
Gallbladder cancer: epidemiology and genetic risk associations
Introduction
Gallbladder cancer (GBC) is a rare, highly-lethal malignant neoplasm of the biliary system. GBC is the most common type of biliary tract malignancy affecting the gallbladder; a sac-like organ located beneath the liver that is responsible for storage of bile produced in the liver. The gallbladder contracts in response to gastrointestinal hormones produced upon entry of food into the small intestine, depositing the bile into the duodenum where it acts to aid digestion, particularly of fats. Though generally uncommon, gallbladder cancer is more frequent in some distinct geographical locations. Accurate worldwide incidences of gallbladder cancer are difficult to obtain due to difficulties in the detection and diagnosis of gallbladder cancer, particularly in low resource settings where specialized abdominal imaging is not available. In 2018, the International Agency for Research on Cancer (IARC) Globocan 2018 database estimated gallbladder cancer to constitute 1.7% of all cancer deaths with 220,000 new cases diagnosed annually (1,2). Worldwide, gallbladder cancer is noted to disproportionately affect females more than males, perhaps due to the higher propensity of females to having gallstone disease (3).
A poorly understood combination of predisposing factors makes gallbladder cancer a unique malignancy. Factors associated with higher rates of gallbladder cancer occurrence include female sex, geographical location, ethnicity, congenital developmental abnormalities, obesity, a personal or family history of gallstones, chronic infection and inflammation in the gallbladder and ill-defined genetic variants (4). The asymptomatic nature of the initial development of gallbladder cancer and its propensity for early and rapid metastasis result in the majority of gallbladder cancer being diagnosed late, contributing to the poor prognosis of the disease. Less than 20% of gallbladder cancer is eligible for potentially curative surgical resection at diagnosis. It has been shown that gallbladder cancer outcomes are most favorable in countries with better health system rankings and higher expenditures on health, which have lower mortality-to-incidence ratios for gallbladder cancer (5).
The lack of preventive and therapeutic treatment options for gallbladder cancer is partly a result of the limited knowledge on etiology, associated risk factors and molecular pathogenesis of gallbladder cancer. In recent years, single nucleotide polymorphism (SNP) association studies have identified potential genetic variations associated with gallbladder cancer development, though the available data is insufficient to confirm any of the associations. The mechanisms by which these distinct SNPs impact carcinogenesis of the gallbladder have yet to be studied in depth.
A deeper understanding of the relationship between environmental and genetic risk is urgently needed to elucidate the multifactorial pathophysiology of gallbladder cancer. Primary prevention will be feasible when high-risk genetic associations and environmental toxins or other risk factors are more clearly identified and the mechanisms by which these contribute to the overall molecular pathogenesis of gallbladder cancer are determined. This review provides a synopsis of gallbladder cancer epidemiology and risk factors, emphasizing the current and pending knowledge of genetic predisposition to gallbladder cancer.
Epidemiology
Globally, epidemiological trends of gallbladder cancer incidence vary substantially by geographical region (Figure 1) (1,6). The highest rates of GBC are observed in Chile (27/100,000) followed by regions of northern India (21.5/100,000); see companion articles in this issue of CCO. Other high-risk regions include Poland (14/100,000), south Pakistan (11.3/100,000), Japan (7/100,000) and Israel (5/100,000). Development of gallbladder cancer has been associated with a broad range of risk factors. Further study of the epidemiology of gallbladder cancer may shed light on its multifactorial etiology and lead to better strategies for prevention and management.
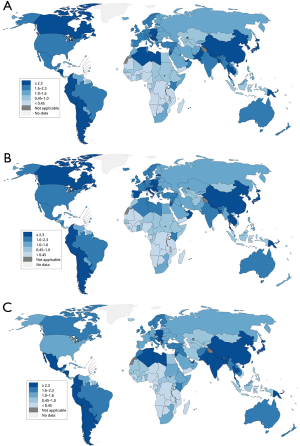
Geographical locations associated with a greater than average incidence rate of gallbladder cancer are focused within Latin America and Asia (7). The lowest rates of GBC are noted in high resource regions with high proportions of persons of European ancestry, including the United States, Australia, Canada, United Kingdom and New Zealand. As previously mentioned, the overall incidence of gallbladder cancer is relatively low in the United States, with an incidence of approximately 2 cases per 100,000 person years (8). Though generally rare in the United States, gallbladder cancer rates are disproportionately higher among some ethnic groups within the United States, particularly in Native Americans (3.3/100,000) compared to non-Native Americans (0.4–1.5/100,000) (9,10). Within the US, gallbladder cancer is more prevalent in Caucasians than Blacks. Hispanic populations of New Mexico experience a greater incidence of gallbladder cancer compared to Caucasian populations in New Mexico (11).
GBC is one of the few cancer types that demonstrate a worldwide gender bias with up to three to six times higher incidence observed in females compared to males. Additionally, the incidence of gallbladder cancer increases consistently with age. More than two thirds of persons diagnosed with gallbladder cancer are over the age of 65 years with the average age of diagnosis being 72 years.
Risk factors
A wide range of conditions, environmental exposures and lifestyle behaviors have been linked to a higher risk of developing GBC (Figure 2). Predisposing conditions affecting the gallbladder and bile ducts are associated with a higher incidence of gallbladder cancer, specifically those that cause chronic irritation and or inflammation of the gallbladder. Gallstones also referred to as cholelithiasis is one of the most strongly associated risk factors for gallbladder cancer with 70–90% of gallbladder cancer cases noting a history of cholelithiasis. It is important to note that the majority of persons with cholelithiasis do not develop gallbladder cancer, with only 0.5% to 3% of gallstone cases resulting in gallbladder cancer (12). The exact mechanism by which cholelithiasis predisposes an individual to gallbladder carcinoma is incompletely understood, though chronic epithelial irritation and mucosal damage is presumed to be involved. Studies suggest that the size of a gallstone directly influences one’s risk of gallbladder cancer. Specifically, gallstones greater than 3 cm are associated with a 9.2 to 10.1 times greater risk of gallbladder cancer compared to gallstones less than 1 cm (13). Persistent low grade inflammation within the gallbladder appears to contribute to the development of gallbladder wall calcification; diffuse calcification results in a porcelain gallbladder, which is associated with an extremely high risk of cancer development (14).
