Epidemiology of gallbladder cancer in India
Introduction
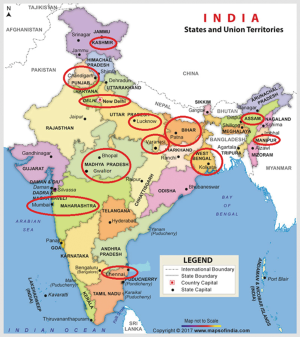
Gallbladder cancer (GBC) arises from the epithelial lining of the gallbladder (GB) and the cystic duct. It is the most common biliary tract malignancy worldwide and manifests as either diffuse thickening of the GB wall or as a GB mass arising from the fundus, neck or body of the GB (1). The incidence of this malignancy is characterized by marked geographical and ethnic variations (2). North, East, Northeast and Central India are among the high incidence areas for gallbladder in contrast to South and West India (3). The incidence in North India is 10–22/100,000 population and is similar to that of other countries with high incidence such as in South America (Chile, Bolivia, Columbia). East Asia (Korea, Japan, China) and central Europe (Slovakia, Poland, Czech Republic) are regions with moderate incidence (Figure 1) (4). The adjoining countries in the Indian sub-continent like Pakistan, Nepal, Bangladesh and Bhutan also have reported high incidence of GBC (5-11). However, Sri Lanka, Maldives, Yemen, Afghanistan, Tajikistan, Turkmenistan, Uzbekistan have low incidence of GBC. Certain ethnic groups like Hispanics, American Indians, Mexican Indians, Alaskan natives as well as Asian Indians are at more than normal risk for development of GBC (2,12-14).

Its clinical presentation is often non-specific resulting in significant delay in diagnosis. It is either detected incidentally at the time of cholecystectomy or when it presents with complications due to local spread of the malignancy in the form of jaundice, hepatomegaly, ascites or duodenal obstruction (15). Aggressive biological nature of the tumor results in rapid spread of the tumor to adjoining vital structures since the GB is located in an anatomically busy area (16). The tumor is thus, often unresectable at presentation resulting in an overall dismal prognosis in India (15). Moreover, chemotherapy, radiotherapy and immunotherapy are not particularly curative. The 5-year survival rate is often <5% in most centers (15). Also, there are no screening programs in place because it is difficult to detect early tumor since the gallbladder mucosa is not amenable to direct endoscopic inspection in contrast to other luminal organs. The radiological features in the form of GB wall thickening are largely non-specific and may masquerade as chronic cholecystitis (17). Studies to understand this fatal disease have been undertaken over the last two decades, which has thrown some more light on understanding of the pathogenesis of this enigmatic disease.
Incidence
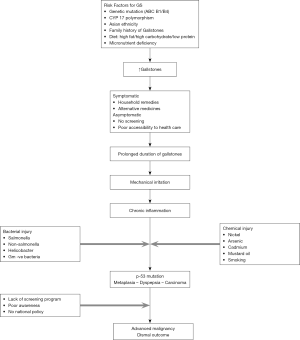
India is a high incidence area for GBC. GBC is one of the three leading cancers among women of North and North-east India. The age standardized rate (ASR) for GBC in women of North and north-east India are 11.8/100,000 population and 17.1/100,000 population respectively (18) (Figure 2). It is similar to the high incidence areas such as Bolivia (14/100,000) and Chile (9.3/100,000) and higher than that found in other parts of Asia: Thailand (7.4), South Korea, Nepal (6.7) and Bangladesh (5.1) per 100,000 population (19). The incidence has been steadily rising in India among women as well as men. The average age-adjusted rate among women has increased from 6.2/100,000 in 2001–2004 to 10.4/100,000 in 2012–2014 (20). This data is from 30 population-based cancer registries from all over India, which were set up by the Indian Council of Medical Research ICMR (18). The incidence rate has shown a rise in Mumbai registry, due to persons ethnically belonging to high incidence area migrating to Mumbai either for employment or for treatment.
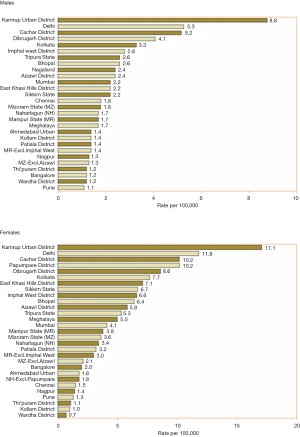
Among the Asian countries, higher number of new cases was registered in China, Japan, India, Republic of Korea and Bangladesh. These five countries represent 88% of all GBC seen in Asia. India accounts for 10% of the global burden of GBC. Among the Asian countries, Maldives, Yemen, Tajikistan, Turkmenistan and Uzbekistan have less than 0.1/100,000 age standardized incidence rate (14). Rising trend in the incidence of GBC in India contrasts strikingly with its decreasing incidence in north American and western European countries (14,21) (Figure 3).
Pathogenesis of GBC in India
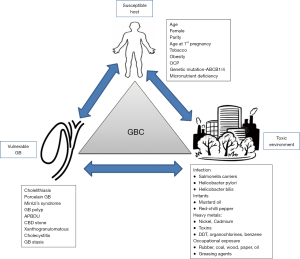
The pathogenesis of GBC is poorly understood in its entirety in India even today. It seems to be a multi-step process in which there is accumulation of genetic and epigenetic alterations due to host as well as environmental factors. These cumulative genetic alterations ultimately trigger mutagenesis. It is due to combination of a vulnerable GB interacting with a toxic environment in a susceptible host (Figure 4). The GB is already a physiologically a vulnerable organ of our body due to the following reasons. It an out pouching of the GI tract and has to empty its content against gravity back into the gut lumen. The fundus of the GB is dependent part in biped erect human beings Fundus is the commonest site for GBC possibly due to higher mucosal contact time. The GB is also an excretory organ of the body as the liver flushes all the environmental toxins and their metabolites into the biliary system. The GB tends to concentrate bile which allows increased mucosal contact to the concentrated toxic substances. A diseased GB also tends to be static, enhancing the mucosal exposure time. The GB is also dependent on a fully functional digestive process, intact endocrine signaling pathway with release of CCK by the duodenum to facilitate its emptying. The GB has a weak muscular structure which further disadvantages the emptying process. Moreover, obstruction along the pathway for flow of bile due to CBD stone, stricture, sphincter of Oddi abnormalities can impede the emptying process. Moreover, bacteria once it enters the GB, is difficult for the body to eradicate. These bacteria tend to cause chronic inflammation, deconjugate conjugated bile acids and toxins and thus locally release toxic metabolites. Presence of GS adds to the vulnerability further of the GB since emptying is more often incomplete in those with chronic cholecystitis which is a constant accompaniment of GSD. The presence of stones adds to the surface area for bacterial colonization and these bacteria are difficult eradicate with antibiotic therapy. Stones also result mechanical injury. Thus, stones promote chronic inflammation.
