
Interventional radiology’s role in the diagnosis and management of patients with gallbladder carcinoma
Introduction
Gallbladder carcinoma is an aggressive, often rare biliary tract malignancy in most western countries, though is more widespread in other regions of the world, with a particularly high incidence observed in Japan, Chile, India, and Bolivia (1-3). It is the 6th most common gastrointestinal malignancy in the United States, and is more common in women (1,2). Adenocarcinoma accounts for 90% of gallbladder carcinoma (4), while squamous cell carcinomas of the gallbladder have been reported to account for 2–12%. Other rarer forms of gallbladder carcinoma include sarcomas, lymphomas, and carcinoid (1).
The diagnosis is usually made late in the disease course as patients are often initially asymptomatic or present with nonspecific complaints including anorexia, weight loss, abdominal pain, and jaundice (5). However, several key risk factors that the treating practitioner should be cognizant of to aid in making an early diagnosis when possible are detailed below. Cholelithiasis is a major risk factor for gallbladder carcinoma, and is seen in 74–92% of cases (1). Although gallstones are a described risk factor for development of gallbladder carcinoma, Comfort et al. showed that less than 1% of patients with asymptomatic gallstones developed gallbladder carcinoma over a 10–25-year period (6). Porcelain gallbladder is another risk factor, and is present in 10–25% of patients with gallbladder carcinoma (7). Smoking increases the risk of developing gallbladder carcinoma by as much as five-fold (6). An additional risk factor for squamous cell carcinoma of the gallbladder is exposure to parasitic infections (8). Unfortunately, these known risk factors are nonspecific and gallbladder carcinomas still often present in advanced stages with local spread and distant metastatic disease. This leads to a dismal prognosis in many patients with an overall mean survival at the time of diagnosis of 6 months and a 5-year survival rate of only 5% (1,9,10).
Treatment with extended (radical) cholecystectomy where surgical resection is extended beyond the gallbladder to include adjacent structures such as portions of the liver can be curative if gallbladder carcinoma is identified in early stages which is approximated to occur in only 10–30% of patients (1). The dismal prognosis for gallbladder carcinoma patients is related in part to the gallbladder lacking a serosal layer adjacent to the liver, enabling hepatic invasion with potential biliary obstruction and metastatic progression (4,10). As the majority of patients presenting with gallbladder carcinoma are not candidates for primary surgical resection, percutaneous interventions are often critical to aid treatments and manage their disease. This review will detail important diagnostic findings, key image-guided procedures and the role interventional radiologists play in the management of patients suffering from gallbladder carcinoma. It will further aid referring practitioners understanding of potential interventional options to optimize their care of patients suffering from metastatic gallbladder carcinoma.
Imaging findings
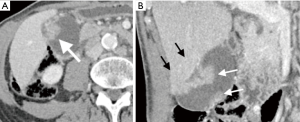
Gallbladder carcinoma is often undetected until late in the disease course with diagnostic imaging. However, an overview of commonly observed findings can be critical for enabling early diagnosis when possible and can help guide treatment and proper management in later disease states. Sonography, CT, and MRI are commonly used diagnostic tools which can show a large gallbladder mass that nearly fills or replaces the lumen and often invades into the adjacent hepatic parenchyma (2,11). On non-contrast CT, gallbladder carcinoma is often hypodense. Following the administration of IV contrast, approximately 40% will show a hypervascular focus of enhancement (2,12,13). MRI can show hypointense or hyperintense signal on T1-weighted images of the gallbladder and hyperintense signal on T2-weighted images (4). CT and MRI both show irregular, intense enhancement in the periphery in early arterial phase, and will be retained in fibrous components of the primary lesion on the portal venous phase (Figure 1) (2). PET-CT typically shows intense hypermetabolic FDG activity at the location of the tumor and metastases (2). Diffuse, focal, or asymmetric wall thickening is seen in 20–30% of cases on initial studies (12). Initial detection of gallbladder carcinoma can occur as a polypoid lesion in 15–25% of cases, with malignant polyps usually measuring greater than 1 cm (14). While these imaging findings alone are not diagnostic of gallbladder carcinoma, they should raise suspicion of gallbladder carcinoma and aid in further diagnostic workup and treatment management.
Percutaneous biopsy and its emerging role in molecular diagnostics
Assessment of tissue specimens remains critical for gallbladder carcinoma diagnosis (15). While the diagnosis is sometimes obtained from analysis of surgical specimens, the uncertain etiology and late stage of presentation often requires a biopsy by either an endoscopic or percutaneous approach. Endoscopic ultrasound (EUS)-guided fine needle aspiration (FNA) can offer sampling of the lesion (5), though the diagnosis is not always clear on imaging with early stage gallbladder carcinoma sometimes mimicking cholecystitis. In these cases, cholecystectomy is often performed with gallbladder carcinoma incidentally detected histologically from the surgical specimens. This is reported to occur in 0.3–3% of laparoscopic cholecystectomies performed for cholelithiasis (16). In a large 1999 international survey, Paolucci et al. found that 409 of 117,840 (0.3%) of cholecystectomies performed for benign disease had incidental GBC (17). An earlier prospective analysis by the Southern Surgeons Club in 1991 (18) found that the majority of new cases of gallbladder carcinoma diagnosed in the United States are found incidentally on pathologic specimens following cholecystectomy for cholelithiasis, with reported rates of 1–2%. When a gallbladder with an incidental carcinoma is removed laparoscopically there is an increased rate of peritoneal and port site dissemination and care should therefore be taken to properly diagnose gallbladder carcinoma prior to surgery when possible to minimize this risk (9).