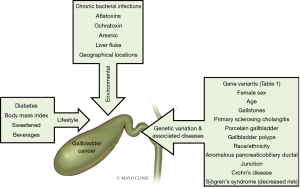
Primary sclerosing cholangitis (PSC), a chronic liver disease that results in inflammation and fibrosis of the bile ducts, is associated with an increased risk of gallbladder cancer (15,16). A 2% lifetime incidence of gallbladder cancer is estimated in patients with PSC (17). Large gallbladder polyps in PSC patients are associated with a substantially higher risk of gallbladder cancer (18). It is recommended that cholecystectomy be performed in PSC patients with gallbladder polyps greater than 8 mm in size (19).
Abnormalities in the structure of the biliary tree have been associated with a greater risk of gallbladder cancer, specifically, anomalous pancreaticobiliary ductal junction, also known as biliopancreatic maljunction or pancreaticobiliary maljunction (20). Anomalous pancreaticobiliary ductal junction is a rare congenital anomaly in which the junction of the pancreatic and biliary ducts is located outside of the duodenal wall. Consequently, the duodenal papillary sphincter muscle does not encompass the full length of the joint channel between the pancreatic and bile ducts. This results in reflux of the pancreatic juice and bile. Since the pressure within the pancreatic duct is usually higher than that within the biliary tract, pancreatic juice continuously bathes the biliary mucosa, inducing mucosal injury and inflammation that predisposes the mucosa to carcinogenesis (21). It is estimated that 10% of all gallbladder cancer cases have this anatomic anomaly. Pancreaticobiliary maljunction is notably more prevalent in Asian populations (22).
Irritation and inflammation of the gallbladder can also arise from chronic bacterial infections caused commonly by Salmonella typhi or Helicobacter bilis. Carriers of Salmonella typhi have a 12-fold increased risk of developing gallbladder cancer (23). Chronic S. typhi infection is twice as common in women as in men. Helicobacter bilis has also been implicated in the formation of cholesterol gallstones (24). In summary, chronic bacterial inflammation of the gallbladder and biliary tree which induces DNA damage and tissue proliferation is considered to be a key contributor to many gallbladder carcinomas.
Increased risk of gallbladder cancer has also been established in several non-hepatobiliary specific diseases. For instances, risk of gallbladder cancer and several autoimmune conditions yielded significant results in an evaluation of association between gallbladder cancer and autoimmune conditions by means of population studies (25). Most recently, in a US population based study, an increased risk of gallbladder cancer was detected in patients diagnosed with Crohn’s disease [odds ratio (OR): 1.83, 95% confidence interval (CI): 1.23–2.71], pernicious anemia (OR: 1.28, 95% CI: 1.09–1.52) and PSC (OR: 2.06, 95% CI: 1.27–3.33), while patients diagnosed with Sjögren’s syndrome were at decreased risk (OR: 0.39, 95% CI: 0.18–0.81) (26). The potential mechanism for the newly observed inverse association with Sjogren’s syndrome remains unclear.
Diabetes mellitus is associated with an increased risk of many different cancer types, including hepatocellular carcinoma and pancreatic adenocarcinoma. The association between diabetes and gallbladder cancer is difficult to demonstrate due to the established associations of diabetes with obesity and gallstone disease. A fairly large body of evidence supports the notion that obesity increases the risk of gallbladder cancer. Several recent studies have focused on increased body size and risk of gallbladder carcinogenesis. Persons who are overweight have a 20% higher risk of gallbladder cancer, compared to persons with normal weight, for obese people the risk is higher by 60% (27). In a different study combining data from 14 cohort studies, the relative risk of gallbladder cancer in overweight persons (BMI =25–29.9 kg/m2) was 1.10 (95% CI: 1.02–1.18) compared to the risk in normal weight persons (BMI =18.5–24.9 kg/m2). There appears to be a dose-response relationship of obesity with risk of GBC, as the relative risk of gallbladder cancer in obese persons (BMI ≥30 kg/m2) was 1.69 (95% CI: 1.54–1.86) (28). A meta-analysis of 20 studies indicated that compared with non-diabetic individuals, both men and women with type 2 diabetes had a similarly increased risk of gallbladder cancer. The increase in risk found was independent of smoking, body mass index or a history of gallstones. In recent large cohort studies, anthropometric factors such as body mass index have been independently associated with risk of gallbladder cancer. For each 5 kg/m2 increase in BMI there was an adjusted risk increase for gallbladder cancer (HR =1.27; 95% CI, 1.19–1.36). Similarly, increasing waist circumference, hip circumference, waist-to-hip ratio, and waist-to-height ratio were all associated with gallbladder cancer. Thus adiposity is associated with an increased risk of gallbladder cancer and the available evidence suggests that weight management may help minimize the risk of gallbladder cancer (29).
An association between consumption of sweetened beverages and gallbladder cancer was suggested in a prospective analysis of 70,832 Swedish adults enrolled in the Swedish Mammography Cohort and the Cohort of Swedish Men, who were free of cancer and diabetes. With a mean follow-up of over 13 years, after adjustment for other risk factors, women and men in the highest category of combined sugar-sweetened and artificially sweetened beverage consumption had a significantly higher risk of gallbladder cancer, with a multivariable hazard ratio of 2.24 (95% CI: 1.02–4.89) for two or more 200 mL servings per day of sweetened beverages compared with no consumption (30).
As noted previously, gallbladder cancer disproportionately affects women in some regions of the world, as a result, reproductive and menstrual factors have been proposed to be involved in the etiology of gallbladder cancer (31). The results of previous studies have been inconsistent. A recent population based cohort study in Japan investigated the association of reproductive and menstrual factors with the risk for gallbladder cancer. Irregular and longer cycles were moderately associated with an increased risk of gallbladder cancer, with HR of 2.12 (95% CI: 1.30–3.47) and 1.76 (95% CI: 1.08–2.89), respectively. In addition, older age at time of first pregnancy tended to be associated with an increased risk of gallbladder cancer, with a HR of 1.84 (95% CI: 1.03–3.29), P-trend =0.036, whereas increased duration of fertility was generally associated with a decreased risk of gallbladder cancer (HR =0.59, 95% CI: 0.35–1.01, P-trend =0.055) (32). Findings from this study highlight the important role that female hormones may play in the etiology of gallbladder cancer, though further studies are needed to characterize the specific mechanisms by which they exert their influence.
