
Evaluation and management of incidental gallbladder cancer
Introduction
Gallbladder cancer (GBC) is a rare disease with an estimated annual incidence of 1–2 people per 100,000 in the United States (1). The incidence, however, is varied depending on geographic area as it is higher in Native American, Chilean, Polish, Asian and Northern Indian populations (2-4). Despite differences in incidence, disease presentation and surgical treatment by region, disease specific survival seems to be independent of geography (5). GBC is an aggressive malignancy with poor outcomes and a 5-year survival that ranges between 5–12% (3). Although many patients with GBC present with advanced disease, the majority of patients in Western countries are diagnosed incidentally after undergoing a cholecystectomy for presumed benign disease. Current standard of care recommends re-resection of the tumor bed with partial hepatectomy for select patients with GBC, as well as complete staging with portal lymphadenectomy. Given the unique method of diagnosis for patients with incidental GBC, many questions arise regarding appropriate imaging modality for staging, the role, timing, and extent of re-resection, and the utility of neoadjuvant and adjuvant treatment. This review will address these issues in the management of incidentally discovered GBC.
Diagnosis
A large majority of patients with GBC in the United States will have their cancer diagnosed incidentally after a cholecystectomy for presumed benign disease. In other countries such as Japan however, a smaller proportion of patients have incidentally diagnosed tumors (5). Preoperative diagnosis of incidental GBC is not feasible in clinical practice as there is no mass seen on preoperative imaging, and cholecystectomy is performed for presumed benign stone disease. If a suspicious mass is observed on preoperative ultrasound, however, then further workup is required as well as referral to a surgical oncologist for a complete evaluation prior to embarking on cholecystectomy. As laparoscopic cholecystectomy has become the most common surgical procedure globally, the incidence of incidental GBC has also risen, with some groups reporting that up to 3% of patients undergoing cholecystectomy harbor an incidental GBC (6). Further, in a Memorial Sloan Kettering Cancer Center (MSKCC) study of 122 patients with GBC over a 15-year period, 72% of patients with newly diagnosed GBC had undergone a recent previous cholecystectomy (7). Incidental GBCs tend to be associated with more favorable pathologic characteristics such as lower tumor grade and less advanced T-stage, compared to non-incidentally diagnosed GBCs (8). Risk factors for GBC include cholelithiasis, elevated body mass index, multiparity, Salmonella infection, and the presence of Helicobacter within the bile of patients (9). The vast majority of GBCs are adenocarcinomas, though approximately 3% can be squamous cell carcinomas, and accurate staging of GBC within the cholecystectomy specimen is key, though assessment of the T stage, or the invasion of the tumor through the layers of the gallbladder wall, can be difficult after a simple cholecystectomy where oncologic margins may be disrupted. Regardless, the T stage of the tumor often dictates subsequent treatment strategies as described later in this review.
Staging
After the diagnosis of GBC has been confirmed on pathologic examination of the resection specimen from the previous cholecystectomy, appropriate staging must be performed prior to initiating treatment. Patients should undergo high quality cross-sectional imaging with computerized tomography (CT) or magnetic resonance imaging (MRI), with positron emission tomography (PET) scans reserved for select cases based on features seen on CT or MRI. In a retrospective Surveillance, Epidemiology and End Results (SEER) database study of almost 3,000 patients with GBC over a 15-year period, CT scan was the most used peri-operative imaging modality, utilized in 61% of patients (10). MRI can also be used to detect vascular invasion, biliary tract involvement, liver invasion, and lymph node involvement with reliable accuracy (9).
The role of PET scan has not been sufficiently proven in a prospective fashion for patients with GBC, however numerous retrospective studies have reported some utility (11,12). For example, in a study from MSKCC, PET scan altered the management of 23% of patients with GBC (11). A Chilean study of 32 patients with incidental GBC demonstrated that PET-CT altered management in 38% of patients though some of these patients did not undergo CT or MRI so the utility of PET-CT is not fully known (13). FGD-PET scans have been shown to be useful, however, in predicting N-stage and M-stage in patients, but not T-stage (14). Based on these studies, we recommend only the selective use of PET scans for patients with questionable, but concerning, features on high-quality triple phase CT or MRI.