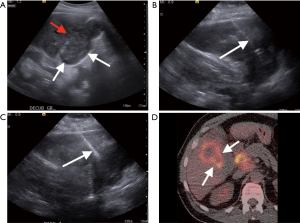
Percutaneous FNA of the gallbladder for the diagnosis of suspected gallbladder carcinoma is controversial, as some authors advocate that it increases the risk of biliary peritonitis (19). However, Rana et al. found that ultrasound guided FNA of gallbladder lesions performed by an experienced interventional radiologist with a pathologist present for rapid on-site evaluation (ROSE) did not experience any biliary peritonitis in 596 cases over a 5-year period. While the potential risk of biliary peritonitis following a percutaneous FNA is unclear, FNAs are currently commonly performed in some practices (19). Figure 2 demonstrates an image guided percutaneous FNA and biopsy of a gallbladder carcinoma invading the liver, which confirmed the diagnosis of gallbladder carcinoma. Since many gallbladder carcinomas present in advanced stages as masses, cellular aspirates are often easy to obtain and yield definitive diagnostic results. The diagnosis can be more challenging in cases where only wall thickening is present. However, the diagnostic yield of a percutaneous biopsy is still relatively high with a success rate reported of greater than 90%. Percutaneous biopsy can also play a role in patients with disseminated disease or in patients who are not candidates for surgical resection as a method to confirm the diagnosis before palliative therapy, experimental trials, or hospice (2).
Molecular testing and genomic profiling are likely to play an increasing role in the treatment planning for patients suffering from gallbladder carcinoma and biopsies will therefore likely be required with increasing frequency. Hundreds of genes have been implicated in the pathogenesis and disease progression of gallbladder carcinoma (20). Despite the advances made in the understanding of genetic aberrations contributing to gallbladder carcinoma, to date there is no established genetic marker in routine clinical use for diagnosis (20). Future studies will likely play an important role in tailoring treatments based on the genetic characteristics of a patient’s gallbladder carcinoma and biopsies are likely to play a critical role as treatments become personalized to each gallbadder carcinoma’s genetic makeup.
Management of biliary leaks
Postoperative bile leaks after resection of gallbladder carcinoma have increased in frequency over the past 3 decades due to the development of laparoscopic biliary surgery, and can also occur after trauma or any procedure affecting the biliary system such as endoscopic retrograde cholangiopancreatography (ERCP), percutaneous transhepatic cholangiography (PTC), ablation of hepatic tumors, or even liver biopsy (21). Additionally, bilomas have even been observed with some hepatobiliary tumors that have obstructed the biliary system independent of a procedure. For example, some patients with gallbladder carcinoma whose tumors have obstructed the biliary system and caused bile duct dilatation have been found to eventually develop bilomas (22).
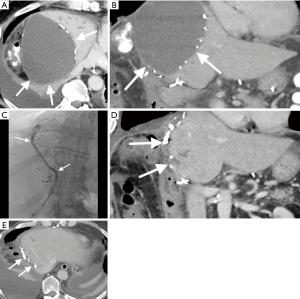
Biliary leaks most often occur within one week to one month postoperatively and the clinical presentation can include right upper quadrant pain, nausea, vomiting, anorexia, and fever. Laboratory findings include leukocytosis and abnormal liver function tests. A bile leak can lead to a biloma, or an organized fluid collection containing bile, which often is accompanied by an inflammatory reaction resulting in fibrosis. Diagnosis often involves CT, however ultrasound, hepatobiliary cholescintigraphy (HIDA), and MRI may also be useful. Management often involves PTC, biliary diversion away from injured bile ducts, and percutaneous drainage of bilomas. PTC is used to study the anatomy of the biliary tree, aid in procedural planning, and identify the site of bile injury. Once the anatomy and bile injury is well understood, the interventional radiologist can advance an 18- to 22-gauge needle to the fluid collection percutaneously under ultrasound, CT, or fluoroscopic guidance. This is followed by serial dilation over a wire and subsequent drain placement. Drains are left in place until the patient’s clinical picture improves, drain output decreases, and imaging demonstrates near or complete resolution of fluid collections. Figure 3 demonstrates presence of a post partial hepatectomy biloma in a patient suffering from biliary tract cancer, which nearly completely resolved following placement of a percutaneous biloma drain.