Tobacco smoking and alcohol consumption are established risk factors for numerous cancers. The risk of gallbladder cancer attributed to smoking and alcohol has been inconsistent, likely due to at least in part to the small sample sizes in the studies performed thus far. A recent meta-analysis of 26 prospective studies evaluating the association of cigarette smoking and alcohol consumption in biliary tract cancers found that smoking was associated with an increased risk of all other types of biliary tract cancer except gallbladder cancer (33). In additional, this work did not find any significant association between alcohol consumption and gallbladder cancer risk. Further work is needed to clarify the associations between smoking and alcohol consumption and gallbladder cancer risk.
Fungal aflatoxins, ochratoxin, arsenic and other environmental risk factors for gallbladder cancer.
Aflatoxins are mycotoxin produced by Aspergillus fungi, mainly Aspergillus flavus and Aspergillus parasiticus, which are ubiquitous in warm, humid regions of the world (34). Aflatoxins contaminate foods prepared from infected cereals, oilseeds, nuts, spices, milk, and meats with fungus and that are kept under conditions that support fungal growth (35). Aflatoxins were first recognized as carcinogenic in 1976 and have been most prominently associated with a synergistic interaction with chronic hepatitis B virus infection in the development of hepatocellular carcinoma. A role for aflatoxins in gallbladder carcinogenesis has been proposed and is supported by an increasing body of evidence (36-39). The presumed mechanism by which aflatoxins contribute to gallbladder carcinogenesis is chronic exposure of the gallbladder epithelium to carcinogenic metabolites of aflatoxin excreted from the liver into bile (36). In a case-control study patients with gallbladder cancer had significantly more circulating aflatoxin-albumin adducts compared to population controls (OR: 13.0; 95% CI: 3.0–52.5) (37).
Ochratoxin A is another mycotoxin that may be associated with GBC development (40). Ochratoxin A is produced by Penicillium and Aspergillus species and found in spices, cereals, and nuts as well as cocoa, beer, and coffee (41). Studies have shown that dried red chili peppers from Chile, Bolivia, and Peru show substantial concentrations of Ochratoxin A, higher than the concentrations of aflatoxins in the same specimens (40). These results suggest the association between Ochratoxin A contamination of red chili peppers and the development of gallbladder cancer may be stronger than the association with aflatoxin exposure (40).
A potential association between arsenic exposure due to natural contamination of groundwater and gallbladder cancer incidence has been postulated for a number of years, in part due to the similarities of the geographic distribution of arsenic exposure and incidence of gallbladder cancer in some countries and regions. There is increasing evidence that arsenic at least partially contributes to gallbladder cancer risk, particularly in women (42). In addition to arsenic, other metals have also been associated with gallbladder cancer risk, including boron, lithium, molybdenum, cadmium, chromium, copper, and vanadium (43).
Preventive factors
A number of medications commonly used on a chronic basis for inflammatory and metabolic conditions have been examined for their potential for use a chemo-preventive agents for prevention of cancer. These include aspirin, non-aspirin non-steroidal anti-inflammatory drugs, cholesterol-lowering statin drugs, and the oral anti-diabetic metformin. Studies performed examining the effectiveness of these agents in prevention against gallbladder cancer have had case control or cohort study designs. Case control studies examine the frequency of use of one or more of the agents in persons who either acquired gallbladder cancer or in a group of relevant matched controls. A lower rate of use of the agent in persons with gallbladder cancer is presumed to be evidence of an association of use of the agent with a chemo preventive effect against cancer. In population-based cohort study designs the rates of gallbladder cancer development are assessed in persons in the population who are or have been on the agent under study compared to the rates of gallbladder cancer development in persons not on the agent. While case control studies are much more feasible, they tend to overestimate the effect of the potentially chemo-preventative agent. Population based studies require more resources to execute but provide more accurate estimates of chemo-preventive effects. For gallbladder cancer, case control studies examining the impact of potential chemo-preventive agents have shown that aspirin use is associated with a reduced risk of gallbladder cancer (OR 0.37; 95% CI: 0.17–0.88). Higher amount and frequency of use, as well as younger age at the onset of aspirin use were also associated with a reduced risk of gallbladder cancer (44).
A reduced risk of gallbladder cancer has also been shown in individuals taking statin drugs, as compared with those not taking statins, with a 14% reduction in risk of gallbladder cancer, HR 0.86 (95% CI: 0.67–1.09) (45).
Survival of patients with gallbladder cancer
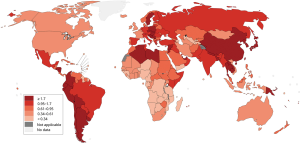
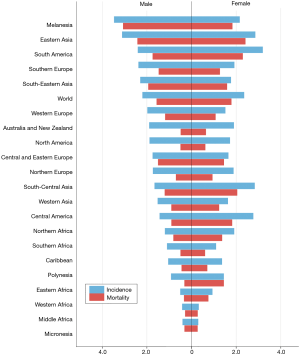
GBC is the most aggressive of biliary tract cancers with the shortest median survival period. Available treatment options vary significantly across regions with high prevalence of gallbladder cancer, resulting in varying patient outcomes by region (Figures 3 and 4). Despite treatment in the most medically advanced regions, gallbladder malignancies are highly lethal. According to the American Cancer Society, only about 1 in 5 gallbladder cancer cases will be discovered when the disease is still localized to the gallbladder (46). The remaining majority of gallbladder cancer cases are diagnosed when the malignancy has spread outside of the gallbladder, which drastically limits the available options for curative treatment and lowers overall survival. Data from the National Cancer Data Base of the American College of Surgeons report the following 5-year survival rates for patients with gallbladder cancer by stage: 80% for stage 0, 50% for stage I, 28% for stage II, 8% for stage IIA, 7% for stage IIB, 4% for stage IVA, and 2% for stage IVB. The poor prognosis of patients with gallbladder cancer highlights the need for improved strategies for integrating knowledge of the factors associated with gallbladder cancer development to develop programs to accelerate detection of gallbladder cancer at earlier stages.