Predicting residual disease
Other than appropriately staging a patient, the most important information to glean preoperatively is the probability of residual cancer in the cholecystectomy bed or portal lymph nodes as patients with residual disease have significantly worse survival compared to those without residual disease (15). Up to 40% of patients with incidental GBC will have residual disease in the gallbladder fossa (7). The primary predictor of residual disease is the pathologic T-stage of the tumor. Patients with T1 tumors can have between a 0–37.5% incidence of residual disease, while patients with T2 or T3 tumors can have between a 10–57% and 36–77% incidence of residual disease, respectively (7,16). Although T-stage can predict the presence of residual disease, in patients with incidental GBC, appropriate measurement of T-stage can often be complicated by the initial non-oncologic resection of the gallbladder specimen. Thus, Ethun et al. developed a more robust score to predict the presence of residual disease based on T-stage as well as pathologic tumor grade, lymphovascular invasion and perineural invasion, which reliably predicted the presence of residual disease, and an increasing score was associated with reduced overall survival (17).
The role of re-resection
The role of re-resection after an incidental GBC is diagnosed is to remove residual microscopic local-regional disease from the surgical bed in an effort to achieve an R0 resection, as well as to perform a complete staging lymphadenectomy. Re-resection is indicated in patients with pathologically confirmed T1b (invasion in the muscularis layer), T2 (invades perimuscular connective tissue without extension beyond serosa or into liver), or T3 (perforation of serosa/liver invasion) disease without evidence of metastatic disease and appropriate performance status to undergo the potential morbidity of a larger operation. The timing of re-resection after the discovery of incidental GBC has been questioned with some groups demonstrating that TNM stage, not the time interval between cholecystectomy and re-resection, is the main prognostic factor for patients (18). Other groups have shown that re-resection should be performed optimally 4 to 8 weeks after initial cholecystectomy as re-resection within 4 weeks of initial cholecystectomy or greater than 8 weeks afterwards was associated with worse outcomes, even when accounting for tumor stage (19).
Although R0 resection is the goal of repeated operation, the morbidity of re-resection must be balanced with improvements in oncologic outcomes afforded with an oncologic resection (20). The European AFC-GBC collaborative group demonstrated in a study of 218 patients with incidental GBC over a 10-year period that patients who underwent re-resection had a 5-year overall survival of 41% versus 15% for those who did not have re-resection, especially in those patients with T2 and T3 disease (21). While for T2 and T3 tumors, re-resection is generally accepted, the role of resection in patients with T1b disease is more controversial. Multiple studies, however, have demonstrated an improvement in overall survival in well-selected patients with T1b tumors who underwent re-resection compared to those who did not (22,23). Thus, current standard of care is to recommend re-resection for incidental T1b, T2 and T3 GBCs.
Staging laparoscopy
Given the real potential for distant or peritoneal spread of GBC, we recommend that staging laparoscopy be performed prior to performing a laparotomy to rule out the possibility of M1 disease in the abdomen (24). This should be done particularly in the setting of T3 disease or those tumors with adverse pathologic characteristics. The utility of staging laparoscopy was demonstrated in a 2011 study of 136 patients with incidental GBC which demonstrated that 20% of patients who underwent staging laparoscopy prior to laparotomy had distant disease, most likely in those patients with T3 disease, poorly differentiated tumors, or a positive cystic duct margin on previous cholecystectomy (25).
Port site excision
The excision of port sites from the original laparoscopic cholecystectomy is not indicated routinely, though previous groups have argued for the routine excision of port sites during re-resection (26). Although resection of the port sites removes potentially contaminated tissue during initial laparoscopic cholecystectomy and thus theoretically may remove residual disease, previous groups have demonstrated that only a small percentage of patients have disease in these specimens (27-30). Further, port site resection was not associated with improved survival, but rather the presence of port site disease is more likely a surrogate for microscopic peritoneal disease (31).
Lymphadenectomy
Given the aggressive nature of this malignancy, many patients will have metastases to regional lymph nodes so complete staging and management includes regional portal lymphadenectomy (32). Extended lymph node dissection, i.e., removing aortocaval lymph nodes, is not routinely indicated. We do recommend, however, that a regional lymphadenectomy be performed to include a portal lymphadenectomy and excision of all lymph nodes in the hepatoduodenal ligament. The likelihood of lymph node disease increases with advancing T-stage of the primary tumor, with T3 tumors having a 45% likelihood of harboring lymph node metastases (16). Lymphadenectomy not only provides means to adequately stage these tumors, but also may control locoregional spread as lymphadenectomy with radical resection is associated with improved survival compared to radical resection alone for patients with tumors that are T1b or T2 (33). Similarly, the resection of 6 or more lymph nodes is associated with improved survival compared to retrieving less than 6 lymph nodes in patients deemed to have lymph node negative disease (7). Dissection of the celiac, retro-pancreatic or para-aortic lymph nodes, however, is not indicated as disease in these lymph node basins is considered distant metastatic spread (34).
