with minimal ablation margins >10 mm.
RAS mutation and thermal ablation
Mutations in the RAS family of proto-oncogenes (KRAS, NRAS, HRAS) are present in 30–45% of the patients with colorectal cancers (8). These mutations cause a constitutive activation of the MAPK pathway, leading to resistance to treatment with EGFR antibodies. RAS mutations are the most well-recognized and relevant prognostic biomarker among patients undergoing CLM resection (26). More recently, published data also suggests an equivalent role in the image-guided ablation setting, as discussed below and summarized in Table 1.
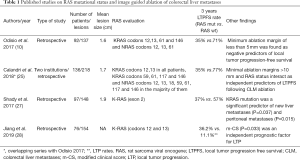
Full table
In 2017, Odisio et al. showed that mutant RAS patients (both NRAS and KRAS) had shorter 3-year LTPFS following liver ablation compared to wild-type RAS patients (35% versus 71% respectively; P=0.001) (10). In this same study, the authors also showed that among all the ablated CLM that presented with LTP, patients with mutant RAS recurred earlier and in smaller tumors when compared with those in wild-type RAS patients. In the same year, Shady et al. also showed mutant RAS as a significant predictor of LTP after CLM ablation with margins of 1–5 mm (P=0.018), with an LTP rate of 80% versus 41% for wild-type (27). The findings of these two seminal papers have been also confirmed by a recent paper, which found lower LTP rates in wild-type RAS patients (9.8%) when compared with mutant RAS patients (27.8%, P=0.004) (28).
The relationship between RAS mutational status and minimal ablation margins has also been investigated (25,27). Calandri et al. (25) performed a two-institutional retrospective analysis of 136 patients submitted to CLM ablation. The authors demonstrated that 3-year LTPFS was significantly better among wild-type RAS tumors compared with mutant RAS tumors, irrespective of the minimal ablation margins achieved (≤10 mm ablation margins: 29% mutant RAS vs. 70% wild-type RAS, P<0.001; >10 mm ablation margins: 48% mutant RAS vs. 94% wild-type RAS, P=0.006). Based on their analysis, achieving minimal ablation margins >10 mm in mutant RAS tumors would provide similar 3-years LTPFS as achieving ≤10 mm ablation margins in wild-type RAS tumors (P=0.36). In the previously mentioned series published by Shady et al. (27), the authors identified KRAS mutation as a significant independent predictor of LTP following RF ablation of CLM with minimal ablation margins of 1–5 mm (P=0.018). The authors also showed that mutant RAS tumors carried a risk of progression of 15.6-fold higher when compared with wild-type RAS tumors ablated with ablation margins of ≥6 mm. Therefore, the authors suggested that minimal ablation margins of ≥6 mm should be absolutely required (27), especially for mutant-type RAS tumors, and proposed minimal ablation margins of ≥10 mm as the treatment endpoint to offer the best possible local tumor control (25,27).
Discussion
Ablative and surgical techniques and RAS: similarities and differences
Surgical data regarding CLM management have shown the role of RAS mutational status as both a prognostic factor of OS and as predictor of recurrence following surgery (9). Proposed strategies in the surgical literature for improving the outcomes of CLM resection according to the mutational RAS status are (I) the identification of specific predictive factors (in the mutant RAS patients); (II) a better understanding of the minimal resection margins required. Passot et al. (29), stratified mutant RAS patients who underwent surgery according to node-positivity of the primary tumor, CLM size >3 cm, and more than 7 cycles of preoperative chemotherapy, showing different OS in the subgroup analyses and proposed a pre-procedural scoring system.
Brudvik et al. demonstrated in a retrospective series that RAS mutations were associated with positive margins in patients undergoing resection of CLM, underlying a more infiltrative tumor behavior (30). Likewise, as for resection of tumors in proximity to vessels, if vascular R1 can be considered as R0 parenchyma in case of wild-type RAS tumors, this is not acceptable for mutant RAS tumors (31,32). Very recently, these and other studies have led to a specific modified clinical score (m-CS) that includes the mutational RAS status, modified from the traditional risk-score (t-cs); m-CS has been successfully validated, becoming a useful tool to predict survival after resection of CLM (33).