Chronic inflammation results in mutagenesis especially of the p-53 pathway in India. Studies from India have shown that p53 mutations are detected in 70% (22). Patients with GS more often have metaplasia and dysplasia compared to those without GS. Moreover, malnutrition associated with micronutrient deficiency probably weakens the immune defenses which reduces immune surveillance for cancer (23).
Thus, a combination of multiple factors may act in tandem to promote carcinogenesis.
Risk factors for GBC
Geographical region
The marked geographical variations in the incidence of this malignancy suggest ethnic predisposition or presence of local environmental risk factors. In India, the incidence of GBC is 10 times higher in north India compared to the southern Indian states [8.9/100,000 population (Delhi) vs. 0.8/100,000 population (Chennai)] (18). The age standardized incidence rate of GBC showed that the incidence rate was high in northern and eastern India (7–14/100,000 population) compared to south and western India (<1/100,000 population). Amongst patients living in north, eastern and central India the risk for developing GBC is higher than that among patients living in southern India (OR 4.82; 95% CI: 3.87–5.99). The ICMR population-based registry (2009–2011) clearly divides India into high risk area and low risk area for GBC (24). The regions have been classified as high risk if the AAR is >5/100,000 population. The AAR varies from 0.2 to 17.1 per 100,000 population in different regions of the country. In a study by Tata Memorial Hospital Mumbai, residence in high-risk areas and period of residence in these regions was associated with increased risk for GBC. In western India, it is detected more often among people who have migrated from high risk areas rather than natural residents of the region. Migration from high risk region to low risk region resulted in individuals carrying higher susceptibility even when they moved to lower risk regions (OR 1.36; 95% CI: 1.02–1.82) (3). The risk was maximum amongst those who always lived-in high-risk region compared to those who never lived in high-risk region (OR 5.58; 95% CI: 4.42–7.05). Indians migrating to other countries also carry high risk for GBC. Studies done in Kuwait, United Kingdom have shown that Indian immigrants are at higher risk for GB malignancy as compared to the native population of these countries (12).
There are various putative factors which have been proposed to explain partly the differences in the incidence across the country (25-34). The quality of evidence for these factors is limited as they come from small case-controlled studies and requires further larger multi centric studies. The high-risk regions extend from the states of Jammu and Kashmir, Punjab, Haryana, Himachal Pradesh, Uttarakhand, UP, Bihar, Bengal, Assam and Manipur. A large part of these states is based along the major rivers of the country namely Sutlej, Ganges, Yamuna and Brahmaputra (Figure 5). These rivers arise from the glaciers and flow from the northern Himalayas towards west and east and have become polluted due to human waste and industrial pollutants. As the Ganges flows towards east, the pollutants concentration as well as bacterial contamination have been found to steadily rise which may account partially for high incidence in this gangetic region of the country. It is also an agricultural driven community. The Ganges supports a very densely populated human civilization on its banks, especially the poorer sections, which subsist on the river for its daily water needs. Untreated sewage, industrial waste and agricultural effluents unfortunately get added to the water along its course (32). The fecal coliform count steadily rises as the river flows towards the east (30). Salmonella typhi (S. typhi) and Helicobacter pylori (H. pylori) are feco-orally transmitted organisms which have been known to be associated with pathogenesis of GBC and are likely to be increased as the river flows downstream (27,30,35-37). In North, North east and eastern India, mustard oil is the staple cooking oil in contrast to coconut oil, sesame or groundnut oil in south and west India. Mustard oil has irritant property on the gut and is often adulterated with butter yellow which is known carcinogen (33). Individuals belonging to the poorer socioeconomic strata are unable to afford branded safe oils and thus consume loose mustard oils which may be contaminated/adulterated. Higher levels of sanguinarine and diethyl nitrosamine, carcinogenic adulterants in mustard oil, have been found in blood and tissue of GBC patients as compared to patients with cholelithiasis. Diethyl nitrosamine has been reported to induce hepatic carcinogenesis. Mustard oil has pro-inflammatory properties and induces tumors (33).
High level of pesticides, heavy metals and nitrates both of which are carcinogenic have been identified as pollutants in Ganges (25). Also, patients with GBC have higher biliary concentration of these pesticides and nitrates compare to those with gallstones without GBC (25). The reasons for low incidence in south and west India are unclear. Role of diet, H. pylori (incidence and virulence) water and soil contamination needs to be systematically assessed.
Age and gender
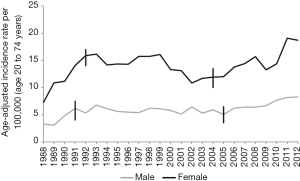
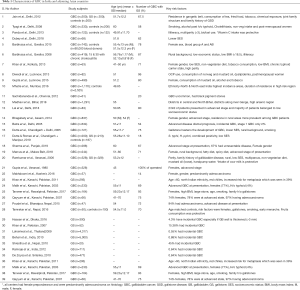
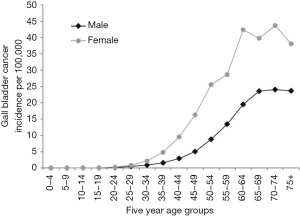
The mean age of presentation of GBC in India is younger than their counterparts in the USA and western European countries. The average age at diagnosis in India was 51±11 years in contrast to 71.2±12.5 years in the West (4–6). Age distribution of patients with GBC seen in various large series from India and adjoining countries are summarized in a tabular format (Table 1). The mean/median age is usually 50–55 years. Increasing age is associated with increasing risk for GBC (38) (Figure 6). The age at presentation of GBC in India is a decade earlier than their western counterparts, but is similar to that in the Latin American countries. This is possibly due to presence of multiple risk factors which act in an additive manner (39). Many patients with GBC have more than 1 risk factors which all may act in tandem thereby hastening the pathogenesis of GBC. The incidence of GBC with respect to age groups shows a rising trend from 30 years onwards (Figure 6). Hence, a high index of suspicion is required even in younger patients in high incidence areas. There is an increasing trend in GBC incident rates in urban Delhi region in both genders (Figure 3) (38).