Preoperative portal vein embolization (PVE)
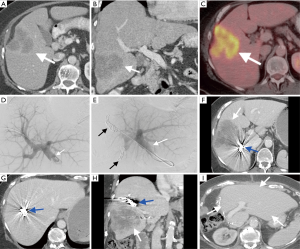
In patients with stage I gallbladder carcinoma who are good surgical candidates, resection is often attempted following cholecystectomy to improve overall survival (OS) (14). Pre-operative planning is critical as surgery often includes resection of hepatic margins bordering the gallbladder fossa with an en bloc nodal dissection (14). Since the extent of hepatic involvement in gallbladder carcinoma is often advanced, more aggressive surgeries are often required, and future liver remnant (FLR) hypertrophy following PVE when possible can facilitate safe resection of larger portions of diseased liver (23). PVE can increase the volume of the FLR, with a goal of FLR hypertrophy of >20% of the initial functional liver and can be accomplished with particles, glue, ethanol, coils or in combination (23-26). The most commonly used agents are a combination of N-butyl-2-cyanoacrylate (NBCA), microspheres and coils (25). Figure 4 demonstrates a patient with bile duct cancer who underwent PVE prior to surgical resection with subsequent dramatic hypertrophy of the FLR.
PVE has been shown to be an effective method of stimulating liver hypertrophy in the setting of hepatobiliary cancers including gallbladder carcinoma (23,24,27). When PVE is not performed, there is an increased risk of postoperative hepatic failure (24).
Percutaneous biliary drainage to manage biliary obstruction
Malignant obstructive jaundice is a common cause of morbidity in hepatobiliary cancers including gallbladder carcinoma. Drainage of biliary obstructions are typically performed either via percutaneous transhepatic biliary drain placement or placement of an endoscopic biliary drain (28). In a recent meta-analysis by Duan et al., they concluded that both percutaneous and endoscopic biliary drains have similar therapeutic success rates (28). Percutaneous biliary drains in the setting of malignant obstruction are often essential in the setting of high bile duct obstructions (intrahepatic or at the porta hepatis) or when endoscopic interventions have failed, whereas endoscopic interventions are preferred in the setting of low biliary obstruction (extrahepatic biliary obstruction) (29). Percutaneous biliary drain placement is often indicated as a method to palliate symptoms of obstruction, including pruritus due to elevated bilirubin or to treat cholangitis (29,30). Because external catheters can negatively impact patient quality of life and can be inadvertently retracted, the ultimate goal for patients with short life expectancies is to provide a completely internal drain (29). The interventional radiologist can percutaneously perform primary biliary stenting by placing a stent across the underlying obstruction and help alleviate some of the patient’s discomfort. Maybody et al. found that following the placement of a single self-expanding metallic stent for high bile duct obstruction, no obstruction was observed 9 months following placement (31). Additional stents can be placed if needed to relieve obstructions, though the average patency has been shown to be decreased to approximately 7 months in these cases (31).
Transarterial therapy for intrahepatic metastases
In the majority of gallbladder carcinoma patients who are no longer surgical candidates, minimally invasive locoregional therapies such as transarterial therapies may be a treatment consideration. Embolization procedures have been widely studied for the treatment of some hepatic metastases. In this approach, embolic materials and/or chemotherapeutic or internal radiation are delivered via the arteries feeding the tumors. Liver malignancies mainly derive their blood supply from the hepatic arteries while healthy liver tissue predominantly receives its blood supply from portal vein branches. This constitutes the principle for selectively targeted intraarterial therapy where the healthy liver is predominantly spared. These treatment methods are often used to treat hepatocellular carcinoma (HCC), and have been shown to improve survival (32). Additionally, in the past decade the use of image-guided locoregional therapies as a palliative option in hepatobiliary cancers such as unresectable intrahepatic cholangiocarcinoma (ICC), has been an increasingly accepted management approach (33).
Catheter-based intraarterial therapies are becoming an increasingly used locoregional therapy approach for the treatment of unresectable intrahepatic biliary tract cancer with studies demonstrating promising survival benefits. Scheuermann et al. performed a retrospective study where they analyzed survival rates among all available therapeutic options for ICC at their institution (34). Out of 273 patients with ICC, 130 (47.6%) underwent surgical resection, 111 (40.7%) received systemic chemotherapy/best supportive care and a total of 32 (11.7%) underwent trans-arterial chemoembolization (TACE) with mitomycin-c (n=29) or drug (doxorubicin)-eluting beads (DEB) (n=3). The median OS of surgical patients was 28 months and 37 months in patients with R0 and N0 disease. There was no survival difference observed between surgical patients with positive resection margins (11 months) or lymph node positive disease (9 months) compared to patients who underwent conventional TACE (cTACE) or DEB-TACE (11 months). Multiple additional studies have also shown improved OS in a variety of patients receiving cTACE including patients treated for palliation (35), and as an adjuvant therapy after radical surgery (36). A small number of prospective studies have been performed with the use of cTACE, and demonstrated promising results in some patients. However, results from large randomized clinical trials are needed to optimally determine which specific patients suffering from ICC would benefit the most from cTACE (37-40).
Additional studies in patients suffering from biliary tract cancer who were treated with DEB-TACE also have shown promising results. The aim of DEB-TACE is to reduce the risk of systemic distribution and increase intra-tumoral drug concentrations of chemotherapeutic agents, improving therapeutic effectiveness through a combination of an embolic effect and drug-elution. Poggi et al. performed a retrospective study of nine patients with hepatic malignancies, seven of which were ICC, who received oxaliplatin-preloaded microspheres combined with systemic chemotherapy (oxaliplatin and gemcitabine) compared to eleven patients who were treated with chemotherapy that is 5-fluorouracil and oxaliplatin based, and found that the median OS after DEB-TACE and chemotherapy was 30 months compared to 12.7 months for chemotherapy alone (41). In a prospective study which compared irinotecan-eluting beads (iDEB-TACE) with cTACE and systemic chemotherapy (gemcitabine and oxaliplatin), the iDEB-TACE group had a prolonged median OS of 11.7 months compared to median OS of systemic chemotherapy of 11.0 months and 5.7 months in the cTACE group suggesting the potential additional benefit of iDEB-TACE compared to cTACE (42). The therapeutic effect of TACE and DEB-TACE in the treatment of metastatic gallbladder carcinoma is not well known.