In the ablation literature, evidence concerning the role of RAS mutational status is more recent than the surgical literature. For more than a decade, publications concerning image-guided ablation have focused attention on its safety and efficacy, paying specific attention to the technical aspects. In recent years, the need to overcome the persistent relatively high LTP rates associated with ablation when compared with the recurrence rates reported in the surgical literature have led the interventional community to try to understand the role of tumor biology, highlighting that beyond technology, patient selection is of paramount importance (10). Despite this, presently, there is no consensus in regards to patient selection and ablation margins according of RAS mutational status (6,16).
Although the RAS mutational status is not considered a contraindication to image-guided ablation, care should be exercised in the selection of the patients. Thus, considering the relationship between ablation margins, mutational status and LTPFS, we recommend that mutant RAS patients should be considered candidates for ablation only if adequate ablation margins can be planned and obtained. Specifically, a three-dimensional minimal ablation margins of ≥5 mm is always required in patients with mutant RAS, and, ideally, a minimal ablation margin of ≥10 mm should be highly desirable. Further refinements on imaging fusion and biomechanical deformation methods should provide more accurate methods to assess minimal ablation margins intra-procedurally in order to improve the ability of achieving sufficient minimal ablation margins on a three-dimensional plane.
The understanding of the relevance of tumor biology on local ablation outcomes may lead, in the near future, to unique tailored approaches for interventional procedures (especially in the case of mutant RAS patients) consisting of sequential treatments between systemic therapies (global treatment) and image-guided ablation (local treatment), in a so-called “glocal” approach (34).
RAS and other biomarkers in the OMD setting
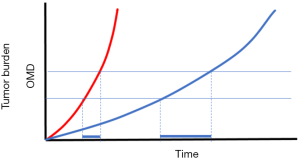
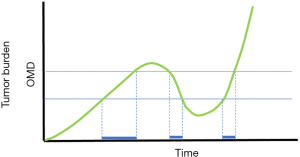
Hellman proposed the term oligometastases for the first time in 1995 (35), suggesting that in some patients with a limited number of clinically detectable metastatic tumors, a limited tumor burden reflects a transitional state before widespread systemic disease. In this setting, local treatments of oligometastases would have the potential to yield improved systemic control (36). According to ESMO consensus guidelines, the oligometastatic condition is defined as the presence of metastases “at up to 2 or occasionally 3 sites and 5 or sometimes more lesions, predominantly visceral and occasionally lymphonodal.” This definition focuses on the global radiological tumor burden without including any tumor biology information. However, tumor biology deeply impacts the OS of all CRC patients including those who fit the OMD criteria, as shown by different aggressiveness and tumor growth kinetics (Figure 1). Furthermore, patients may be potentially considered in the OMD setting at different times during their disease history, even after selective selection of systemic therapies that may have changed the global genetic tumor profile and subsequently its aggressiveness (Figure 2). In the near future, efforts should be made to identify prognosticators for OMD. For instance, the use of circulating tumor DNA analysis in a longitudinal manner might prove to be a valuable surrogate of tumor aggressiveness in the OMD setting. In order to better select patients, a deeper knowledge of tumor genetics is required. Also, as recently understood and demonstrated, not all RAS mutations are equal (37,38), potentially with different impact on disease free survival and OS after local therapies. Finally, efforts are required to understand the interactions between different genes mutations, such as the case of co-mutations of TP53 and RAS, which lead to a decreased OS after CLM resections (39).