Genetic association studies in gallbladder cancer
Due to the rapid increase in our understanding of the human genome and the development of advanced genetic sequencing techniques, the genetic basis of cancer susceptibility has become an area of particular research interest. Cancers are complex diseases known to arise due to unique combinations of lifestyle, environmental and genetic factors, though the significance of each in regard to carcinogenesis and disease progression is not completely understood. As a method of further exploring inherited genetic variants, researchers have organized genetic association studies intended to pinpoint associations between inherited genetic variants and the incidence or outcomes of a particular disease such as gallbladder cancer. Based on the available epidemiologic evidence linking gallbladder cancer development to diseases causing chronic gallbladder or biliary tract inflammation, such as gallstones and PSC, as well as the known associations of these diseases with particular racial groups or geographic regions, it can be presumed that a proportion of the risk for gallbladder cancer development is inherited. While this may be the case, it is also important to decipher the environmental factors that contribute to and interact with the variants responsible for inherited susceptibility. This concept of gene x environment interactions is very important, but studies are limited by the relatively low numbers of gallbladder cancer patients that have been enrolled in genetic association studies thus far, as well as by the lack of detailed, comprehensive information on lifetime environmental exposures in both the enrolled persons with gallbladder cancer and the large numbers of control persons needed for such studies,
The two most widely utilized approaches to demonstrating inherited genetic association are classified as candidate gene and genome wide studies. In the case of candidate gene studies, specific variants are selected based on their hypothesized biological role in the disease and are genotyped using a case-control study design. Statistical analyses are then performed to identify associations between the specific genetic variant(s) and the presence of disease. The most frequently occurring and most commonly studied type of genetic variant is the SNP. Because candidate gene studies are based on the ability to predict functional candidate genes and variants, this approach has been subject to the criticism that current biomedical knowledge is typically insufficient to make accurate and reliable predictions of many causative risk variants.
In contrast, in the second approach, designated as genome-wide association studies (GWAS), the entire genomes of numerous patients with the disease of interest and disease-free controls are screened simultaneously for an extensive number of known genetic variants interspersed throughout the entire human genome. This notion assumes that common variants in several genes will each contribute a small rise, or fall, in the risk of disease and the accumulation of risk from each variant will account for the overall risk of disease development. SNPs of interest in GWAS are identified by comparing the distribution of individual variants in cases and controls. SNPs that show statistically significant differences in frequency between cases and controls are considered to be associated with the disease. The associations found as a result of a genome-wide study do not necessarily imply that nearby genes are drivers of the disease, though they can serve as critical clues in uncovering the mechanisms involved in the development of the disease.
With the use of both candidate gene and genome-wide studies, great strides have been made in identifying genetic risk associations for many cancers. Comparatively, our understanding of the role of inherited genetic variability in gallbladder cancer incidence is substantially behind that for other cancers, in part because of the relatively low incidence of gallbladder cancer. Studies are particularly needed in geographic regions and among racial and ethnic groups that have higher risk of gallbladder cancer. Table 1 summarizes the genetic variants that have been associated with GBC thus far. The following discussion highlights the most recent work on genetic variants associated with gallbladder cancer.
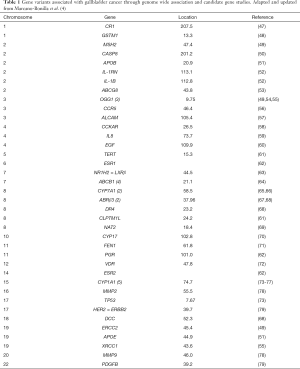
Full table
Relatively little knowledge has come from GWAS in gallbladder cancer. The most recent GWAS focusing on gallstone disease broadly, with a sub analysis of gallbladder cancer in the presence gallstone disease, in admixed Chilean latinos concluded that the ABCG8 and TRAF3 genes are significantly associated with gallstone disease and gallbladder cancer in admixed Latinos (80). These genes were previously associated with gallbladder cancer in Chinese, India and European populations. Other studies have shown that the degree of specific Native American ancestry is a major determinant of gallbladder cancer risk in Chile. Specifically, individuals of Mapuche indigenous ancestry have higher rates of gallbladder cancer. Indeed, each 1% increase in the proportion of Mapuche ancestry was associated with a 3.7% increased mortality risk by gallbladder cancer (95% CI: 3.1–4.3%, P=6×10−27) (81).
A case-control GWAS of gallbladder cancer cases and controls of Indian descent observed genome-wide significant associations for several novel markers in the chromosomal region 7q21.12 responsible for both the ABCB1 and ABCB4 genes, with the most notable SNPs after replication and meta-analysis being rs1558375 (GWAS P=3.8×10−9; replication P=0.01; combined P=2.3×10−10); rs17209837 (GWAS P=2.0×10−8; replication P=0.02; combined P=2.3×10−9), and rs4148808 (GWAS P=2.4×10−8; replication P=0.008; combined P=2.7×10−9) (64). The ABCB1 and ABCB4 genes encode transporters for phospholipids across the hepatobiliary epithelium, which presumably function to modulate bile solubility and the propensity for gallstone formation. The association of the ABCB1 and ABCB4 genes with gallbladder cancer suggests the importance of hepatobiliary phospholipid transport in the pathogenesis of gallbladder cancer in Indian populations.
Another GWAS performed in a Japanese population identified an association of the SNP rs7504990 in the deleted in colon cancer (DCC) gene which encodes a netrin 1 receptor with increased risk of gallbladder cancer in a small sample of colon cancer patients associated with greater risk of gallbladder cancer (OR: 7.0; 95% CI: 3.4–14.1, P=7.46×10−8) (82).
Besides the three genome wide studies described here, all other published genetic association studies on GBC have utilized the candidate gene approach. Genetic variants associated with gallbladder cancer risk that have been studied using the candidate gene approach include variants in genes predicted to influence pathways involving gut hormones, inflammation, apoptosis, DNA repair, drug metabolism, hedgehog signaling, and Wnt signaling.
One candidate gene study performed in Bolivia, which has a high incidence of gallbladder cancer, examined the influence of genetic polymorphisms in the cytochrome P450 (CYP1A1), glutathione S-transferase mu 1 (GSTM1), theta 1 (GSTT1) and tumor suppressor protein p53 (TP53) genes on GBC susceptibility in a case-control study of 32 patients with gallbladder cancer compared to 86 healthy Bolivian subjects using PCR-restriction fragment length polymorphism assays (48). The frequency of the GSTM1 null genotype was significantly higher in gallbladder cancer patients than in healthy subjects (OR 2.35; 95% CI: 1.03–5.37; age-adjusted OR 3.53; 95% CI: 1.29–9.66; age- and sex-adjusted OR 3.40; 95% CI: 1.24–9.34). No significant differences were observed in the frequencies of CYP1A1, GSTT1, or TP53 polymorphisms between the two groups. The GSTM1 null genotype was associated with increased gallbladder cancer risk in Bolivians (48).
