Achieving margin negative resection—doing less is justified: oncological outcomes of wedge excision of liver in gallbladder cancer (GBC) surgery
Introduction
All oncological resections require adequate margin of excision to prevent local recurrence and its oncological consequences. Margin is taken mainly to account for pseudo-extensions or sub-mucosal spread which mainly depend on tumour biology. In oncology, due to rapid evolution in the understanding of biology of most cancers, increasingly conservative surgeries are now performed with equally good oncological outcomes.
Gallbladder cancer (GBC) is an inherently aggressive disease and has propensity to metastasize and fail at distant sites in up to 85% of operated cases (1). Surgery for GBC is still evolving. Routine bile duct resection which was advocated previously for nodal clearance is now performed only with an aim to achieve negative margins (2). However, the controversy of liver wedge resection or segment IVb/V anatomical resection for an oncologically adequate margin in GBC surgery is yet unresolved in absence of randomised controlled trials (RCTs).
We aimed to evaluate the peri-operative and oncological outcomes of liver wedge excision in operated patients of GBC at our institution.
Methods
Patients who underwent an upfront radical cholecystectomy (with liver wedge of 2.5–3 centimetres or clear margin on liver) from June 2010 to December 2015 at a single tertiary care centre were selected from a prospectively maintained surgical database. Patients with an incidental diagnosis of GBC requiring revision surgery or patients who had received neo-adjuvant chemotherapy (NACT) prior to surgery were excluded to avoid confounding since it would be difficult to ascertain the exact stage of disease at presentation. Data collection conformed to ethical guidelines of the declaration of Helsinki. Approval from the institutional review board (IRB) was sought (Project No. 1880). All patients signed an informed consent prior to surgery.
Patients with clinic-radiological suspicion or histologically proven GBC underwent a contrast enhanced computerised tomography (CECT) abdomen, pelvis and thorax for staging.
Intra-operatively, patients with histologically proven malignancy underwent inter-aortocaval nodal sampling as the initial step. If the nodes were reported to be negative on frozen section examination a radical cholecystectomy comprising of complete peri-portal lymphadenectomy (hepatoduodenal nodal stations 8, 12 and 13) along with a liver wedge excision of 2.5–3 cm beyond palpable disease was performed.
Patients without a confirmed diagnosis and with only radiological suspicion of malignancy underwent a cholecystectomy first for confirmation of the diagnosis on frozen section. After confirmation of malignancy these patients underwent a radical cholecystectomy as described above. In exceptional situations where the gallbladder was densely adhered to bed, an excision of gallbladder and liver wedge en-bloc was performed. Cystic duct margin was assessed on frozen section in all cases to confirm negativity and extra hepatic bile duct excision (EHBDE) was done in case of positive margin.
Post-operative course, morbidity and mortality were recorded according to Clavien Dindo classification (3).
Adjuvant chemotherapy was considered for patients with ≥ pT2 tumors and node positive disease after discussion in the multidisciplinary tumor (MDT) board. The American Joint Commission on Cancer (AJCC) 7th edition was used for pathological staging (4).
All patients were followed up at regular intervals, every 3 monthly for the first 2 years, and every 6 monthly for next 3 years. During each follow up visit, physical examination, ultrasonography (USG) of abdomen and pelvis and tumor marker carbohydrate antigen (CA19-9) were done. Patients with suspicion of recurrence underwent CECT thorax abdomen and pelvis or positron emission tomography (PET) scan depending on MDT decision. Patients who recurred were offered palliative chemotherapy.
Analysis
The data was analyzed using SPSS (Statistical Package for the Social Sciences) version 20.00. Overall survival (OS) was calculated from date of diagnosis to date of death or last follow-up date while disease free survival (DFS) was calculated from date of diagnosis to date of recurrence or death. OS and DFS were calculated by using Kaplan-Meier survival curves.