Full table
Women are at 2–6 times higher risk for developing GBC (40). The observed risk is higher among women compared to men (OR 6.04; 95% CI: 4.52–8.07 versus OR 3.17; 95% CI: 2.23–4.50). Among patients with gallstones, women are at 2.4 times higher risk for GBC (41). The female:male ratio varies from 3:1 to 4.5:1 in various Indian series (42). However, a study from South India a low incidence area had a male: female ratio of 1.6:1 (43). Women are exposed to higher levels of estrogen and progesterone during their lifetime more so during pregnancies. Indian women are younger in age at the time of marriage, younger in age at time of 1st pregnancy and have a higher number of pregnancies than their western counterparts in developed countries. In a recent compilation of data from Asia comprising of 116,048 patients the female:male ratio is 1.2:1 at presentation (14). The GB mucosa has been found to have estrogen and progesterone receptors which may promote GB stasis, stone formation, and this in turn increases exposure time of the GB mucosa to bacterial and chemical toxins (44). Women in India, also are less educated, have less access to economic resources, lesser access to nutrition and poorer access to medical care. These factors may further marginalize them in a socio-economically poor strata. Women in India also tend to be undernourished and thus are likely to have suboptimal immune status and micronutrient deficiencies both of which promotes carcinogenesis.
Gallstones
Gallstones were noticed to be associated with GBC since early 19th century. However, though the constant association has been verified in various studies all over the globe, the cause and effect has been a matter of debate. Incidence of symptomatic gallstones is 20 times higher in north India compared to South India. The nature of stone in north India is of predominantly cholesterol/mixed compared to South India which has pigment stones (45). Various studies in India have documented presence of gall stone in 70–90% of patients with GBC. Table 1 (15,34,39). Certain studies have a lower rate, possibly due problems with detecting them on ultrasound when they are entrapped within a mass. Surgical series have usually found higher prevalence of gallstone in association with GBC (46).
Whether gallstones are coincidental finding or a co-factor in the pathogenesis of GBC needs careful examination (16). There is little doubt that the association of gallstones in patients with GBC is real and not due to random chance, since it has been substantiated in various case control as well as cohort studies across the globe. The association could be co-incidental, causative, or could be due to reverse causation. Reverse causation is unlikely, because once GBC develops, the survival of the patient is significantly shortened leaving little time for development of large gallstones which are usually seen in these patients. After development of GBC, patients are anorexic and on low lithogenic diet and thus are unlikely to develop gallstones (46). A diseased gallbladder in the setting of GBC is likely to be hypomotile and hence may accumulate sludge rather than develop stones
The association is likely to be causative rather than co-incidental because the Bradford Hill criterion for causation seems to be fulfilled. Firstly, the strength of association has been high namely odds ratio of 5–7 nearly in various case control and cohort studies (16). The age-adjusted risk for GBC in men and women with gallstones was 16.2 and 46.4 in American Indians, 2.3 and 3 in American Blacks, 4.5 and 11.5 in Swedish whites respectively (12,14). Secondly, temporality is established by the fact that in cohort studies the development of stones precedes the development of GBC (47). After the onset of GBC, it is unlikely for such large stones (2–3 cm) to develop in a fairly short span of time. Untreated GB Cancer usually progresses rapidly over 6–12 months. It is not possible to develop such large stones within 6 months once GBC develops, since GBC progresses very rapidly. The consistency of the association has been documented by different researchers, in different geographical areas, with different robust study designs. The dose response is established by virtue of the fact that increasing stone size, increasing duration, multiple gallstones, higher stone volume (>6 mL) are all associated with higher risk for GBC (16). Complicated gallstones in the form of presence of, Mirrizi’s syndrome as well as xanthogranulomatous cholecystitis is associated with increased risk of GBC (12). There is experimental evidence in Syrian hamsters, in whom on insertion of beeswax pellets into the gallbladder lumen, GBC was found to develop in 54% of them starting from 21 weeks of exposure. Carcinoma gallbladder in hamsters was induced by insertion of Cholesterol pellets and feeding oral dimethyl nitrosamine (48). The specificity of the association also exists in the form of high presence of gallstones in 60–90% of patients with GBC in contrast to other diseases. The coherence is established by the fact that there is high incidence of pre-neoplastic lesions among patients with gallstones compared to those without gallstones (49). Also, the prevalence of GBC parallels the prevalence of gallstones in the region (2) (Figure 7). Moreover, the prevalence of pre-neoplastic lesions is higher in high incidence area of GBC compared to low incidence area for GBC (49,50). In our study, which has been just completed in 200 patients with GBC, 213 patients of gallstones and 187 patients of dyspepsia across two centers in North India and North East India, we found that metaplasia was present in 86% and 64% of routine cholecystectomy specimen for symptomatic gallstone of patients operated. In another study from north India, 48% of patients had metaplasia and 16% had dysplasia among patients with gallstones undergoing routine cholecystectomy (41). The prevalence of pre-neoplastic lesions in Chile is very high (95%) and very low in Canada (<1%); which is in keeping with the local incidence of GBC (49,50). Thus, there is enough evidence to suggest that gallstones are causative rather than just incidental in patients with GBC. Gallstones may promote GBC by causing direct mechanical injury of the GB mucosa during GB contraction especially when the stones are large, irregular, and occupy a larger volume of the GB (16). Also, gallstone surface may provide a surface to form bacterial bio-films which allow persistence of the bacteria. Often bacteria such as salmonella once they are established in the GB, are difficult to eradicate with antibiotic treatment in the presence of GS. Cholecystectomy is advocated in patients who have S. typhi carrier state and GS (51). Pathogens such as H. pylori and S. typhi are known for their potential to initiate carcinogenesis (51-54). The chemical composition of bile may also be altered due to deconjugation of bile acids, conjugated toxins, and accumulation of heavy metals and metabolites in the GB which may be compounded by biliary stasis often found to accompany in patients with gallstones (25,32). Thus, gallstones may be contributing to a multiple hit processes in the development of GBC in India.
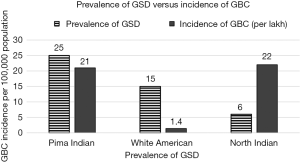
In India, the incidence of GBC is out of proportion to the prevalence of gallstones indicating that co-factors may play a significant role in the development of GBC (2,12) (Figure 8). Gallstones alone, in the absence of cofactors, may result only in mechanical injury which the GB mucosa may recover by natural healing process. Combination of multiple repeated insults of varying nature may overwhelm the tissue repair mechanism giving way to chronic inflammation, mutagenesis and carcinogenesis. Poor nutritional status and micronutrients deficiency may also result in poor immune surveillance to check tumor development. Thus, in India, GS is likely to playing a sizable role in causing of GBC along with multiple factors. Thus, removal of gallbladder, which is harboring GS, even if the patient is asymptomatic in high risk area, may be a worthwhile step in some selected cases in preventing GBC related mortality (16).