Another candidate gene study performed in a north Indian population assessed the ability of five polymorphisms in the telomerase reverse transcriptase (TERT) region on 5p15.33 and one on the 8q24.21 locus to predict GBC risk and treatment response in 523 gallbladder cancer cases patients undergoing chemoradiotherapy and 274 controls (61). Binary logistic regression analysis showed significant associations of the SNPs TERT rs2736100C > A (OR 0.69; 95% CI: 0.52–0.92, P=0.01), CLPTM1L rs401681C > T (OR 0.59; 95% CI: 0.41–0.85, P=0.004), and CASC8 rs6983267G > T (OR 1.6; 95% CI: 1.2–2.2, P=0.001) with gallbladder cancer risk. Further, multivariate logistic regression showed that haplotype CLPTM1LC, rs401681C, rs31489 TERT T, rs2853676A, rs2736100 MIR4457 G rs4635969 (OR 7.5; 95% CI: 1.8–31.5, P=0.006) was significantly associated with poor treatment response. In survival analysis, Kaplan–Meier survival curves showed significantly poorer survival and COX regression suggested significantly higher hazard ratio in TT genotype carriers of CASC8 rs6983267 (OR 4.3; 95% CI: 1. 1–17.1, P=0.04) as compared to the major allele and heterozygous (GG + GT) genotypes in metastatic GBC cases. Overall, the study showed that genetic variants at 5p15.33 and 8q24.21 significantly influence GBC risk and treatment response in the north-Indian population.
Regarding receptor tyrosine kinase signaling pathways, polymorphisms in the platelet derived growth factor-B (PDGFB) gene have been significantly associated with gallbladder cancer in men and women overall and in the human epidermal growth factor receptor-2 (HER2) gene have been significantly associated with gallbladder cancer in women (79). The ORs for these effects ranged from 2.5 to 10 and there were interactions observed between PDGFB and HER2 gene variants (79).
One of the drawbacks of a focus on candidate gene studies for assessing the effect of genetic variants on cancer risk is the phenomenon of selective publication bias. Studies with positive results are substantially more likely to be published than studies with negative results. Consequently the literature on this subject tends to have a bias towards positive studies.
Though the individual SNPs identified as associated with an increased risk of gallbladder cancer may not account for a major proportion of the overall risk of development of gallbladder cancer in any one person, when considered collectively polygenic risk scores can be calculated that integrate the magnitude of risk associated with each identified SNP association into a cumulative score. Scores can be generated using a multiplicative model that allows accurate prediction of individual risk based on the number of risk alleles carried by that individual (83-85). Information of this kind could potentially allow stratification of individuals according to their risk level for gallbladder cancer and facilitate strategies for cancer surveillance, early detection and prevention of gallbladder cancer. Such knowledge would allow for personalized risk information to be provided to each patient. Additional large-scale GWAS are necessary to achieve clinically relevant risk estimation, specifically in different racial groups as genetic associations found in one racial group cannot be generalized to all other racial groups.
As common genetic variants and their respective genes are identified, it is crucial to determine the molecular impact of the variants on gene expression and protein function, with consequent impacts on cell-signaling and metabolic pathways. This information will potentially provide critical insight into the molecular and cellular influences that contribute to GBC pathogenesis. Ultimately, such knowledge will unveil strategies for more precise and effective preventive and therapeutic targeting of gallbladder cancer.
Summary, conclusions and future directions
The epidemiology of gallbladder cancer demonstrates substantial geographic variability due to large differences in regional prevalence of the main environmental risk factors. The risk factors with the strongest links to the development of gallbladder cancer are those that induce inflammation over prolonged periods of time. Genetic variants associated with increased risk of GBC have been explored using small scale approaches, but conclusive determinations of relative risk will require larger, more comprehensive studies. Though many variables have been linked to gallbladder carcinogenesis, gallstone disease is the single risk factor most commonly identified in cases. In countries such as Chile where gallstones occur almost universally in women and lifetime GBC risk in women is very high, prophylactic cholecystectomy is recommended as the standard of care for all adult women. Strategies such as this can be extremely effective for those patients identified as being at highest risk for gallbladder cancer development, with the goal of earlier detection or prevention. By identifying SNPs associated with increased risk, clinicians could identify at-risk patients and implement appropriate surveillance strategies to monitor and detect gallbladder cancer during the earliest stages, when effective therapeutic interventions are feasible.
While the available information on genomic variations in patients with gallbladder cancer continues to increase, substantially more work is needed to reduce the variance in the results obtained due to the use of different methodologies and studies in different populations. Large gaps remain in our achieving a comprehensive understanding of the processes that govern gallbladder carcinogenesis, the outcomes of patients with gallbladder cancer, and both response and resistance to treatments for gallbladder cancer. Importantly, additional genetic risk association studies are needed, specifically large GWASs to identify and validate known as well as novel candidate SNPs associated with gallbladder cancer. Furthermore, since the associations vary according to population and are influenced by environmental exposures, these genome-wide studies must be replicated in different at-risk populations. Functional studies must also be performed on the variants or genetic regions implicated through gene association studies, in order to guide the field to potential therapeutic targets for the development of targeted precision therapies for gallbladder cancer.
Acknowledgments
Funding: Funding for this project was from the National Institutes of Health to LR Roberts (R01 CA 186566), the Mayo Clinic Hepatobiliary SPORE (P50 CA 210964), the Mayo Clinic Cancer Center (P30 CA 15083), the Mayo Clinic Center for Clinical and Translational Science (UL1 TR 002377) and The Cholangiocarcinoma Foundation.
Footnote
Conflicts of Interest: Dr. Lewis Roberts has received grant support from ARIAD Pharmaceuticals, Bayer, BTG International, Exact Sciences, Gilead Sciences, Glycotest, Inc., RedHill, Inc., Target PharmaSolutions, and Wako Diagnostics; he has provided advisory services to Bayer, Exact Sciences, Gilead Sciences, GRAIL, Inc., QED Therapeutics and TAVEC. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer 2019;144:1941-53. [PubMed]
- Piehler JM, Crichlow RW. Primary carcinoma of the gallbladder. Surg Gynecol Obstet 1978;147:929-42. [PubMed]
- Marcano-Bonilla L, Mohamed EA, Mounajjed T, et al. Biliary tract cancers: epidemiology, molecular pathogenesis and genetic risk associations. Chin Clin Oncol 2016;5:61. [Crossref] [PubMed]
- Wang CC, Tsai MC, Wang SC, et al. Favorable gallbladder cancer mortality-to-incidence ratios of countries with good ranking of world's health system and high expenditures on health. BMC Public Health 2019;19:1025. [Crossref] [PubMed]
- Ferlay J, Lam F, Colombet M, et al. Global Cancer Observatory: Cancer Today. Lyon, France: International Agency for Research on Cancer. 2018.