Yttrium-90 radioembolization (Y90-RE) is a form of selective internal radiation therapy (SIRT) where small embolic particles (20–40 µm) containing the radionucleotide Y90 emits β-radiation that is delivered to tumors intra-arterially (43). It is becoming a commonly utilized option for the treatment of primary hepatic malignancies and metastatic hepatic lesions. Y90 radioembolization can currently be performed with glass-based microspheres (TheraSphere, BTG, Canada) and resin-based microspheres (SIR-Sphere, Sirtex, Australia). Given the severe radiation potency of radioembolization and risk of non-target delivery of the microspheres, patients undergo a shunt evaluation using technetium-99 macro-agglutinated albumin (Tc-MAA), SPECT and angiographic imaging prior to receiving the Y90 therapy (44,45). SIR-Spheres are currently FDA approved for the treatment of unresectable metastatic liver tumors from primary colorectal cancer with adjuvant intra-hepatic artery chemotherapy (IHAC) of floxuridine while TheraSpheres are FDA approved as a Humanitarian Device for the treatment of unresectable HCC. Applications of Y90 in patients with other malignancies such as metastatic hepatobiliary cancers require institutional review board (IRB) approval (33).
Most of the current literature investigates the use of Y-90 in the treatment of HCC, metastatic colorectal cancer, and metastatic neuroendocrine carcinoma (NEC) (32). The efficacy of Y-90 against biliary tract cancers in improving OS and treatment response is currently variable in part due to limited data or the majority of the data derived from small retrospective or prospective cohort studies (46). However, some studies using Y-90 against biliary cancers such as ICC have shown promising results in unresectable disease and for downstaging the disease to enable secondary resection (47,48). Multiple studies have shown that Y90 can be effectively used in some patients with unresectable ICC (44,49-51). There is some suggestion that the greatest survival benefits are in patients with little to no cancer related symptoms at baseline and no portal vein thrombosis prior to treatment (49).
While data exists regarding the role and benefit of embolization in the treatment of intrahepatic metastatic disease for hepatobiliary malignancies (52), the optimal treatment method is still debated (53). In a multi-institutional pooled cohort analysis, Hyder et al. showed that intraarterial therapy (Y90 and TACE) for advanced ICC was safe and led to good disease control in the majority of patients (54). The first randomized controlled trial to compare the efficacy of Y90 and cTACE in 24 patients with unresectable ICC by radiographic response on contrast-enhanced MRI (ceMRI) in 24 patients is underway and will hopefully aid our understanding of the best treatment options for these patients (40). Treatments for metastatic gallbladder carcinoma have been utilized at some institutions with Y90 following IRB approval. Figure 5 demonstrates a patient with metastatic gallbladder carcinoma to the liver who was treated with SIRspheres.
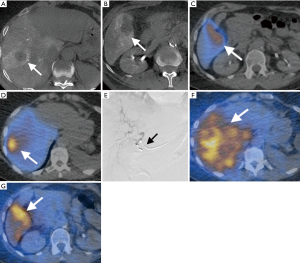
Future studies are necessary though to best assess which GBC patients are most likely to benefit from this treatment approach.
Thermal ablation as treatment for intrahepatic metastases
Percutaneous image guided thermal ablation such as microwave ablation and radiofrequency ablation (RFA) are being utilized with increasing frequency for the treatment of patients with liver metastases who are not surgical candidates and may become more commonly used in metastatic gallbladder carcinoma treatment given its propensity for hepatic involvement (3,32). Patients with less than three to four lesions measuring less than 3–4 cm in diameter as seen on cross-sectional imaging are typically considered the ideal candidates for this approach (32). A 2011 study by Kim et al. found that RFA was effective in controlling small (<3 cm in diameter) ICC with a median survival of 38.5 months (55). Microwave ablation has been shown to be an effective treatment for primary hepatic malignancies and malignancies with an avidity for liver metastasis suggesting that this treatment may become a viable option for certain patients with metastatic gallbladder carcinoma (56). Future studies will be necessary to identify the optimal ablation technique and whether patients with particular gallbladder carcinoma variants and presentations are most likely to respond favorably to this therapy.
Hepatic artery infusion (HAI) pumps
HAI pumps are implantable devices that can be safely placed intra-arterially as an adjunct to other therapies (57). HAI have been placed in cases of hepatic metastatic disease, enabling local, continuous infusion of cytotoxic agents to maximize direct tumor effects and minimize systemic side effects (57). In patients with unresectable advanced gallbladder carcinoma, this technique has been described in the literature (58) with some case reports showing disease regression and extended survival by 9–15 months (58-61). Ideal candidates are reported as those with less than 70% hepatic tumor burden, preserved hepatic function, no portal hypertension or portal vein thrombosis (57). In patients with unresectable intrahepatic cholangiocarcinoma confined to the liver or with limited regional nodal disease, a combination of systemic therapy plus HAI chemotherapy showed greater survival than systemic therapy alone (62). Further data related to the utilization of HAI is evolving.