Obesity
Obesity, body mass index (BMI) of >30, is associated with two times increased risk for GBC. The relative risk is 1.88 (95% CI: 1.66–2.13). Zatonski et al. in a large multicentric study showed that obesity is associated with increased risk for GBC and the adjusted relative ratio (RR) was 2.1 (95% CI: 1.2–3.8) between the highest quartile and the lowest quartile for BMI (13). The increase in risk for GBC for 1 unit increase in BMI was 1.06 (55). Various studies from the India, have been of case-control design. Patients with GBC have lower BMI than those with GSD or healthy control counterparts. This low BMI is reflective of weight loss secondary to malignancy but most patients are not reported to be obese to start with. In a large population based case control study from India involving 333 patients with GBC, BMI in fact showed an inverse relationship with GBC (56). There is no cohort study, which has examined this issue. Undernutrition, however, may be associated with suboptimal immune status and a pro-inflammatory state secondary to micronutrient and anti-oxidant deficiency; both of which in turn can promote malignancy.
Parity
Higher parity is associated with increased risk with GBC globally as well as in India. Age adjusted relative risk (RR) for parity ranges from 1.3 to 4.2 (26,35,57). Parity was higher in GBC when compared to patients with gallstones (5.5±2 vs. 3.3±2, P=0.001) (35). Increasing parity was associated with increasing risk (2,26). In a large population based case control study, more than four pregnancies were associated with increased risk for any gallbladder diseases (RR 1.86; 95% CI: 1.3–2.65) when compared to healthy controls (58). Another study from Varanasi, the median number of pregnancies was 6 in contrast to 4 among patients with gallstones (OR 6.66; 95% CI: 1.8–23.4). Postmenopausal status was associated with increased risk for GBC (OR 3.17; 95% CI: 1.56–6.47) (56). Thus it is not surprising that, Indian women with higher parity rates than their global counterparts are at an increased risk of GBC.
Family history
History of GBC or gallstone disease (GSD) in first degree relatives has been associated with increased risk for GBC by 5 times (summary RR 4.8; 95% CI: 2.6–8.9) (2). Hsing et al. has shown that family history of gallstones is associated with 5.3 times increased risk for GBC (95% CI: 1.5–18.9) (59). In a large population based study in Gangetic belt, family history of gallbladder disease is associated with increased gallbladder disease (OR 1.79; 95% CI: 1.3–2.4) (58). Kumar et al. from Varanasi demonstrated that family history of biliary disease is associated with increased risk for GBC (OR 3.48; 95% CI: 1.38–8.98) (36).
Rural residence
Residence in rural areas is associated with increased risk for GBC. It has been assessed in various case control studies from different regions of the country. Study by Kumar et al. found that 80% of patients with GBC resided in rural areas compared to 54% patients with gallstones (OR 3.52; 95% CI: 2.48–4.99) (36). In another study in Delhi, residence in rural area was 59% versus 32% among patients with gallstones (35).
Barbhuiya et al. suggested that the incidence in rural population was 5.56/100,000 population in comparison to 3.62/100,000 population amongst urban population (RR 1.62; 95% CI: 1.4–1.8) (60). Rural residence is associated with lower literacy rates, poorer socio-economic status and poorer access to medical care in India. Though a large proportion of Indians live in rural areas, these studies which compares patients with GBC and GSD, reflects the additional risk conferred by rural residence to our patients with GBC.
Socio-economic status
Various studies in India have suggested that patients with GBC are likely to belong to lower socio-economic status. Lower socio-economic status has been associated with increased risk for GBC in Chile as well as in India (2). In a study by Dutta et al. in north India, 32% of patients belong to lower socio-economic status compared to 11% patients of gallstones 35). Socio-economic status was defined according to Kuppuswamy scale, which has been standardized for India. In another study by Dubey et al., 75% of patients with GBC belong to lower-middle or lower-socio-economic class (61). Low socio-economic status is associated with lower literacy rate, overcrowding, poor access to health care, poorer sanitation and poorer access to clean drinking water than those belonging to upper socioeconomic status (SES). It is, hence, associated to higher exposure to feco-oral infections like S. typhi and H. pylori (30,36). Certain studies have evaluated the literacy rate among patients with GBC and found that lower literacy rate is associated with higher RR for GBC (1.49, 95% CI: 1.3–1.7) (60).
Helicobacter infection
Helicobacter species has been associated with increased risk of GBC. The odds ratio ranges from 2.7–12 in various series from India and globally (52-54). Various methods have been used to evaluate H. pylori, which include rapid urease test in gastric biopsy, H. pylori serology, PCR in bile and gallbladder tissue. All studies from India have shown a definite but small risk of H. pylori in the causation of GBC (Table 2). However, a study from Japan showed a strong association between H. bilis and GBC (OR 6.5) in comparison to patients with other gallbladder diseases. H. bilis in contrast to H. pylori is resistant to the action of bile and survives in the gallbladder for long duration (53). Mishra et al. found that the prevalence of H. pylori in patients with GBC was higher than that in gallstone (54). We also found that prevalence of H. pylori in GBC was significantly higher than controls (59% vs. 20%) in our recently concluded bicentric study between Chandigarh (North India) and Manipur (north east India) (unpublished data). However, the prevalence among gallstones was also 64%. In studies, where prevalence of H. pylori is compared between patients with GBC and healthy controls, it has been consistently found that patients with GBC have higher H. pylori prevalence, however, when the control group comprises of gallstone patients, the prevalence has been usually similar or higher. This may indicate that Helicobacter infection may be also associated with GSD which may narrow the difference between GBC and GS. It may also just be a marker of high feco-oral transmission in this sub-group of patients. H. pylori positivity was higher in the areas with gastric metaplasia in the gallbladder mucosa (62). H. pylori, especially the virulent strains induce a pro-inflammatory state, results in detrimental immune alterations for the hosts and hence promotes carcinogenesis in the same lines as gastric cancer pathogenesis (63,64). We feel that the pathogenesis of GBC seems to be more akin to gastric cancer rather than colon cancer.

Full table
S. typhi infection
Several studies have studied the association of S. typhi infection and GBC. Follow up of patients from the Aberdeen outbreak suggested that S. typhi carrier state was associated with 167 times increased risk for hepatobiliary cancer (65). Various studies from Chile, have demonstrated the role of S. typhi carrier state as a risk factor for GBC (66). Recent meta-analysis which evaluated 17 studies showed that summary OR was 3.5 (95% CI: 2.48–5) for studies using serology and 4.1 (95% CI: 2.41–7.12) for studies using culture techniques (51). Recent studies on the role of non-typhoid salmonella, have reported their presence in the gallbladder tissue on PCR in patients with GBC (67). These species of salmonella are resistant to common antibiotics and result in persistent GB inflammation. Salmonella group of organisms is associated with mutagenesis as they deconjugate bile acids and metabolites rendering them as highly active intermediates which bind to DNA. All previous studies from India on role of S. typhi have been summarized in table below (Table 3). From an epidemiological perspective the prevalence of S. typhi infection is higher in low and middle income countries especially among the lower socio-economic strata (68).