- Arroyo GF, Gentile A, Parada LA. Gallbladder cancer: South American experience. Chin Clin Oncol 2016;5:67. [Crossref] [PubMed]
- Yang JD, Kim B, Sanderson SO, et al. Biliary tract cancers in Olmsted County, Minnesota, 1976-2008. Am J Gastroenterol 2012;107:1256-62. [Crossref] [PubMed]
- Weiss KM, Ferrell RE, Hanis CL, et al. Genetics and epidemiology of gallbladder disease in New World native peoples. Am J Hum Genet 1984;36:1259-78. [PubMed]
- Alberts SR, Kelly JJ, Ashokkumar R, et al. Occurrence of pancreatic, biliary tract, and gallbladder cancers in Alaska Native people, 1973-2007. Int J Circumpolar Health 2012;71:17521. [Crossref] [PubMed]
- Nemunaitis JM, Brown-Glabeman U, Soares H, et al. Gallbladder cancer: review of a rare orphan gastrointestinal cancer with a focus on populations of New Mexico. BMC Cancer 2018;18:665. [Crossref] [PubMed]
- Hsing AW, Bai Y, Andreotti G, et al. Family history of gallstones and the risk of biliary tract cancer and gallstones: a population-based study in Shanghai, China. Int J Cancer 2007;121:832-8. [Crossref] [PubMed]
- Lai CH, Lau WY. Gallbladder cancer--a comprehensive review. Surgeon 2008;6:101-10. [Crossref] [PubMed]
- Berk RN, Armbuster TG, Saltzstein SL. Carcinoma in the porcelain gallbladder. Radiology 1973;106:29-31. [Crossref] [PubMed]
- Lewis JT, Talwalkar JA, Rosen CB, et al. Prevalence and risk factors for gallbladder neoplasia in patients with primary sclerosing cholangitis: evidence for a metaplasia-dysplasia-carcinoma sequence. Am J Surg Pathol 2007;31:907-13. [Crossref] [PubMed]
- Chascsa DM, Lindor KD. Cancer risk, screening and surveillance in primary sclerosing cholangitis. Minerva Gastroenterol Dietol 2019. [Epub ahead of print]. [PubMed]
- Said K, Glaumann H, Bergquist A. Gallbladder disease in patients with primary sclerosing cholangitis. J Hepatol 2008;48:598-605. [Crossref] [PubMed]
- Buckles DC, Lindor KD, Larusso NF, et al. In primary sclerosing cholangitis, gallbladder polyps are frequently malignant. Am J Gastroenterol 2002;97:1138-42. [Crossref] [PubMed]
- Eaton JE, Thackeray EW, Lindor KD. Likelihood of malignancy in gallbladder polyps and outcomes following cholecystectomy in primary sclerosing cholangitis. Am J Gastroenterol 2012;107:431-9. [Crossref] [PubMed]
- Kamisawa T, Kuruma S, Tabata T, et al. Pancreaticobiliary maljunction and biliary cancer. J Gastroenterol 2015;50:273-9. [Crossref] [PubMed]
- Kamisawa T, Kuruma S, Chiba K, et al. Biliary carcinogenesis in pancreaticobiliary maljunction. J Gastroenterol 2017;52:158-63. [Crossref] [PubMed]
- Kimura W. Congenital dilatation of the common bile duct and pancreaticobiliary maljunction: clinical implications. Langenbecks Arch Surg 2009;394:209-13. [Crossref] [PubMed]
- Koshiol J, Wozniak A, Cook P, et al. Salmonella enterica serovar Typhi and gallbladder cancer: a case-control study and meta-analysis. Cancer Med 2016;5:3310-235. [Crossref] [PubMed]
- Murphy G, Michel A, Taylor PR, et al. Association of seropositivity to Helicobacter species and biliary tract cancer in the ATBC study. Hepatology 2014;60:1963-71. [Crossref] [PubMed]
- Castro FA, Liu X, Forsti A, et al. Increased risk of hepatobiliary cancers after hospitalization for autoimmune disease. Clin Gastroenterol Hepatol 2014;12:1038-45.e7. [Crossref] [PubMed]
- McGee EE, Castro FA, Engels EA, et al. Associations between autoimmune conditions and hepatobiliary cancer risk among elderly US adults. Int J Cancer 2019;144:707-17. [Crossref] [PubMed]
- Li ZM, Wu ZX, Han B, et al. The association between BMI and gallbladder cancer risk: a meta-analysis. Oncotarget 2016;7:43669-79. [PubMed]
- Liu H, Zhang Y, Ai M, et al. Body Mass Index Can Increase the Risk of Gallbladder Cancer: A Meta-Analysis of 14 Cohort Studies. Med Sci Monit Basic Res 2016;22:146-55. [Crossref] [PubMed]
- Jackson SS, Van Dyke AL, Zhu B, et al. Anthropometric Risk Factors for Cancers of the Biliary Tract in the Biliary Tract Cancers Pooling Project. Cancer Res 2019;79:3973-82. [Crossref] [PubMed]
- Larsson SC, Giovannucci EL, Wolk A. Sweetened Beverage Consumption and Risk of Biliary Tract and Gallbladder Cancer in a Prospective Study. J Natl Cancer Inst 2016;108. [Crossref] [PubMed]
- Shin A, Song YM, Yoo KY, et al. Menstrual factors and cancer risk among Korean women. Int J Epidemiol 2011;40:1261-8. [Crossref] [PubMed]
- Makiuchi T, Sobue T, Kitamura T, et al. Reproductive factors and gallbladder/bile duct cancer: a population-based cohort study in Japan. Eur J Cancer Prev 2017;26:292-300. [Crossref] [PubMed]
- McGee EE, Jackson SS, Petrick JL, et al. Smoking, Alcohol, and Biliary Tract Cancer Risk: A Pooling Project of 26 Prospective Studies. J Natl Cancer Inst 2019. [Epub ahead of print]. [Crossref] [PubMed]
- Aflatoxins. Available online: http://www.cancer.gov/about-cancer/causes-prevention/risk/substances/aflatoxins
- Organization WH. Public Health Strategies for Preventing Aflatoxin Exposure. Available online: http://www.who.int/ipcs/events/2005/workshop_report.pdf