Major hepatectomy and bile duct excision
With improvements in surgical technique and anesthetic advancements, the ability to safely perform larger resections of the liver and extrahepatic biliary system is entirely feasible. The ability to perform an extended resection however needs to be balanced with the oncologic need and benefit of these resections. Major hepatectomy is not routinely indicated unless needed to achieve an R0 resection, as an R0 resection is associated with improved survival. This has been demonstrated by multiple studies, including a retrospective study of 99 patients from Toronto, Canada over a 12-year period (35). Standard re-resection should consist of a segment 4b/5 resection, as a larger resection or major hepatectomy has not been associated with improved survival as shown by a MSKCC study of 109 patients over a 15-year period (36). For the same reason, routine bile duct resection is also not indicated, unless to achieve an R0 resection, as it has been associated with increased morbidity without added survival benefit or greater lymph node yield (20,36). The most likely clinical scenario that necessitates a bile duct resection is a positive cystic duct margin at the time of the original cholecystectomy that cannot be converted to negative without a bile duct resection and reconstruction.
Perioperative therapy
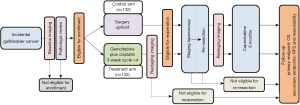
The role of chemotherapy in the perioperative, specifically adjuvant or neoadjuvant setting for patients with GBC has been questioned, and now has been recently studied in several randomized trials (15). After resection, the majority of disease recurrences are distant, which highlights the systemic nature of GBC and need for multimodality therapy (37). A meta-analysis of twenty studies and 6,712 patients supported the administration of adjuvant therapy after resection, but there was no consensus on the ideal regimen (38). Until recently, the regimen utilized was extrapolated from the ABC-02 trial which demonstrated in a randomized controlled trial of 410 patients with advanced biliary tract malignancies (149 patients with GBC) that patients who received a combination of cisplatin-gemcitabine had an overall survival of 11.7 months versus 8.1 months with gemcitabine alone (39). This doublet regimen became the standard of care for GBC and often was utilized and extrapolated to the adjuvant setting. Recently, several randomized trials have been conducted, or are currently accruing, that help define the standard of care adjuvant therapy regimen. Along the vein of the ABC-02 regimen, the PRODIGE 12 study assessed the value of gemcitabine-oxaliplatin compared to placebo in the adjuvant setting, but was reported as a negative trial (40). The BILCAP (BILiary CAPecitabine) randomized controlled trial in 447 patients with resected biliary tract malignancies reported that 6 months of adjuvant capecitabine improved overall survival compared to placebo (41). Although this trial reported a statistically non-significant p-value (P=0.097) in the intent-to-treat analysis, the per-protocol analysis was clinically and statistically significant. Thus, this regimen has now become the standard of care recommendation after re-resection of incidental GBC (42). Concurrently, a Japanese study assessed the value of S-1 compared to observation and the ACTICAA-1 study is assessing the value of chemotherapy augmentation with gemcitabine-cisplatin compared to capecitabine (43,44). These trials are awaiting maturation of follow-up/final results and complete accrual, respectively. The utility of adjuvant radiation however has not been proven, and given its role in controlling locoregional disease and GBC’s tendency to spread to distant sites, current recommendations are that chemoradiation be used in the setting of microscopically disease-positive surgical margins (R1 resections) (42). Given the unique situation of incidental GBC, the margin positive resection rate is in single digits, thus limiting the application of radiation for this disease. Chemoradiation in the setting of GBC was studied recently in 2015 with the Southwest Oncology Group (SWOG) S0809 Phase II trial which reported a well-tolerated adjuvant regimen of gemcitabine and capecitabine with radiotherapy and showed a 2-year overall survival of 67% and a median overall survival of 35 months (45). Efficacy of this regimen would need to be further tested in a Phase III setting, ideally with a pre-planned subgroup analysis for incidentally diagnosed GBC. There currently is no evidence for the use of chemotherapy in the neoadjuvant setting prior to re-resection. Furthermore, all of the above-mentioned trials group all biliary tract malignancies together, despite each tumor type having distinct molecular signatures and biologic behaviors. Accordingly, the authors of this review are presently leading a phase III trial studying neoadjuvant gemcitabine-cisplatin with adjuvant capecitabine compared to adjuvant capecitabine alone for patients with resectable, incidental GBC (NCT03579758, Figure 1).