Full table
Smoking
Smoking has been seen to be associated with increased risk of GBC in various studies globally as well as in India. In our previous study, smoking was an independent risk factor for GBC and the summary RR was 11 (95% CI: 1.7–71) for those who smoked more than 10 cigarettes per day for minimum 5 years compared to non-smokers (35). In a meta-analysis published by Aune D, they found a dose of response in relationship of smoking and GBC (69). In another study from east India, chewing tobacco was associated with increased risk for GBC (41,70).
Chemical exposure
In a large population-based cohort study conducted in states of Bihar and Uttar Pradesh in which the soil and water analysis was performed to assess levels of nickel, cadmium, chromium and Dichloro diphenyl trichloroethane (DDT) (32). It was found that the levels of these pollutants were higher in those regions with higher prevalence of GB diseases in the soil as well as water samples (58).
Pandey et al. also showed higher presence of heavy metals and toxins in the GB bile among patients with GB stasis compared to those without GB stasis (26). The leather tanneries in the city of Kanpur also release heavy metal compounds into the flowing river. These pollutants tend to be excreted by the liver in the conjugated detoxified form into the bile. The gallbladder tends to concentrate these toxins. In the presence of bacteria, which release beta glucuronidase enzyme, the conjugated toxins get deconjugated, rendering them toxic to the mucosa. Exposure to mining, radon gas, rubber industries, paint, chemicals, paper and wood industries have been associated with increased risk for GBC (4,16,71).
Structural biliary abnormalities
Anomalous pancreaticobiliary junction is a congenital malformation in which the pancreatic duct joins the biliary duct outside the duodenal wall. It has been associated with increased risk of GBC in Japan as well as in other East Asian countries and warrants prophylactic cholecystectomy. The reflux of pancreatic juice into the gallbladder results in chemical irritation in gallbladder mucosa and K-ras mutation and resultant papillary adenocarcinomas (16). Pancreatic enzymes and secondary bile acids result in chronic mucosal injury which results in hyperplasia and dysplasia. Patients with APBDU and GBC tend to be younger and have lower prevalence of gallstones (72). In India, however, the prevalence of anomalous pancreaticobiliary junction is very low. In a study from our center, of 3,827 endoscopic retrograde cholangiopancreatography (ERCPs), only 2.6% had APBDU (73). Studies from Japan have suggested that a fair number of patients with GBC have APBDU (74).
GB polyps are present in around 5% of adult patients. Most of them (95%) are non-neoplastic in nature. Benign adenomas constitute <5% of all GB polyps and their size ranges from 0.5–2 cm. Presence of large polyps (>10 mm), sessile polyps, solitary polyps, associated gallstones, older age, rapidly increasing size suggests neoplastic nature of the polyp (75). Endoscopic ultrasound is useful in differentiate benign versus malignant polyp. Presence of hypoechoic, heterogenous lesion with height/width ratio (0.8), increased vascularity suggests increased risk for neoplasia (76). If the polyps are suggestive of neoplastic polyp, there is associated wall thickness or family history of malignancy such polyps are best removed by cholecystectomy. Other polyps need close 3–6 months follow up to assess for increase in polyp size (75,76).
Chronic inflammation of the gall bladder wall leads to dystrophic calcification imparting a bluish tinge to the gallbladder. It is also fragile and has been hence termed as ‘porcelain’ gallbladder. The calcification may be focal or diffuse. It is prevalent in 0.1–0.2% of patients with GB disease. The prevalence of GBC in older reports was as high as 25% in patients with porcelain gallbladder. However, a recent systematic review of 111 studies suggest that rate of GBC is only 6%. Gallbladders with focal, stippled or multiple punctate calcification, those with associated wall thickness and symptomatic porcelain gallbladder are more likely to harbor malignancy and thus may benefit from prophylactic cholecystectomy (77,78). However, there is no data available in India on this aspect.
Primary sclerosing cholangitis is associated with increased risk for biliary cancers, cholangio-carcinoma as well as GBC. Chronic biliary inflammation in this condition promotes carcinogenesis. These patients need annual surveillance for GBC and are likely to benefit with prophylactic cholecystectomy (76). There are no studies in India regarding this association.
Medications
Role of oral contraceptives in GBC has been studied extensively globally, but not to a significant extent in Indian studies. In a study from Lucknow, 87% of the GBC cases were oral contraceptive users (79) Methyl dopa and isoniazid have been implicated, however, the association is weak and there are no studies from India to substantiate the association (71).
Genetic factors
Genetic factors have been studied extensively in the last decade. p-53 mutation is the dominant pathway for development of GBC. In India, 50–70% the tumor show overexpression of p-53 (22,80). Exome sequencing of GBC has found ERBB pathway to be most dysregulated pathway in this disease. CERBB2 mutation was found in 9.4% and is associated with poor outcome. Study in North India identified K-ras mutation in codon 13 instead of codon 12 or 61 contrary to findings seen globally (81). HER2/neu overexpression was found in 14% which had therapeutic implications for molecular targeting. Micro satellite instability was detected in 10% of patients with GBC (82). In another study from North India loss of heterozygosity (LOH) was found more often in pre-neoplastic lesions than those without pre-neoplastic lesions (83). In a large cohort study of 1,042 GBC patients from West India, genome-wide associations were found in the chromosome regions 7q21.12 which harbors ABCB1 and ABCB4 genes. The most common SNP on meta-analysis was rs1558375, rs17209837 and rs4148808. Genome-wide association studies (GWAS) heritability analysis suggested that the risk of GBC in a sibling was with ABCB1 and ABCB4 mutations was 3 times higher (RR 3.15; 95% CI: 1.8–5.49). This suggests the importance of hepatobiliary phospholipid transporter genes in the pathogenesis of GBC possibly by increased risk for GS. Over 1,281 mutations have been identified in GBC, however, this significance is yet to be ascertained (84). Comparison of genetic alterations identified in India viz-a-viz the global status is reviewed in detail in a recent article by Sharma et al. (84).