Conclusions
RAS mutational status is emerging as a relevant prognosticator for LTP and OS after CLM image guided ablation. Available evidence suggests wider minimal ablations margins should be obtained in mutant RAS CLM patients in order to obtain improved LTPFS rates. Further studies are required to investigate specific interactions between RAS and other gene mutations, specific predictive factors of poor outcomes in the OMD setting in the mutant RAS CLM patient population and synergies between local and systemic therapies.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. [Crossref] [PubMed]
- Abdalla EK, Adam R, Bilchik AJ, et al. Improving Resectability of Hepatic Colorectal Metastases: Expert Consensus Statement. Ann Surg Oncol 2006;13:1271-80. [Crossref] [PubMed]
- Pawlik TM, Scoggins CR, Zorzi D, et al. Effect of Surgical Margin Status on Survival and Site of Recurrence After Hepatic Resection for Colorectal Metastases. Ann Surg 2005;241:715-22. [Crossref] [PubMed]
- Adam R, Delvart V, Pascal G, et al. Rescue Surgery for Unresectable Colorectal Liver Metastases Downstaged by Chemotherapy: A Model to Predict Long-term Survival. Ann Surg 2004;240:644-57; discussion 657-8. [PubMed]
- Ruers T, Van Coevorden F, Punt CJ, et al. Local Treatment of Unresectable Colorectal Liver Metastases: Results of a Randomized Phase II Trial. J Natl Cancer Inst 2017. [Crossref] [PubMed]
- Van Cutsem E, Cervantes A, Adam R, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol 2016;27:1386-422. [Crossref] [PubMed]
- Puijk RS, Ruarus AH, Vroomen LGPH, et al. Colorectal liver metastases: surgery versus thermal ablation (COLLISION) - a phase III single-blind prospective randomized controlled trial. BMC Cancer 2018;18:821. [Crossref] [PubMed]
- Karapetis CS, Khambata-Ford S, Jonker DJ, et al. K-ras Mutations and Benefit from Cetuximab in Advanced Colorectal Cancer. N Engl J Med 2008;359:1757-65. [Crossref] [PubMed]
- Vauthey JN, Zimmitti G, Kopetz SE, et al. RAS Mutation Status Predicts Survival and Patterns of Recurrence in Patients Undergoing Hepatectomy for Colorectal Liver Metastases. Ann Surg 2013;258:619-26. [Crossref] [PubMed]
- Odisio BC, Yamashita S, Huang SY, et al. Local tumour progression after percutaneous ablation of colorectal liver metastases according to RAS mutation status: Local tumour progression after ablation of colorectal liver metastases. Br J Surg 2017;104:760-8. [Crossref] [PubMed]
- Petre EN, Sofocleous C. Thermal Ablation in the Management of Colorectal Cancer Patients with Oligometastatic Liver Disease. Visc Med 2017;33:62-8. [Crossref] [PubMed]
- Solbiati L, Goldberg SN, Ierace T, et al. Hepatic metastases: percutaneous radio-frequency ablation with cooled-tip electrodes. Radiology 1997;205:367-73. [Crossref] [PubMed]
- Poulou LS. Percutaneous microwave ablation vs. radiofrequency ablation in the treatment of hepatocellular carcinoma. World J Hepatol 2015;7:1054. [Crossref] [PubMed]
- Ahmed M, Solbiati L, Brace CL, et al. Image-guided Tumor Ablation: Standardization of Terminology and Reporting Criteria—A 10-Year Update. Radiology 2014;273:241-60. [Crossref] [PubMed]
- Sofocleous CT, Petre EN, Gonen M, et al. CT-guided Radiofrequency Ablation as a Salvage Treatment of Colorectal Cancer Hepatic Metastases Developing after Hepatectomy. J Vasc Interv Radiol 2011;22:755-61. [Crossref] [PubMed]
- Gillams A, Goldberg N, Ahmed M, et al. Thermal ablation of colorectal liver metastases: a position paper by an international panel of ablation experts, the interventional oncology sans frontières meeting 2013. Eur Radiol 2015;25:3438-54. [Crossref] [PubMed]
- Veltri A, Sacchetto P, Tosetti I, et al. Radiofrequency Ablation of Colorectal Liver Metastases: Small Size Favorably Predicts Technique Effectiveness and Survival. Cardiovasc Intervent Radiol 2008;31:948-56. [Crossref] [PubMed]
- Shady W, Petre EN, Gonen M, et al. Percutaneous Radiofrequency Ablation of Colorectal Cancer Liver Metastases: Factors Affecting Outcomes—A 10-year Experience at a Single Center. Radiology 2016;278:601-11. [Crossref] [PubMed]