Results
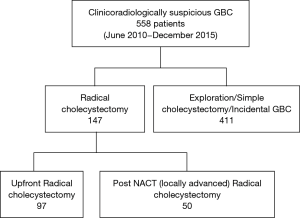
Five hundred and fifty-eight patients underwent surgery for GBC in the above time period. One hundred and forty-seven patients underwent radical cholecystectomy for primary GBC of which fifty patients had received NACT prior to surgery and were therefore excluded (Figure 1).
Ninety-seven patients were selected for our study. Male to female ratio was 1:2.88 (25 and 72), with median age of 52 years (range, 30–79 years).
All the patients underwent an open radical cholecystectomy. Median duration of surgery was 230 minutes (range, 120–480 minutes) with a median blood loss of 600 mL (range, 100–3,000 mL). Five patients underwent EHBDE and two required additional organ resection to achieve negative margin. Median post-operative stay was 6 days (4–25 days). Post-operative morbidity as per Clavien Dindo score of III and above was 9.2% (n=9) with 3.1% (n=3) patients requiring radiological or surgical intervention for bile leak. The post-operative mortality rate was 2.1% (n=2), of which one patient died secondary to biliary sepsis and pancreatitis while other patient died due to unrelated issues leading to multi-organ failure.
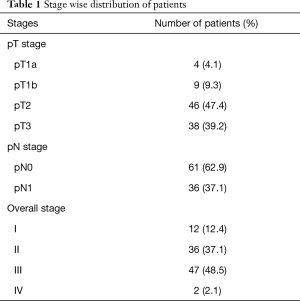
The pT stage, N stage and overall stage wise distribution of cases is shown in Table 1. The liver parenchymal cut margin was negative in all patients except one. The median number of lymph nodes retrieved was 7 (range, 3–19).
Full table
Adjuvant chemotherapy was given to 65 patients. One patient with microscopically positive liver margin received adjuvant radiation in addition.
At a median follow up of 47 months, 56 (57.7%) patients were disease free and alive where as 16 (16.5%) were alive with disease. Two (2.1%) patients died in postoperative period, 17 (17.5%) patients died of disease, and 6 (6.2%) died of unrelated causes. Thirty-three patients developed recurrence of which 11 patients had loco-regional recurrence (duodenal-1, hepatoduodenal ligament and hilar recurrence-5, peri-portal nodal recurrence-4, gallbladder bed-1) and 22 patients had distant metastases. One patient (1.03%) recurred in the gallbladder bed and developed obstructive jaundice for which percutaneous trans-hepatic biliary drainage was done followed by chemoradiation (CTRT) for tumor control. The remainder of the loco-regional recurrences were discontiguous with the gallbladder bed.
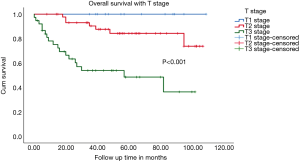
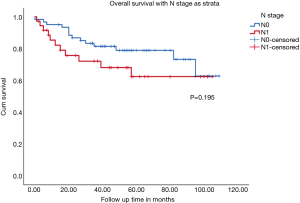
The 3-year OS of patients with pT2 disease was 84.8% and of pT3 was 52.6% (Figure 2). For patients with node positive disease, the 3-year OS was 69.4% while that of patients with node negative disease was 77% (Figure 3). Stage-wise the 3-year OS of stage III patients was 59.6% while of stage II was 86.1% (Figure 4).
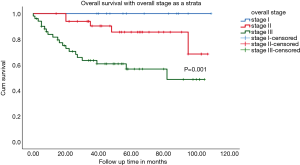
DFS when stratified according to the pathological T stage was 84% for pT2 and 45% for pT3 stage. DFS for node positive disease was 58.3% while it was 76.6% for node negative disease. Stage II patients had a DFS of 69.4% and stage III 38.3%.