Caveats in managing patients with gallstones in India
Prevalence of GBC is of endemic proportions in certain parts of India and the incidence is still on the rise. We have an incidence rate similar to that of Latin American countries such as Chile and Bolivia. They have adopted the policy of screening for gallstones among women >40 years and men >50 years. The incidence of GBC is low in Western Europe and Northern America hence they have adopted the policy of expectant management in patients with asymptomatic stones. In India, due to lack of cohort studies, there is no uniform policy on management of gallstone and hence treatment has to be individualized. Patients living in remote areas, patients with life expectancy >20 years, gallstones >30 mm, those with a non-functioning gallbladder, family history of malignancy and those with GB polyps and, those on transplant programs should be preferably offered prophylactic cholecystectomy (85). Patients with symptomatic gallstones should be advised early cholecystectomy. They should be educated to refrain from alternative therapies and house hold remedies, and seek medical attention at the earliest. In India, there is a large pool of expertise available for safe laparoscopic cholecystectomy nowadays, and hence decision for prophylactic cholecystectomy should be more liberal than restrictive. Among all the risk factors, gallstones are most easy to identify and target for preventing GBC. A subset of patients with GS who are at high risk for GBC may be offered prophylactic cholecystectomy.
Despite a high incidence of GBC in our country, incidental GBC is fairly underreported due to no active screening program to detect incidental GBC among patients with symptomatic gallstones undergoing routine cholecystectomy. These patients can be easily detected if there is a higher index of suspicion prior to, during as well as after surgery (86). Patients with gallstones undergoing routine cholecystectomy should a have a routine check list to identify those with red flags, so that they can be dealt with a higher index of suspicion.
To conclude, India is a high incidence area for GBC. GBC affects patients at a younger age than their western counterparts in developed nations. Within India, north, north east, east and central Indian regions are at higher risk. Environmental risk factors such as soil and water contamination by industrial wastes, agricultural effluents and human sewage have been identified as putative risk factors enhancing carcinogenesis among patients with GS of this region. Selected patients with GS in these regions are easily identifiable targets who may be offered prophylactic cholecystectomy to prevent GBC. Large multicentric comprehensive studies are required in India to assess the attributable risk of each of the identified putative risk factors. This will help in formulating cost effective national strategies in preventing GBC related mortality in the country.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Wistuba II, Gazdar AF. Gallbladder cancer: lessons from a rare tumour. Nat Rev Cancer 2004;4:695-706. [Crossref] [PubMed]
- Randi G, Franceschi S, La Vecchia C. Gallbladder cancer worldwide: geographical distribution and risk factors. Int J Cancer 2006;118:1591-602. [Crossref] [PubMed]
- Mhatre SS, Nagrani RT, Budukh A, et al. Place of birth and risk of gallbladder cancer in India. Indian J Cancer 2016;53:304. [Crossref] [PubMed]
- Hundal R, Shaffer EA. Gallbladder cancer: epidemiology and outcome. Clin Epidemiol 2014;6:99-109. [PubMed]
- Alvi AR, Siddiqui NA, Zafar H. Risk factors of gallbladder cancer in Karachi-a case-control study. World J Surg Oncol 2011;9:164. [Crossref] [PubMed]
- Khan MR, Raza SA, Ahmad Z, et al. Gallbladder intestinal metaplasia in Pakistani patients with gallstones. Int J Surg 2011;9:482-5. [Crossref] [PubMed]
- Malik IA. Clinicopathological features and management of gallbladder cancer in Pakistan: A prospective study of 233 cases. J Gastroenterol Hepatol 2003;18:950-3. [Crossref] [PubMed]
- Tanveer SM, Mukarram HS, Nayyar HS, et al. Incidental gallbladder cancer: Missing links in Pakistani population. Int J Hepatobiliary Pancreat Dis 2017;7:1. [Crossref]
- Qayyum A. Patients with Gall Bladder Cancer: A Clinical Experience. Pak J Med Sci 2007;23:298-300.
- Tamrakar D, Paudel I, Adhikary S, et al. Risk Factors for Gallbladder Cancer in Nepal a Case Control Study. Asian Pac J Cancer Prev 2016;17:3447-53. [PubMed]
- Hasan MM, Laila SZ, Mamun MMH. Incidence of Gallbladder Carcinoma in Thick Walled Gallbladder in Comparison with that of Normal Thickness - A Study of 300 Cases. J Bangladesh Coll Physicians Surg 2017;34:193-8. [Crossref]
- Kapoor VK, Mcmichael AJ. Gallbladder cancer: An ‘Indian’ disease. Natl Med J India 2003;16:209-13. [PubMed]
- Zatonski WA, Lowenfels AB, Boyle P, et al. Epidemiologic Aspects of Gallbladder Cancer: A Case-Control Study of the SEARCH Program of the International Agency for Research on Cancer. J Natl Cancer Inst 1997;89:1132-8. [Crossref] [PubMed]
- Mahdavifar N, Mohammadian-Hafshejani A, Ghafari M, et al. Incidence and mortality of gallbladder cancer and its relationship with human development index(HDI) in Asia in 2012. WCRJ 2017;4:e974.
- Batra Y, Pal S, Dutta U, et al. Gallbladder cancer in India: A dismal picture. J Gastroenterol Hepatol 2005;20:309-14. [Crossref] [PubMed]
- Dutta U. Gallbladder cancer: Can newer insights improve the outcome?: Gallbladder cancer: Newer insights. J Gastroenterol Hepatol 2012;27:642-53. [Crossref] [PubMed]
- Levy AD, Murakata LA, Rohrmann CA. Gallbladder Carcinoma: Radiologic-Pathologic Correlation. RadioGraphics 2001;21:295-314. [Crossref] [PubMed]
- National Cancer registry programme. Consolidated report of population based cancer registries: 2012-14 [Internet]. Available online: http://ncdirindia.org/NCRP/ALL_NCRP_REPORTS/PBCR_REPORT_2012_2014/index.htm
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Phadke P, Mhatre S, Budukh A, et al. Trends in gallbladder cancer incidence in the high- and low-risk regions of India. Indian J Med Paediatr Oncol 2019;40:90. [Crossref]
- Henley SJ, Weir HK, Jim MA, et al. Gallbladder Cancer Incidence and Mortality, United States 1999-2011. Cancer Epidemiol Biomarkers Prev 2015;24:1319-26. [Crossref] [PubMed]
- Mishra PK, Jatawa SK, Raghuram GV, et al. Correlation of aberrant expression of p53, Rad50, and cyclin-E proteins with microsatellite instability in gallbladder adenocarcinomas. Genet Mol Res 2009;8:1202-10. [Crossref] [PubMed]
- Elmadfa I, Meyer AL. The Role of the Status of Selected Micronutrients in Shaping the Immune Function. Endocr Metab Immune Disord Drug Targets 2019. [Epub ahead of print]. [Crossref] [PubMed]
- Murthy NS, Rajaram D, Gautham MS, et al. Trends in incidence of gallbladder cancer – Indian scenario. Gastrointestinal Cancer: Targets and Therapy 2011;1:1-9.