Celiac plexus blocks and pain palliation
Palliation can be critical for improving quality of life in patients suffering from gallbladder carcinoma as the majority present with incurable disease. Pain is one of the most frequent symptoms encountered with aggressive abdominal malignancies and efforts to minimize these symptoms can greatly reduce patient suffering. The celiac plexus is the largest visceral plexus, and serves as a major relay center for nociception in the upper abdomen (63). Although medical management continues to be the first line standard of care for oncologic pain, celiac plexus blocks using lidocaine or steroids and celiac plexus neurolysis using agents such as ethanol or phenol have all been utilized in upper abdominal cancers as a method to treat intractable pain (64-67). While celiac plexus blocks provide short-term pain relief, celiac plexus neurolysis has been shown to offer effective, immediate onset, long lasting pain reduction (64-66,68). In a recent case report by Kanthed et al., they found that in a patient with unresectable gallbladder carcinoma with intractable abdominal pain, significant pain relief was experienced immediately following celiac plexus neurolysis, the need for opioid intake was reduced, and the patient reported improved quality of life on follow-up visits (68). Polati et al. found that analgesic drug consumption was significantly lower and quality of life scores significantly improved following celiac plexus blocks in patients with pancreatic cancer (64). A recent retrospective analysis by Koyyalagunta et al. investigating the use of alcohol and phenols in celiac plexus neurolysis found that approximately 45% of patients with pancreatic cancer who underwent the procedure had a 30% or greater reduction in their pain until death (66). Interestingly, patients who underwent celiac plexus neurolysis also had significant improvements in anxiety and depression, suggesting there may be treatment benefits beyond pain control (66). Unilateral or bilateral blocks or neurolysis can be used, and selection should be based on clinical indications and the patient’s overall clinical picture. While pain relief following celiac plexus neurolysis is a clear benefit to these patients, it is important to note that severe coagulopathy and thrombocytopenia are contraindications that must be considered before performing this procedure (69). Other contraindications that can limit the ability to perform the celiac plexus neurolysis include vascular aneurysms in close proximity to the celiac plexus, intra-abdominal infection or sepsis, patients with bowel obstruction, and inability to visualize anatomic structures (63,69). This procedure ultimately has potential to bring pain relief to many patients suffering from incurable abdominal malignancies and it is important to be aware of the potential benefit of this procedure for metastatic gallbladder carcinoma patients who are good candidates.
Conclusions
The interventional radiologist plays an important and increasing role in the diagnosis, management, and treatment of gallbladder carcinoma. While surgical resection of gallbladder carcinoma remains the gold standard for treatment and leads to the best OS when possible, the majority of gallbladder carcinoma patients present with nonspecific findings, require tissue for accurate diagnosis and present in a late stage of disease when they are no longer surgical candidates. When the patient with metastatic gallbladder carcinoma is still determined to be a candidate for surgery, the interventional radiologist plays a critical role in aiding surgical success and management of surgical complications. Procedures such as PVE aid hypertrophy of the FLR and enable safe larger hepatic resections. Post-surgical bile leaks can pose significant mortality and can be effectively managed via percutaneous drain placements.
In the majority of cases where patients are no longer surgical candidates and may still need tissue samples for pathologic diagnosis, interventional radiologists can play an even larger role in the management, and treatment of metastatic gallbladder carcinoma. Percutaneous biopsy enables pathologic diagnosis of metastatic or primary disease. Gallbladder carcinoma symptoms can be relieved through percutaneous procedures such as biliary drain placement to relieve obstruction, and celiac plexus blocks can be performed for pain relief. Interventional radiologists can further treat gallbladder carcinoma metastases through a variety of approaches including embolizations, HAI, and percutaneous thermal ablations though further studies are necessary to determine effectiveness of these treatments in various metastatic gallbladder carcinoma settings.