Conclusions
The rising use of laparoscopic cholecystectomy in the current era has led to an increase in the number of incidentally diagnosed GBCs. Appropriate preoperative staging and pathologic assessment is paramount as it guides further treatment strategies. Re-resection with partial hepatectomy and regional lymphadenectomy is indicated for patients with non-metastatic T1b, T2 and T3 lesions with the goal of surgery being to achieve an R0 resection. The use of adjuvant capecitabine can improve oncologic outcomes and further study is needed to define the role of neoadjuvant chemotherapy prior to re-resection.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Fong Y, Malhotra S. Gallbladder cancer: recent advances and current guidelines for surgical therapy. Adv Surg 2001;35:1-20. [PubMed]
- Misra S, Chaturvedi A, Misra NC, et al. Carcinoma of the gallbladder. Lancet Oncol 2003;4:167-76. [Crossref] [PubMed]
- Piehler JM, Crichlow RW. Primary carcinoma of the gallbladder. Surg Gynecol Obstet 1978;147:929-42. [PubMed]
- Murthy NS, Rajaram D, Gautham M, et al. Trends in incidence of gallbladder cancer – Indian scenario. Gastrointest Cancer 2011;1:1-9.
- Butte JM, Matsuo K, Gonen M, et al. Gallbladder cancer: differences in presentation, surgical treatment, and survival in patients treated at centers in three countries. J Am Coll Surg 2011;212:50-61. [Crossref] [PubMed]
- Toyonaga T, Chijiiwa K, Nakano K, et al. Completion radical surgery after cholecystectomy for accidentally undiagnosed gallbladder carcinoma. World J Surg 2003;27:266-71. [Crossref] [PubMed]
- Ito H, Ito K, D'Angelica M, et al. Accurate staging for gallbladder cancer: implications for surgical therapy and pathological assessment. Ann Surg 2011;254:320-5. [Crossref] [PubMed]
- Ethun CG, Le N, Lopez-Aguiar AG, et al. Pathologic and Prognostic Implications of Incidental versus Nonincidental Gallbladder Cancer: A 10-Institution Study from the United States Extrahepatic Biliary Malignancy Consortium. Am Surg 2017;83:679-86. [PubMed]
- Miller G, Jarnagin WR. Gallbladder carcinoma. Eur J Surg Oncol 2008;34:306-12. [Crossref] [PubMed]
- Mayo SC, Shore AD, Nathan H, et al. National trends in the management and survival of surgically managed gallbladder adenocarcinoma over 15 years: a population-based analysis. J Gastrointest Surg 2010;14:1578-91. [Crossref] [PubMed]
- Corvera CU, Blumgart LH, Akhurst T, et al. 18F-fluorodeoxyglucose positron emission tomography influences management decisions in patients with biliary cancer. J Am Coll Surg 2008;206:57-65. [Crossref] [PubMed]
- Shukla PJ, Barreto SG, Arya S, et al. Does PET-CT scan have a role prior to radical re-resection for incidental gallbladder cancer? HPB (Oxford) 2008;10:439-45. [Crossref] [PubMed]
- Butte JM, Redondo F, Waugh E, et al. The role of PET-CT in patients with incidental gallbladder cancer. HPB (Oxford) 2009;11:585-91. [Crossref] [PubMed]
- Lamarca A, Barriuso J, Chander A, et al. (18)F-fluorodeoxyglucose positron emission tomography ((18)FDG-PET) for patients with biliary tract cancer: Systematic review and meta-analysis. J Hepatol 2019. [Crossref] [PubMed]
- Duffy A, Capanu M, Abou-Alfa GK, et al. Gallbladder cancer (GBC): 10-year experience at Memorial Sloan-Kettering Cancer Centre (MSKCC). J Surg Oncol 2008;98:485-9. [Crossref] [PubMed]
- Pawlik TM, Gleisner AL, Vigano L, et al. Incidence of finding residual disease for incidental gallbladder carcinoma: implications for re-resection. J Gastrointest Surg 2007;11:1478-86; discussion 1486-7. [Crossref] [PubMed]
- Ethun CG, Postlewait LM, Le N, et al. A Novel Pathology-Based Preoperative Risk Score to Predict Locoregional Residual and Distant Disease and Survival for Incidental Gallbladder Cancer: A 10-Institution Study from the U.S. Extrahepatic Biliary Malignancy Consortium. Ann Surg Oncol 2017;24:1343-50. [Crossref] [PubMed]