- Shukla VK, Prakash A, Tripathi BD, et al. Biliary heavy metal concentrations in carcinoma of the gall bladder: case-control study. BMJ 1998;317:1288-9. [Crossref] [PubMed]
- Pandey M. Risk factors for gallbladder cancer: a reappraisal Eur J Cancer Prev 2003;12:15-24. [Crossref] [PubMed]
- Nath G, Singh YK, Kumar K, et al. Association of carcinoma of the gallbladder with typhoid carriage in a typhoid endemic area using nested PCR. J Infect Dev Ctries 2008;2:302-7. [Crossref] [PubMed]
- Pandey M, Shukla VK. Diet and gallbladder cancer: a case-control study. Eur J Cancer Prev 2002;11:365-8. [Crossref] [PubMed]
- Gupta SK, Shukla VK. Gallbladder cancer etiopathology and treatment. Health Administrator 2005;XVII:134-42.
- Pandey M, Vishwakarma RA, Khatri AK, et al. Bile, bacteria, and gallbladder carcinogenesis. J Surg Oncol 1995;58:282-3. [Crossref] [PubMed]
- Rehana Z, Malik A, Ahmad M. Genotoxicity of the Ganges water at Narora (U.P.), India. Mutat Res 1996;367:187-93. [Crossref] [PubMed]
- Gupta SK, Singh SP, Shukla VK. Copper, zinc, and Cu/Zn ratio in carcinoma of the gallbladder. J Surg Oncol 2005;91:204-8. [Crossref] [PubMed]
- Shukla Y, Arora A. Enhancing effects of mustard oil on preneoplastic hepatic foci development in Wistar rats. Hum Exp Toxicol 2003;22:51-5. [Crossref] [PubMed]
- Shukla VK, Khandelwal C, Roy SK, et al. Primary carcinoma of the gall bladder: A review of a 16-year period at the university hospital. J Surg Oncol 1985;28:32-5. [Crossref] [PubMed]
- Dutta U, Garg PK, Kumar R, et al. Typhoid carriers among patients with gallstones are at increased risk for carcinoma of the gallbladder. Am J Gastroenterol 2000;95:784-7. [Crossref] [PubMed]
- Kumar S. Infection as a risk factor for gallbladder cancer. J Surg Oncol 2006;93:633-9. [Crossref] [PubMed]
- Routh D. Gallbladder Carcinoma: A Reason to Worry in North and North-Eastern India. Clin Surg 2017;2:1661.
- Malhotra RK, Manoharan N, Shukla NK, et al. Gallbladder cancer incidence in Delhi urban: A 25-year trend analysis. Indian J Cancer 2017;54:673-7. [Crossref] [PubMed]
- Dutta U, Nagi B, Garg PK, et al. Patients with gallstones develop gallbladder cancer at an earlier age Eur J Cancer Prev 2005;14:381-5. [Crossref] [PubMed]
- Konstantinidis IT. Trends in Presentation and Survival for Gallbladder Cancer During a Period of More Than 4 Decades: A Single-Institution Experience. Arch Surg 2009;144:441-7; discussion 447. [Crossref] [PubMed]
- Jain K, Sreenivas V, Velpandian T, et al. Risk factors for gallbladder cancer: A case-control study. Int J Cancer 2013;132:1660-6. [Crossref] [PubMed]
- Bhagabaty S, Sharma J, Krishnatreya M, et al. A Profiles of gall bladder cancer reported in the hospital cancer registry of a Regional Cancer Center in the North-East India. Int J Res Med Sci 2014;2:1683. [Crossref]
- Sachidananda S, Krishnan A, Janani K, et al. Characteristics of Gallbladder Cancer in South India. Indian J Surg Oncol 2012;3:228-30. [Crossref] [PubMed]
- Baskaran V, Vij U, Sahni P, et al. Do the Progesterone Receptors Have a Role to Play in Gallbladder Cancer? Int J Gastrointest Cancer 2005;35:61-8. [Crossref] [PubMed]
- Jayanthi V, Sarika S, Varghese J, et al. Composition of gallbladder bile in healthy individuals and patients with gallstone disease from north and South India. Indian J Gastroenterol 2016;35:347-53. [Crossref] [PubMed]
- Gupta S, Udupa KN, Gupta S. Primary carcinoma of the gallbladder: A review of 328 cases. J Surg Oncol 1980;14:35-44. [Crossref] [PubMed]
- Attili AF, de Santis A, Capri R, et al. The natural history of gallstones: The GREPCO experience. Hepatology 1995;21:655-60. [Crossref] [PubMed]
- Kowalewski K, Todd EF. Carcinoma of the Gallbladder Induced in Hamsters by Insertion of Cholesterol Pellets and Feeding Dimethylnitrosamine. Proc Soc Exp Biol Med 1971;136:482-6. [Crossref] [PubMed]
- Duarte I, Llanos O, Domke H, et al. Metaplasia and precursor lesions of gallbladder carcinoma. Frequency, distribution, and probability of detection in routine histologic samples. Cancer 1993;72:1878-84. [Crossref] [PubMed]
- Dowling GP, Kelly JK. The histogenesis of adenocarcinoma of the gallbladder. Cancer 1986;58:1702-8. [Crossref] [PubMed]
- Nagaraja V, Eslick GD. Systematic review with meta-analysis: the relationship between chronic Salmonella typhi carrier status and gall-bladder cancer. Aliment Pharmacol Ther 2014;39:745-50. [Crossref] [PubMed]
- Bansal VK, Misra MC, Chaubal G, et al. Helicobacter pylori in gallbladder mucosa in patients with gallbladder disease. Indian J Gastroenterol 2012;31:57-60. [Crossref] [PubMed]
- Matsukura N, Yokomuro S, Yamada S, et al. Association between Helicobacter bilis in bile and biliary tract malignancies: H. bilis in bile from Japanese and Thai patients with benign and malignant diseases in the biliary tract. Jpn J Cancer Res 2002;93:842-7. [Crossref] [PubMed]
- Mishra RR, Tewari M, Shukla HS. Helicobacter pylori and pathogenesis of gallbladder cancer. J Gastroenterol Hepatol 2011;26:260-6. [Crossref] [PubMed]
- Engeland A, Tretli S, Austad G, et al. Height and Body Mass Index in Relation to Colorectal and Gallbladder Cancer in Two Million Norwegian Men and Women. Cancer Causes Control 2005;16:987-96. [Crossref] [PubMed]