This review discusses key interventional procedures that all healthcare providers should be familiar with to aid in the diagnosis, management, and treatment of gallbladder carcinoma. As molecular diagnostics and tailored patient specific therapies continue to develop, the role that minimally invasive interventional procedures will play in the care of patients suffering from gallbladder carcinoma will continue to grow.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Revzin MV, Scoutt L, Smitaman E, et al. The gallbladder: uncommon gallbladder conditions and unusual presentations of the common gallbladder pathological processes. Abdom Imaging 2015;40:385-99. [Crossref] [PubMed]
- Furlan A, Ferris JV, Hosseinzadeh K, et al. Gallbladder carcinoma update: Multimodality imaging evaluation, staging, and treatment options. AJR Am J Roentgenol 2008;191:1440-7. [Crossref] [PubMed]
- Misra S, Chaturvedi A, Misra NC, et al. Carcinoma of the gallbladder. Lancet Oncol 2003;4:167-76. [Crossref] [PubMed]
- Levy AD, Murakata LA, Charles Rohrmann MA. Gallbladder Carcinoma: Radiologic-Pathologic Correlation. Radiographics 2001;21:295-314. [Crossref] [PubMed]
- Kim HJ, Lee SK, Jang JW, et al. Diagnostic role of endoscopic ultrasonography-guided fine needle aspiration of gallbladder lesions. Hepatogastroenterology 2012;59:1691-5. [PubMed]
- Comfort MW, Gray HK, Wilson JM. The silent gallstone; a 10- to 20 year follow-up study of 112 cases. Ann Surg 1948;128:931-7. [Crossref] [PubMed]
- Berk RN, Armbuster TG, Saltzstein SL. Carcinoma in the Porcelain Gallbladder. Radiology 1973;106:29-31. [Crossref] [PubMed]
- Roppongi T. Minute Squamous Cell Carcinoma of the Gallbladder: a Case Report. Jpn J Clin Oncol 2000;30:43-5. [Crossref] [PubMed]
- Varshney S, Buttirini G, Gupta R. Incidental carcinoma of the gallbladder. Eur J Surg Oncol 2002;28:4-10. [Crossref] [PubMed]
- Hundal R, Shaffer EA. Gallbladder cancer: Epidemiology and outcome. Clin Epidemiol 2014;6:99-109. [PubMed]
- Rodríguez-Fernández A, Gómez-Río M, Medina-Benítez A, et al. Application of modern imaging methods in diagnosis of gallbladder cancer. J Surg Oncol 2006;93:650-64. [Crossref] [PubMed]
- Franquet T, Montes M, Ruiz de Azua Y, et al. Primary gallbladder carcinoma: Imaging findings in 50 patients with pathologic correlation. Gastrointest Radiol 1991;16:143-8. [Crossref] [PubMed]
- Yun EJ, Cho SG, Park S, et al. Gallbladder carcinoma and chronic cholecystitis: Differentiation with two-phase spiral CT. Abdom Imaging 2004;29:102-8. [Crossref] [PubMed]
- Reid KM, Ramos-De la Medina A, Donohue JH. Diagnosis and surgical management of gallbladder cancer: A review. J Gastrointest Surg 2007;11:671-81. [Crossref] [PubMed]
- Aloia TA, Járufe N, Javle M, et al. Gallbladder Cancer: Expert consensus statement. HPB (Oxford) 2015;17:681-90. [Crossref] [PubMed]
- Pilgrim CH, Usatoff V, Evans P. Consideration of anatomical structures relevant to the surgical strategy for managing gallbladder carcinoma. Eur J Surg Oncol 2009;35:1131-6. [Crossref] [PubMed]
- Paolucci V, Schaeff B, Schneider M, et al. Tumor seeding following laparoscopy: International survey. World J Surg 1999;23:989-95; discussion 996-7. [Crossref] [PubMed]
- Southern Surgeons Club. A prospective analysis of 1518 laparoscopic cholecystectomies. The Southern Surgeons Club. N Engl J Med 1991;324:1073-8. [Crossref] [PubMed]
- Rana C, Krishnani N, Kumari N. Ultrasound-guided fine needle aspiration cytology of gallbladder lesions: a study of 596 cases. Cytopathology 2016;27:398-406. [Crossref] [PubMed]
- Sharma A, Sharma KL, Gupta A, et al. Gallbladder cancer epidemiology, pathogenesis and molecular genetics: Recent update. World J Gastroenterol 2017;23:3978-98. [Crossref] [PubMed]
- Nikpour AM, Knebel RJ, Cheng D. Diagnosis and management of postoperative biliary leaks. Semin Intervent Radiol 2016;33:307-12. [Crossref] [PubMed]
- Soyer P, Gouhiri MH, Boudiaf M, et al. Carcinoma of the gallbladder: Imaging features with surgical correlation. AJR Am J Roentgenol 1997;169:781-5. [Crossref] [PubMed]
- Ebata T, Yokoyama Y, Igami T, et al. Portal vein embolization before extended hepatectomy for biliary cancer: Current technique and review of 494 consecutive embolizations. Dig Surg 2012;29:23-9. [Crossref] [PubMed]
- Cotroneo AR, Innocenti P, Marano G, et al. Pre-hepatectomy portal vein embolization: Single center experience. Eur J Surg Oncol 2009;35:71-8. [Crossref] [PubMed]
- Li D, Madoff DC. Portal vein embolization for induction of selective hepatic hypertrophy prior to major hepatectomy: rationale, techniques, outcomes and future directions. Cancer Biol Med 2016;13:426-42. [Crossref] [PubMed]