Discussion
Lymphatic pathways in GBC have been extensively studied and many routes have been proposed for the involvement of segment IVb and V of liver in GBC. Gallbladder lymphatics drain by three pathways, however, none of these pathways traverse through liver (5). Only one animal study using dyes has demonstrated that in cases of obstruction of lymphatics due to tumour, gallbladder lymphatics drain through liver bed and then into hepatoduodenal ligament (6).
Venous drainage pathway as a rationale for segment IVb/V resection has also been postulated by some authors (6). Sugita et al. found that the veins emerging from neck of gallbladder drain through hepatic hilum and into segment IVa, anterior portal branch and right branch, whereas veins from body and fundus drain into segments IVa and V (7). These veins eventually drain into sinusoids and then into hepatic veins. Yoshimitsu et al. also reinforced the chloecysto-venous pathway as the main route of liver metastasis (8). This has formed the main basis for justifying a formal segment IVb/V resection in surgery for GBC.
Embryologically, segments IVb and V of liver and gallbladder are not directly related but they take their respective position later in the development due to anatomic reasons (9).
Though venous drainage pathway of gallbladder to segments IV and V looks attractive, venous drainage is not limited only to these segments, as these eventually drain into hepatic veins, and hence spread can occur in any segment of the pathway (10). Even in cases of colorectal cancer liver metastases and hepatocellular carcinoma, where vascular invasions are quite common, there is no convincing evidence to establish oncological superiority of anatomical over non anatomical resections or vice versa (11).
Few retrospective series have compared outcomes of segment IVb/V and wedge liver resection. Goetze et al. showed that survival of T2 tumors was better with segment IVb/V than with wedge resection with 5-year survival of 54% and 46% respectively but it was not statistically significant (12). While Horiguchi et al. showed no advantage of segment IVb/V resection compared to wedge resection in pT2 N0 patients (13).
Comparison of bed recurrences (Table 2) following segment IVb/V resection with wedge resection shows that even segment IVb/V resection have almost equal chances of bed recurrences (1,13,14).

Full table
Though small anatomic studies have favoured segment IVb/V resection over gallbladder wedge resection, neither surgical nor oncological outcomes of ours and similar studies favour segment IVb/V over wedge resection. It is important here to emphasize the fact that the entire cystic plate should be excised because incomplete excision of the cystic plate violates the subserosal plane of the gallbladder and thus may leave behind tumor cells in this plane. In addition, complete excision of the cystic plate facilitates removal of the adipose tissue within the triangle of Calot, which usually contains cystic duct node (15).
Until date, there has been no RCT to compare the outcomes of liver wedge versus segment IVb/V excision. A group of investigators from Saint Vincent’s Hospital, Korea is presently recruiting patients for a trial comparing liver wedge excision versus segment 4b/5 bisegmentectomy with respect to recurrence rates and survival rates (16). This trial is expected to give results by 2023. In our study, the 3-year OS of patients with pT2 disease was 84.8% and of pT3 was 52.6% with wedge excision. Survival outcomes of our series are comparable to international literature (Table 3) (17-20). This emphasises the fact that with wedge excision we are able to achieve equally good oncological outcomes. Even the peri-operative outcomes in our series compare well with published literature (13,18). Post-operative morbidity as per Clavien Dindo score of III and above was 9.2% in our series with 3.1% patients requiring intervention for bile leak. This is significantly less as compared to the post-operative morbidity of 19% seen in a study from Memorial Sloan-Kettering Cancer Center (MSKCC), which, among other factors, compared major hepatectomy and segment 4b/5 bisegmentectomy in GBC (21). In this study patients undergoing major hepatectomy had a median survival of 27 months, compared to 45 months for patients who did not undergo major hepatic resection. In our cohort of patients who underwent wedge resection of liver the median survival was observed to be 50 months. This again emphasizes the fact that extent of liver resection doesn’t improve survival as long as we have negative resection margins. In another retrospective study from the same centre where they analysed surgical trends in GBC over two decades, there was reduced likelihood of patients requiring a major hepatectomy for achieving a complete resection, without an adverse impact on OS (22).