- Tyagi B, Manoharan N, Raina V. Risk factors for gallbladder cancer : A population based case-control study in Delhi. Indian J Med Paediatr Oncol 2008;29:16. [Crossref]
- Lambe M, Trichopoulos D, Hsieh CC, et al. Parity and cancers of the gall bladder and the extrahepatic bile ducts. Int J Cancer 1993;54:941-4. [Crossref] [PubMed]
- Unisa S, Jagannath P, Dhir V, et al. Population-based study to estimate prevalence and determine risk factors of gallbladder diseases in the rural Gangetic basin of North India. HPB 2011;13:117-25. [Crossref] [PubMed]
- Hsing AW, Gao YT, Han TQ, et al. Gallstones and the risk of biliary tract cancer: a population-based study in China. Br J Cancer 2007;97:1577-82. [Crossref] [PubMed]
- Barbhuiya MA, Singh TD, Poojary SS, et al. Gallbladder cancer incidence in Gwalior district of India: Five-year trend based on the registry of a regional cancer center. Indian J Cancer 2015;52:430-7. [Crossref] [PubMed]
- Dubey A, Rawat K, Pathi N, et al. Carcinoma of gall bladder: Demographic and clinicopathological profile in indian patients. Oncol J India 2018;2:3. [Crossref]
- Misra V, Misra SP, Dwivedi M, et al. Helicobacter pylori in areas of gastric metaplasia in the gallbladder and isolation of H. pylori DNA from gallstones. Pathology 2007;39:419-24. [Crossref] [PubMed]
- de Martel C, Plummer M, Parsonnet J, et al. Helicobacter species in cancers of the gallbladder and extrahepatic biliary tract. Br J Cancer 2009;100:194-9. [Crossref] [PubMed]
- Kim SS, Ruiz VE, Carroll JD, et al. Helicobacter pylori in the pathogenesis of gastric cancer and gastric lymphoma. Cancer Lett 2011;305:228-38. [Crossref] [PubMed]
- Caygill CPJ, Hill MJ, Braddick M, et al. Cancer mortality in chronic typhoid and paratyphoid carriers. Lancet 1994;343:83-4. [Crossref] [PubMed]
- Koshiol J, Wozniak A, Cook P, et al. Salmonella enterica serovar Typhi and gallbladder cancer: a case-control study and meta-analysis. Cancer Med 2016;5:3310-3235. [Crossref] [PubMed]
- Iyer P, Barreto SG, Sahoo B, et al. Non-typhoidal Salmonella DNA traces in gallbladder cancer. Infect Agent Cancer 2016;11:12. [Crossref] [PubMed]
- Mogasale V, Maskery B, Ochiai RL, et al. Burden of typhoid fever in low-income and middle-income countries: a systematic, literature-based update with risk-factor adjustment. Lancet Glob Health 2014;2:e570-80. [Crossref] [PubMed]
- Aune D, Vatten LJ, Boffetta P. Tobacco smoking and the risk of gallbladder disease. Eur J Epidemiol 2016;31:643-53. [Crossref] [PubMed]
- Khan I, Panda N, Banerjee M, et al. Epidemiological Factors in Gall Bladder Cancer in Eastern India-A Single Centre Study. Indian J Surg Oncol 2013;4:67-72. [Crossref] [PubMed]
- Shaffer EA. Gallbladder Cancer: The Basics. Gastroenterol Hepatol (N Y) 2008;4:737-41. [PubMed]
- Chang LY, Wang HP, Wu MS, et al. Anomalous pancreaticobiliary ductal union--an etiologic association of gallbladder cancer and adenomyomatosis. Hepatogastroenterology 1998;45:2016-9. [PubMed]
- Kochhar R, Singhal M, Nagi B, et al. Prevalence of Type III Anomalous Pancreaticobiliary Junction in a Tertiary Care Hospital of North India. JOP 2009;10:383-6. [PubMed]
- Mori K, Nagakawa T, Ohta T, et al. Association between gallbladder cancer and anomalous union of the pancreaticobiliary ductal system. Hepatogastroenterology 1993;40:56-60. [PubMed]
- Dutta U, Poornachandra KS. Gallbladder polyps: how to pick up the sinister ones. J Gastroenterol Hepatol 2009;24:175-8. [Crossref] [PubMed]
- Hickman L, Contreras C. Gallbladder Cancer. Surg Clin North Am 2019;99:337-55. [Crossref] [PubMed]
- DesJardins H, Duy L, Scheirey C, et al. Porcelain Gallbladder: Is Observation a Safe Option in Select Populations? J Am Coll Surg 2018;226:1064-9. [Crossref] [PubMed]
- Schnelldorfer T. Porcelain Gallbladder: A Benign Process or Concern for Malignancy? J Gastrointest Surg 2013;17:1161-8. [Crossref] [PubMed]
- Dwivedi S, Madeshiya A, Singh D, et al. Gall Bladder Cancer and some epidemiological factors: A cross sectional study. Biomed Res 2013;24:5.
- Misra S, Chaturvedi A, Misra NC, et al. Carcinoma of the gallbladder. Lancet Oncol 2003;4:167-76. [Crossref] [PubMed]
- Singh MK, Chetri K, Pandey UB, et al. Mutational spectrum of K-ras oncogene among Indian patients with gallbladder cancer. J Gastroenterol Hepatol 2004;19:916-21. [Crossref] [PubMed]
- Priya TP, Kapoor VK, Krishnani N, et al. Fragile histidine triad (FHIT) gene and its association with p53 protein expression in the progression of gall bladder cancer. Cancer Invest 2009;27:764-73. [Crossref] [PubMed]
- Jain K, Mohapatra T, Das P, et al. Sequential Occurrence of Preneoplastic Lesions and Accumulation of Loss of Heterozygosity in Patients With Gallbladder Stones Suggest Causal Association With Gallbladder Cancer Ann Surg 2014;260:1073-80. [Crossref] [PubMed]
- Sharma A, Sharma KL, Gupta A, et al. Gallbladder cancer epidemiology, pathogenesis and molecular genetics: Recent update. World J Gastroenterol 2017;23:3978-98. [Crossref] [PubMed]
- Mohandas KM, Patil PS. Cholecystectomy for asymptomatic gallstones can reduce gall bladder cancer mortality in northern Indian women. Indian J Gastroenterol 2006;25:147-51. [PubMed]
- Jha V, Sharma P, Mandal KA. Incidental gallbladder carcinoma: Utility of histopathological evaluation of routine cholecystectomy specimens. South Asian J Cancer 2018;7:21-3. [PubMed]