- May BJ, Madoff DC. Controversies of preoperative portal vein embolization. Hepat Oncol 2016;3:155-66. [Crossref] [PubMed]
- Hwang S, Ha TY, Ko GY, et al. Preoperative Sequential Portal and Hepatic Vein Embolization in Patients with Hepatobiliary Malignancy. World J Surg 2015;39:2990-8. [Crossref] [PubMed]
- Duan F, Cui L, Bai Y, et al. Comparison of efficacy and complications of endoscopic and percutaneous biliary drainage in malignant obstructive jaundice: A systematic review and meta-analysis. Cancer Imaging 2017;17:27. [Crossref] [PubMed]
- Deipolyi AR, Covey AM. Palliative Percutaneous Biliary Interventions in Malignant High Bile Duct Obstruction. Semin Intervent Radiol 2017;34:361-8. [Crossref] [PubMed]
- Levy JL, Sudheendra D, Dagli M, et al. Percutaneous biliary drainage effectively lowers serum bilirubin to permit chemotherapy treatment. Abdom Radiol (NY) 2016;41:317-23. [Crossref] [PubMed]
- Maybody M, Brown KT, Brody LA, et al. Primary patency of wallstents in malignant bile duct obstruction: Single vs. two or more noncoaxial stents. Cardiovasc Intervent Radiol 2009;32:707-13. [Crossref] [PubMed]
- Brown KT, Do RK, Gonen M, et al. Randomized trial of hepatic artery embolization for hepatocellular carcinoma using doxorubicin-eluting microspheres compared with embolization with microspheres alone. J Clin Oncol 2016;34:2046-53. [Crossref] [PubMed]
- Savic LJ, Chapiro J, Geschwind JH. Intra-arterial embolotherapy for intrahepatic cholangiocarcinoma: update and future prospects. HepatoBiliary Surg Nutr 2017;6:7-21. [Crossref] [PubMed]
- Scheuermann U, Kaths JM, Heise M, et al. Comparison of resection and transarterial chemoembolisation in the treatment of advanced intrahepatic cholangiocarcinoma - A single-center experience. Eur J Surg Oncol 2013;39:593-600. [Crossref] [PubMed]
- Park SY, Kim JH, Yoon HJ, et al. Transarterial chemoembolization versus supportive therapy in the palliative treatment of unresectable intrahepatic cholangiocarcinoma. Clin Radiol 2011;66:322-8. [Crossref] [PubMed]
- Shen WF, Zhong W, Liu Q, et al. Adjuvant transcatheter arterial chemoembolization for intrahepatic cholangiocarcinoma after curative surgery: Retrospective control study. World J Surg 2011;35:2083-91. [Crossref] [PubMed]
- Kiefer MV, Albert M, McNally M, et al. Chemoembolization of intrahepatic cholangiocarcinoma with cisplatinum, doxorubicin, mitomycin C, ethiodol, and polyvinyl alcohol. Cancer 2011;117:1498-505. [Crossref] [PubMed]
- Vogl TJ, Naguib NN, Nour-Eldin NE, et al. Transarterial chemoembolization in the treatment of patients with unresectable cholangiocarcinoma: Results and prognostic factors governing treatment success. Int J Cancer 2012;131:733-40. [Crossref] [PubMed]
- Burger I, Hong K, Schulick R, et al. Transcatheter arterial chemoembolization in unresectable cholangiocarcinoma: Initial experience in a single institution. J Vasc Interv Radiol 2005;16:353-61. [Crossref] [PubMed]
- Kloeckner R, Ruckes C, Kronfeld K, et al. Selective internal radiotherapy (SIRT) versus transarterial chemoembolization (TACE) for the treatment of intrahepatic cholangiocellular carcinoma (CCC): Study protocol for a randomized controlled trial. Trials 2014;15:311. [Crossref] [PubMed]
- Poggi G, Amatu A, Montagna B, et al. OEM-TACE: A new therapeutic approach in unresectable intrahepatic cholangiocarcinoma. Cardiovasc Intervent Radiol 2009;32:1187-92. [Crossref] [PubMed]
- Kuhlmann JB, Euringer W, Spangenberg HC, et al. Treatment of unresectable cholangiocarcinoma: Conventional transarterial chemoembolization compared with drug eluting bead-transarterial chemoembolization and systemic chemotherapy. Eur J Gastroenterol Hepatol 2012;24:437-43. [PubMed]
- Chakravarty R, Dash A. Availability of Yttrium-90 from Strontium-90: A Nuclear Medicine Perspective. Cancer Biother Radiopharm 2012;27:621-41. [Crossref] [PubMed]
- Hoffmann RT, Paprottka PM, Schön A, et al. Transarterial hepatic yttrium-90 radioembolization in patients with unresectable intrahepatic cholangiocarcinoma: Factors associated with prolonged survival. Cardiovasc Intervent Radiol 2012;35:105-16. [Crossref] [PubMed]
- Schiro BJ, Amour ES, Harnain C, et al. Management of High Hepatopulmonary Shunts in the Setting of Y90 Radioembolization. Tech Vasc Interv Radiol 2019;22:58-62. [Crossref] [PubMed]
- Currie BM, Soulen MC. Decision Making: Intra-arterial Therapies for Cholangiocarcinoma-TACE and TARE. Semin Intervent Radiol 2017;34:92-100. [Crossref] [PubMed]