Full table
Since only wedge excision may be adequate to achieve negative margins rather than entire anatomical segment IVb/V, whether liver resection can altogether be omitted in highly selected tumours involving only peritoneal aspect of gall bladder (pT2) is an interesting aspect that needs to be studied. It has been shown that these tumours behave differently from that occurring on the hepatic aspect (23). It has also been reclassified as T2a, in 8th edition of AJCC staging manual (24). Goel et al. evaluated the role of PET CT in revision surgery for incidental GBC and found that if there was no uptake in gallbladder bed, none of them had disease in liver wedge on final histopathology in pT1b tumors (25). They postulated omission of gallbladder bed excision in this subset during revision surgery. Whether this can be extrapolated to upfront resection in GBC is another interesting aspect to be studied, which may add value to evolving treatment paradigms in GBC management.
We had only one patient who recurred in gallbladder bed. In addition, all but one of our patients had negative margins. Hence, disease biology probably plays a more important role in recurrence than margin status. However, the retrospective nature of our study and limited numbers prevents us from making definite conclusions. Till we have larger prospective study or an RCT comparing segment IVb/V versus wedge excision of liver bed, we should aim to achieve an oncologically margin negative resection, which has been shown by the authors to be one of the main predictor of overall outcome (26). The results of the ongoing Korean study may throw some light on this issue (16).
Conclusions
Long-term outcomes of radical cholecystectomy with liver wedge resection performed at our centre parallels published international literature emphasizing oncological equivalence of liver wedge resection with negative margins. This procedure is associated with overall lower morbidity (3%) without compromising on oncological outcomes. Our experience with wedge resection gains significance in the absence of any level I evidence and can prompt multicentre RCTs in future that may help in standardizing surgery for GBC.
Acknowledgments
We acknowledge the help extended by the Department of Statistics, TMH, Mumbai.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Data collection conformed to ethical guidelines of the declaration of Helsinki. Approval from the institutional review board (IRB) was sought (Project No. 1880). All patients signed an informed consent prior to surgery.
References
- Jarnagin WR, Ruo L, Little SA, et al. Patterns of initial disease recurrence after resection of gallbladder carcinoma and hilar cholangiocarcinoma. Cancer 2003;98:1689-700. [Crossref] [PubMed]
- Shukla PJ, Barreto SG. Systematic Review: Should Routine Resection of the Extrahepatic Bile Duct Be Performed in Gallbladder Cancer? Saudi J Gastroenterol 2010;16:161-7. [Crossref] [PubMed]
- Clavien PA, Sanabria JR, Strasberg SM. Proposed classification of complications of surgery with examples of utility in cholecystectomy. Surgery 1992;111:518-26. [PubMed]
- Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 2010;17:1471-4.
- Ito M, Mishima Y, Sato T. An anatomical study of the lymphatic drainage of the gallbladder. Surg Radiol Anat 1991;13:89-104. [Crossref] [PubMed]
- Terazawa T, Miyake H, Kurahashi M, et al. Direct lymphatic spreading route into the liver from the gallbladder: an animal experiment using pig. J Med Invest 2004;51:210-7. [Crossref] [PubMed]
- Sugita M, Ryu M, Satake M, et al. Intrahepatic inflow areas of the drainage vein of the gallbladder: analysis by angio-CT. Surgery 2000;128:417-21. [Crossref] [PubMed]
- Yoshimitsu K, Honda H, Kuroiwa T, et al. Liver metastasis from gallbladder carcinoma: anatomic correlation with cholecystic venous drainage demonstrated by helical computed tomography during injection of contrast medium in the cholecystic artery. Cancer 2001;92:340-8. [Crossref] [PubMed]
- Larsen WJ. Human Embryology. 3rd edition. New York: Churchill Livingstone, 2001:575.