- Tsuchiya Y, Terao M, Okano K, et al. Mutagenicity and mutagens of the red chili pepper as gallbladder cancer risk factor in Chilean women. Asian Pac J Cancer Prev 2011;12:471-6. [PubMed]
- Nogueira L, Foerster C, Groopman J, et al. Association of aflatoxin with gallbladder cancer in Chile. JAMA 2015;313:2075-7. [Crossref] [PubMed]
- Foerster C, Koshiol J, Guerrero AR, et al. The case for aflatoxins in the causal chain of gallbladder cancer. Med Hypotheses 2016;86:47-52. [Crossref] [PubMed]
- Koshiol J, Gao YT, Dean M, et al. Association of Aflatoxin and Gallbladder Cancer. Gastroenterology 2017;153:488-94.e1. [Crossref] [PubMed]
- Ikoma T, Tsuchiya Y, Asai T, et al. Ochratoxin A Contamination of Red Chili Peppers from Chile, Bolivia and Peru, Countries with a High Incidence of Gallbladder Cancer. Asian Pac J Cancer Prev 2015;16:5987-91. [Crossref] [PubMed]
- Ringot D, Chango A, Schneider YJ, et al. Toxicokinetics and toxicodynamics of ochratoxin A, an update. Chem Biol Interact 2006;159:18-46. [Crossref] [PubMed]
- Ganesan N, Bambino K, Boffetta P, et al. Exploring the potential carcinogenic role of arsenic in gallbladder cancer. Eur J Cancer Prev 2019. [Epub ahead of print]. [Crossref] [PubMed]
- Lee MH, Gao YT, Huang YH, et al. A Metallomic Approach to Assess Associations of Serum Metal Levels With Gallstones and Gallbladder Cancer. Hepatology 2019. [Epub ahead of print]. [Crossref] [PubMed]
- Liu E, Sakoda LC, Gao YT, et al. Aspirin use and risk of biliary tract cancer: a population-based study in Shanghai, China. Cancer Epidemiol Biomarkers Prev 2005;14:1315-8. [Crossref] [PubMed]
- Liu Z, Alsaggaf R, McGlynn KA, et al. Statin use and reduced risk of biliary tract cancers in the UK Clinical Practice Research Datalink. Gut 2019;68:1458-64. [Crossref] [PubMed]
- Society AC. Available online: https://www.cancer.org/content/dam/CRC/PDF/Public/8629.00.pdf. Accessed August 13, 2019 2019.
- Srivastava A, Mittal B. Complement receptor 1 (A3650G RsaI and intron 27 HindIII) polymorphisms and risk of gallbladder cancer in north Indian population. Scand J Immunol 2009;70:614-20. [Crossref] [PubMed]
- Sakai K, Loza E, Roig GV, et al. CYP1A1, GSTM1, GSTT1 and TP53 Polymorphisms and Risk of Gallbladder Cancer in Bolivians. Asian Pac J Cancer Prev 2016;17:781-4. [Crossref] [PubMed]
- Srivastava K, Srivastava A, Mittal B. Polymorphisms in ERCC2, MSH2, and OGG1 DNA repair genes and gallbladder cancer risk in a population of Northern India. Cancer 2010;116:3160-9. [Crossref] [PubMed]
- Srivastava K, Srivastava A, Mittal B. Caspase-8 polymorphisms and risk of gallbladder cancer in a northern Indian population. Mol Carcinog 2010;49:684-92. [PubMed]
- Andreotti G, Chen J, Gao YT, et al. Polymorphisms of genes in the lipid metabolism pathway and risk of biliary tract cancers and stones: a population-based case-control study in Shanghai, China. Cancer Epidemiol Biomarkers Prev 2008;17:525-34. [Crossref] [PubMed]
- Vishnoi M, Pandey SN, Choudhuri G, et al. IL-1 gene polymorphisms and genetic susceptibility of gallbladder cancer in a north Indian population. Cancer Genet Cytogenet 2008;186:63-8. [Crossref] [PubMed]
- Srivastava A, Tulsyan S, Pandey SN, et al. Single nucleotide polymorphism in the ABCG8 transporter gene is associated with gallbladder cancer susceptibility. Liver Int 2009;29:831-7. [Crossref] [PubMed]
- Jiao X, Huang J, Wu S, et al. hOGG1 Ser326Cys polymorphism and susceptibility to gallbladder cancer in a Chinese population. Int J Cancer 2007;121:501-5. [Crossref] [PubMed]
- Srivastava A, Srivastava K, Pandey SN, et al. Single-nucleotide polymorphisms of DNA repair genes OGG1 and XRCC1: association with gallbladder cancer in North Indian population. Ann Surg Oncol 2009;16:1695-703. [Crossref] [PubMed]
- Srivastava A, Pandey SN, Choudhuri G, et al. CCR5 Delta32 polymorphism: associated with gallbladder cancer susceptibility. Scand J Immunol 2008;67:516-22. [Crossref] [PubMed]
- Yadav A, Gupta A, Rastogi N, et al. Association of cancer stem cell markers genetic variants with gallbladder cancer susceptibility, prognosis, and survival. Tumour Biol 2016;37:1835-44. [Crossref] [PubMed]
- Xu HL, Hsing AW, Vogtmann E, et al. Variants in CCK and CCKAR genes to susceptibility to biliary tract cancers and stones: a population-based study in Shanghai, China. J Gastroenterol Hepatol 2013;28:1476-81. [Crossref] [PubMed]
- Castro FA, Koshiol J, Hsing AW, et al. Inflammatory gene variants and the risk of biliary tract cancers and stones: a population-based study in China. BMC Cancer 2012;12:468. [Crossref] [PubMed]
- Vishnoi M, Pandey SN, Modi DR, et al. Genetic susceptibility of epidermal growth factor +61A>G and transforming growth factor beta1 -509C>T gene polymorphisms with gallbladder cancer. Hum Immunol 2008;69:360-7. [Crossref] [PubMed]
- Yadav S, Chandra A, Kumar A, et al. Association of TERT-CLPTM1L and 8q24 Common Genetic Variants with Gallbladder Cancer Susceptibility and Prognosis in North Indian Population. Biochem Genet 2018;56:267-82. [Crossref] [PubMed]