- Wang X, Sofocleous CT, Erinjeri JP, et al. Margin Size is an Independent Predictor of Local Tumor Progression After Ablation of Colon Cancer Liver Metastases. Cardiovasc Intervent Radiol 2013;36:166-75. [Crossref] [PubMed]
- Hammill CW, Billingsley KG, Cassera MA, et al. Outcome after laparoscopic radiofrequency ablation of technically resectable colorectal liver metastases. Ann Surg Oncol 2011;18:1947-54. [Crossref] [PubMed]
- Solbiati L, Ahmed M, Cova L, et al. Small liver colorectal metastases treated with percutaneous radiofrequency ablation: local response rate and long-term survival with up to 10-year follow-up. Radiology 2012;265:958-68. [Crossref] [PubMed]
- Hamada A, Yamakado K, Nakatsuka A, et al. Radiofrequency ablation for colorectal liver metastases: prognostic factors in non-surgical candidates. Jpn J Radiol 2012;30:567-74. [Crossref] [PubMed]
- Bale R, Widmann G, Schullian P, et al. Percutaneous stereotactic radiofrequency ablation of colorectal liver metastases. Eur Radiol 2012;22:930-7. [Crossref] [PubMed]
- Shady W, Petre EN, Do KG, et al. Percutaneous Microwave versus Radiofrequency Ablation of Colorectal Liver Metastases: Ablation with Clear Margins (A0) Provides the Best Local Tumor Control. J Vasc Interv Radiol 2018;29:268-275.e1. [Crossref] [PubMed]
- Calandri M, Yamashita S, Gazzera C, et al. Ablation of colorectal liver metastasis: Interaction of ablation margins and RAS mutation profiling on local tumour progression-free survival. Eur Radiol 2018;28:2727-34. [Crossref] [PubMed]
- Yamashita S, Chun YS, Kopetz SE, et al. Biomarkers in colorectal liver metastases. Br J Surg 2018;105:618-27. [Crossref] [PubMed]
- Shady W, Petre EN, Vakiani E, et al. Kras mutation is a marker of worse oncologic outcomes after percutaneous radiofrequency ablation of colorectal liver metastases. Oncotarget 2017;8:66117-27. [Crossref] [PubMed]
- Jiang BB, Yan K, Zhang ZY, et al. The value of KRAS gene status in predicting local tumor progression of colorectal liver metastases following radiofrequency ablation. Int J Hyperthermia 2019;36:211-9. [Crossref] [PubMed]
- Passot G, Denbo JW, Yamashita S, et al. Is hepatectomy justified for patients with RAS mutant colorectal liver metastases? An analysis of 524 patients undergoing curative liver resection. Surgery 2017;161:332-40. [Crossref] [PubMed]
- Brudvik KW, Mise Y, Chung MH, et al. RAS Mutation Predicts Positive Resection Margins and Narrower Resection Margins in Patients Undergoing Resection of Colorectal Liver Metastases. Ann Surg Oncol 2016;23:2635-43. [Crossref] [PubMed]
- Viganò L, Procopio F, Cimino MM, et al. Is Tumor Detachment from Vascular Structures Equivalent to R0 Resection in Surgery for Colorectal Liver Metastases? An Observational Cohort. Ann Surg Oncol 2016;23:1352-60. [Crossref] [PubMed]
- Xu D, Wang HW, Yan XL, et al. Sub-millimeter surgical margin is acceptable in patients with good tumor biology after liver resection for colorectal liver metastases. Eur J Surg Oncol 2019. [Epub ahead of print]. [Crossref] [PubMed]
- Brudvik KW, Jones RP, Giuliante F, et al. RAS Mutation Clinical Risk Score to Predict Survival After Resection of Colorectal Liver Metastases Ann Surg 2019;269:120-6. [Crossref] [PubMed]
- Veltri A, Calandri M. Thermal ablation and systemic therapies in the metastatic liver: time for a "glocal" approach. Eur Radiol 2019;29:5042-4. [Crossref] [PubMed]
- Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol 1995;13:8-10. [Crossref] [PubMed]
- Reyes DK, Pienta KJ. The biology and treatment of oligometastatic cancer. Oncotarget 2015;6:8491-524. [Crossref] [PubMed]
- Hobbs GA, Der CJ. RAS Mutations Are Not Created Equal. Cancer Discov 2019;9:696-8. [Crossref] [PubMed]
- Poulin EJ, Bera AK, Lu J, et al. Tissue-Specific Oncogenic Activity of KRAS A146T. Cancer Discov 2019;9:738-55. [Crossref] [PubMed]
- Chun YS, Passot G, Yamashita S, et al. Deleterious Effect of RAS and Evolutionary High-risk TP53 Double Mutation in Colorectal Liver Metastases. Ann Surg 2019;269:917-23. [Crossref] [PubMed]