- Yoshimitsu K, Honda H, Kaneko K, et al. Anatomy and clinical importance of cholecystic venous drainage: helical CT observations during injection of contrast medium into the cholecystic artery. AJR Am J Roentgenol 1997;169:505-10. [Crossref] [PubMed]
- Tang H, Li B, Zhang H, et al. Comparison of Anatomical and Nonanatomical Hepatectomy for Colorectal Liver Metastasis: A Meta-Analysis of 5207 Patients. Sci Rep 2016;6:32304. [Crossref] [PubMed]
- Goetze TO, Paolucci V. Adequate extent in radical re-resection of incidental gallbladder carcinoma: analysis of the German Registry. Surg Endosc 2010;24:2156-64. [Crossref] [PubMed]
- Horiguchi A, Miyakawa S, Ishihara S, et al. Gallbladder bed resection or hepatectomy of segments 4a and 5 for pT2 gallbladder carcinoma: analysis of Japanese registration cases by the study group for biliary surgery of the Japanese Society of Hepato-Biliary-Pancreatic Surgery. J Hepatobiliary Pancreat Sci 2013;20:518-24. [Crossref] [PubMed]
- Wiggers JK, Groot Koerkamp B, Ovadia Z, et al. Patterns of recurrence after resection of gallbladder cancer without routine extrahepatic bile duct resection. HPB 2014;16:635-40. [Crossref] [PubMed]
- Shirai Y, Sakata J, Wakai T, et al. "Extended" radical cholecystectomy for gallbladder cancer: long-term outcomes, indications and limitations. World J Gastroenterol 2012;18:4736-43. [Crossref] [PubMed]
- You DD. The Effect in Wedge Resection and IVb/V Resection of the Liver for Gallbladder Cancer. ClinicalTrials.gov Identifier: NCT02920554.
- Kayahara M, Nagakawa T. Recent trends of gallbladder cancer in Japan: an analysis of 4,770 patients. Cancer 2007;110:572-80. [Crossref] [PubMed]
- Fong Y, Jarnagin W, Blumgart LH. Gallbladder Cancer: Comparison of Patients Presenting Initially for Definitive Operation With Those Presenting After Prior Noncurative Intervention. Ann Surg 2000;232:557-69. [Crossref] [PubMed]
- Bartlett DL, Fong Y, Fortner JG, et al. Long-term results after resection for gallbladder cancer. Implications for staging and management. Ann Surg 1996;224:639-46. [Crossref] [PubMed]
- Hwang KY, Yoon YI, Hwang S, et al. Survival analysis following resection of AJCC stage III gallbladder carcinoma based on different combinations of T and N stages. Korean J Hepatobiliary Pancreat Surg 2015;19:11-6. [Crossref] [PubMed]
- D’Angelica M, Dalal KM, DeMatteo RP, et al. Analysis of the Extent of Resection for Adenocarcinoma of the Gallbladder. Ann Surg Oncol 2009;16:806-16. [Crossref] [PubMed]
- Creasy JM, Goldman DA, Gonen M, et al. Evolution of surgical management of gallbladder carcinoma and impact on outcome: results from two decades at a single-institution. HPB (Oxford) 2019. [Epub ahead of print].
- Shindoh J, de Aretxabala X, Aloia TA, et al. Tumor location is a strong predictor of tumor progression and survival in T2 gallbladder cancer: an international multicenter study. Ann Surg 2015;261:733-9. [Crossref] [PubMed]
- AJCC Cancer Staging Manual. Amin MB, Edge S, Greene F, et al. editors. Springer. Cited 2017 Jul 9. Available online: http://www.springer.com/in/book/9783319406176
- Goel M, Tamhankar A, Rangarajan V, et al. Role of PET CT scan in redefining treatment of incidental gall bladder carcinoma. J Surg Oncol 2016;113:652-8. [Crossref] [PubMed]
- Patkar S, Ostwal V, Ramaswamy A, et al. Emerging role of multimodality treatment in gall bladder cancer: Outcomes following 510 consecutive resections in a tertiary referral center. J Surg Oncol 2018;117:372-9. [Crossref] [PubMed]