- Srivastava A, Sharma KL, Srivastava N, et al. Significant role of estrogen and progesterone receptor sequence variants in gallbladder cancer predisposition: a multi-analytical strategy. PLoS One 2012;7:e40162. [Crossref] [PubMed]
- Sharma KL, Misra S, Kumar A, et al. Association of liver X receptors (LXRs) genetic variants to gallbladder cancer susceptibility. Tumour Biol 2013;34:3959-66. [Crossref] [PubMed]
- Mhatre S, Wang Z, Nagrani R, et al. Common genetic variation and risk of gallbladder cancer in India: a case-control genome-wide association study. Lancet Oncol 2017;18:535-44. [Crossref] [PubMed]
- Srivastava A, Pandey SN, Choudhuri G, et al. Role of genetic variant A-204C of cholesterol 7alpha-hydroxylase (CYP7A1) in susceptibility to gallbladder cancer. Mol Genet Metab 2008;94:83-9. [Crossref] [PubMed]
- Srivastava A, Choudhuri G, Mittal B. CYP7A1 (-204 A>C; rs3808607 and -469 T>C; rs3824260) promoter polymorphisms and risk of gallbladder cancer in North Indian population. Metabolism 2010;59:767-73. [Crossref] [PubMed]
- Rai R, Sharma KL, Misra S, et al. Association of adrenergic receptor gene polymorphisms in gallbladder cancer susceptibility in a North Indian population. J Cancer Res Clin Oncol 2014;140:725-35. [Crossref] [PubMed]
- Rai R, Kim JJ, Misra S, et al. A Multiple Interaction Analysis Reveals ADRB3 as a Potential Candidate for Gallbladder Cancer Predisposition via a Complex Interaction with Other Candidate Gene Variations. Int J Mol Sci 2015;16:28038-49. [Crossref] [PubMed]
- Pandey SN, Modi DR, Choudhuri G, et al. Slow acetylator genotype of N-acetyl transferase2 (NAT2) is associated with increased susceptibility to gallbladder cancer: the cancer risk not modulated by gallstone disease. Cancer Biol Ther 2007;6:91-6. [Crossref] [PubMed]
- Dwivedi S, Agrawal S, Singh S, et al. Association of Cytochrome-17 (MspA1) Gene Polymorphism with Risk of Gall Bladder Stones and Cancer in North India. Asian Pac J Cancer Prev 2015;16:5557-63. [Crossref] [PubMed]
- Jiao X, Wu Y, Zhou L, et al. Variants and haplotypes in Flap endonuclease 1 and risk of gallbladder cancer and gallstones: a population-based study in China. Sci Rep 2015;5:18160. [Crossref] [PubMed]
- Li Z, Yuan WT, Ning SJ, et al. Vitamin D receptor genetic variants are associated with susceptibility of gallbladder adenocarcinoma in a Chinese cohort. Genet Mol Res 2014;13:5387-94. [Crossref] [PubMed]
- Tsuchiya Y, Kiyohara C, Sato T, et al. Polymorphisms of cytochrome P450 1A1, glutathione S-transferase class mu, and tumour protein p53 genes and the risk of developing gallbladder cancer in Japanese. Clin Biochem 2007;40:881-6. [Crossref] [PubMed]
- Kimura A, Tsuchiya Y, Lang I, et al. Effect of genetic predisposition on the risk of gallbladder cancer in Hungary. Asian Pac J Cancer Prev 2008;9:391-6. [PubMed]
- Park SK, Andreotti G, Sakoda LC, et al. Variants in hormone-related genes and the risk of biliary tract cancers and stones: a population-based study in China. Carcinogenesis 2009;30:606-14. [Crossref] [PubMed]
- Pandey SN, Choudhuri G, Mittal B. Association of CYP1A1 Msp1 polymorphism with tobacco-related risk of gallbladder cancer in a north Indian population. Eur J Cancer Prev 2008;17:77-81. [Crossref] [PubMed]
- Sharma KL, Agarwal A, Misra S, et al. Association of genetic variants of xenobiotic and estrogen metabolism pathway (CYP1A1 and CYP1B1) with gallbladder cancer susceptibility. Tumour Biol 2014;35:5431-9. [Crossref] [PubMed]
- Sharma KL, Misra S, Kumar A, et al. Higher risk of matrix metalloproteinase (MMP-2, 7, 9) and tissue inhibitor of metalloproteinase (TIMP-2) genetic variants to gallbladder cancer. Liver Int 2012;32:1278-86. [Crossref] [PubMed]
- Mishra K, Behari A, Kapoor VK, et al. Platelet Derived Growth Factor-B and Human Epidermal Growth Factor Receptor-2 Polymorphisms in Gall Bladder Cancer. Asian Pac J Cancer Prev 2015;16:5647-54. [Crossref] [PubMed]
- Bustos BI, Perez-Palma E, Buch S, et al. Variants in ABCG8 and TRAF3 genes confer risk for gallstone disease in admixed Latinos with Mapuche Native American ancestry. Sci Rep 2019;9:772. [Crossref] [PubMed]
- Lorenzo Bermejo J, Boekstegers F, Gonzalez Silos R, et al. Subtypes of Native American ancestry and leading causes of death: Mapuche ancestry-specific associations with gallbladder cancer risk in Chile. PLoS Genet 2017;13:e1006756. [Crossref] [PubMed]
- Cha PC, Zembutsu H, Takahashi A, et al. A genome-wide association study identifies SNP in DCC is associated with gallbladder cancer in the Japanese population. J Hum Genet 2012;57:235-7. [Crossref] [PubMed]
- Chen CY, Han J, Hunter DJ, et al. Explicit Modeling of Ancestry Improves Polygenic Risk Scores and BLUP Prediction. Genet Epidemiol 2015;39:427-38. [Crossref] [PubMed]
- Michailidou K, Lindstrom S, Dennis J, et al. Association analysis identifies 65 new breast cancer risk loci. Nature 2017;551:92-4. [Crossref] [PubMed]
- Mavaddat N, Michailidou K, Dennis J, et al. Polygenic Risk Scores for Prediction of Breast Cancer and Breast Cancer Subtypes. Am J Hum Genet 2019;104:21-34. [Crossref] [PubMed]