- Rayar M, Sulpice L, Edeline J, et al. Intra-arterial Yttrium-90 Radioembolization Combined with Systemic Chemotherapy is a Promising Method for Downstaging Unresectable Huge Intrahepatic Cholangiocarcinoma to Surgical Treatment. Ann Surg Oncol 2015;22:3102-8. [Crossref] [PubMed]
- Kulik LM, Atassi B, Van Holsbeeck L, et al. Yttrium-90 microspheres (TheraSphere®) treatment of unresectable hepatocellular carcinoma: Downstaging to resection, RFA and bridge to transplantation. J Surg Oncol 2006;94:572-86. [Crossref] [PubMed]
- Ibrahim SM, Mulcahy MF, Lewandowski RJ, et al. Treatment of unresectable cholangiocarcinoma using yttrium-90 microspheres: Results from a pilot study. Cancer 2008;113:2119-28. [Crossref] [PubMed]
- Saxena A, Bester L, Chua TC, et al. Yttrium-90 radiotherapy for unresectable intrahepatic cholangiocarcinoma: A preliminary assessment of this novel treatment option. Ann Surg Oncol 2010;17:484-91. [Crossref] [PubMed]
- Mouli S, Memon K, Baker T, et al. Yttrium-90 radioembolization for intrahepatic cholangiocarcinoma: Safety, response, and survival analysis. J Vasc Interv Radiol 2013;24:1227-34. [Crossref] [PubMed]
- Soydal C, Kucuk ON, Bilgic S, et al. Radioembolization with90Y resin microspheres for intrahepatic cholangiocellular carcinoma: prognostic factors. Ann Nucl Med 2016;30:29-34. [Crossref] [PubMed]
- Simo KA, Halpin LE, McBrier NM, et al. Multimodality treatment of intrahepatic cholangiocarcinoma: A review. J Surg Oncol 2016;113:62-83. [Crossref] [PubMed]
- Hyder O, Marsh JW, Salem R, et al. Intra-arterial therapy for advanced intrahepatic cholangiocarcinoma: A multi-institutional analysis. Ann Surg Oncol 2013;20:3779-86. [Crossref] [PubMed]
- Kim JH, Won HJ, Shin YM, et al. Radiofrequency ablation for the treatment of primary intrahepatic cholangiocarcinoma. AJR Am J Roentgenol 2011;196:W205-9. [Crossref] [PubMed]
- Martin RCG, Scoggins CR, McMasters KM. Safety and efficacy of microwave ablation of hepatic tumors: A prospective review of a 5-year experience. Ann Surg Oncol 2010;17:171-8. [Crossref] [PubMed]
- Lewis HL, Bloomston M. Hepatic Artery Infusional Chemotherapy. Surg Clin North Am 2016;96:341-55. [Crossref] [PubMed]
- Ohta K, Sawamura A, Miyahara E, et al. A case of inoperable advanced gall bladder cancer responding to intra-arterial infusion of 5-fluorouracil (5-FU) and leucovorin (LV). Gan To Kagaku Ryoho 2001;28:516-9. [PubMed]
- Oku T, Yoshizaki N, Waga E, Sumiyoshi T, et al. A case of liver metastasis from gallbladder cancer with marked response to arterial infusion chemotherapy. Gan To Kagaku Ryoho 2003;30:1515-8. [PubMed]
- Maeda T, Sano O, Yamanaka T, et al. A case of unresectable advanced gall bladder cancer successfully treated by hepatic arterial chemotherapy with reservoir (HACR) using CDDP and 5-FU. Gan To Kagaku Ryoho 1999;26:1913-6. [PubMed]
- Shikata A, Mori K, Watahiki Y, et al. A case of unresectable advanced cancer of the gall bladder successfully treated by arterial infusion therapy with cisplatin. Gan To Kagaku Ryoho 1997;24:1820-4. [PubMed]
- Konstantinidis IT, Groot Koerkamp B, Do RK, et al. Unresectable intrahepatic cholangiocarcinoma: Systemic plus hepatic arterial infusion chemotherapy is associated with longer survival in comparison with systemic chemotherapy alone. Cancer 2016;122:758-65. [Crossref] [PubMed]
- Kambadakone A, Thabet A, Gervais DA, et al. CT-guided Celiac Plexus Neurolysis: A Review of Anatomy, Indications, Technique, and Tips for Successful Treatment. RadioGraphics 2011;31:1599-621. [Crossref] [PubMed]
- Polati E, Luzzani A, Schweiger V, et al. The Role of Neurolytic Celiac Plexus Block in the Treatment of Pancreatic Cancer Pain. Transplant Proc 2008;40:1200-4. [Crossref] [PubMed]
- Karm MH, Cho HS, Lee JY, et al. A case report: Clinical application of celiac plexus block in bile duct interventional procedures. Medicine (Baltimore) 2016;95:e4106. [Crossref] [PubMed]
- Koyyalagunta D, Engle MP, Yu J, et al. The Effectiveness of Alcohol Versus Phenol Based Splanchnic Nerve Neurolysis for the Treatment of Intra-Abdominal Cancer Pain. Pain Physician 2016;19:281-92. [PubMed]
- Tomozawa Y, Jahangiri Y, Pathak P, et al. Long-Term Toxicity after Transarterial Radioembolization with Yttrium-90 Using Resin Microspheres for Neuroendocrine Tumor Liver Metastases. J Vasc Interv Radiol 2018;29:858-65. [Crossref] [PubMed]
- Kanthed P, Parmar P. Splanchnic neurolysis for gallbladder cancer pain. Indian J Pain 2017;30:204-6. [Crossref]
- Wang PJ, Shang MY, Qian Z, et al. CT-guided percutaneous neurolytic celiac plexus block technique. Abdom Imaging 2006;31:710-8. [Crossref] [PubMed]