- Barreto SG, Pawar S, Shah S, et al. Patterns of failure and determinants of outcomes following radical re-resection for incidental gallbladder cancer. World J Surg 2014;38:484-9. [Crossref] [PubMed]
- Ethun CG, Postlewait LM, Le N, et al. Association of Optimal Time Interval to Re-resection for Incidental Gallbladder Cancer With Overall Survival: A Multi-Institution Analysis From the US Extrahepatic Biliary Malignancy Consortium. JAMA Surg 2017;152:143-9. [Crossref] [PubMed]
- Pawlik TM, Choti MA. Biology dictates prognosis following resection of gallbladder carcinoma: sometimes less is more. Ann Surg Oncol 2009;16:787-8. [Crossref] [PubMed]
- Fuks D, Regimbeau JM, Le Treut YP, et al. Incidental gallbladder cancer by the AFC-GBC-2009 Study Group. World J Surg 2011;35:1887-97. [Crossref] [PubMed]
- Hari DM, Howard JH, Leung AM, et al. A 21-year analysis of stage I gallbladder carcinoma: is cholecystectomy alone adequate? HPB (Oxford) 2013;15:40-8. [Crossref] [PubMed]
- Abramson MA, Pandharipande P, Ruan D, et al. Radical resection for T1b gallbladder cancer: a decision analysis. HPB (Oxford) 2009;11:656-63. [Crossref] [PubMed]
- Weber SM, DeMatteo RP, Fong Y, et al. Staging laparoscopy in patients with extrahepatic biliary carcinoma. Analysis of 100 patients. Ann Surg 2002;235:392-9. [Crossref] [PubMed]
- Butte JM, Gonen M, Allen PJ, et al. The role of laparoscopic staging in patients with incidental gallbladder cancer. HPB (Oxford) 2011;13:463-72. [Crossref] [PubMed]
- Rathanaswamy S, Misra S, Kumar V, et al. Incidentally detected gallbladder cancer- the controversies and algorithmic approach to management. Indian J Surg 2012;74:248-54. [Crossref] [PubMed]
- Fuks D, Regimbeau JM, Pessaux P, et al. Is port-site resection necessary in the surgical management of gallbladder cancer? J Visc Surg 2013;150:277-84. [Crossref] [PubMed]
- Giuliante F, Ardito F, Vellone M, et al. Port-sites excision for gallbladder cancer incidentally found after laparoscopic cholecystectomy. Am J Surg 2006;191:114-6. [Crossref] [PubMed]
- Maker AV, Butte JM, Oxenberg J, et al. Is port site resection necessary in the surgical management of gallbladder cancer? Ann Surg Oncol 2012;19:409-17. [Crossref] [PubMed]
- Paolucci V, Schaeff B, Schneider M, et al. Tumor seeding following laparoscopy: international survey. World J Surg 1999;23:989-95; discussion 996-7. [Crossref] [PubMed]
- Ethun CG, Postlewait LM, Le N, et al. Routine port-site excision in incidentally discovered gallbladder cancer is not associated with improved survival: A multi-institution analysis from the US Extrahepatic Biliary Malignancy Consortium. J Surg Oncol 2017;115:805-11. [Crossref] [PubMed]
- Jensen EH, Abraham A, Jarosek S, et al. Lymph node evaluation is associated with improved survival after surgery for early stage gallbladder cancer. Surgery 2009;146:706-11; discussion 711-3. [Crossref] [PubMed]
- Downing SR, Cadogan KA, Ortega G, et al. Early-stage gallbladder cancer in the Surveillance, Epidemiology, and End Results database: effect of extended surgical resection. Arch Surg 2011;146:734-8. [Crossref] [PubMed]
- Kondo S, Nimura Y, Hayakawa N, et al. Regional and para-aortic lymphadenectomy in radical surgery for advanced gallbladder carcinoma. Br J Surg 2000;87:418-22. [Crossref] [PubMed]
- Dixon E, Vollmer CM Jr, Sahajpal A, et al. An aggressive surgical approach leads to improved survival in patients with gallbladder cancer: a 12-year study at a North American Center. Ann Surg 2005;241:385-94. [Crossref] [PubMed]
- D'Angelica M, Dalal KM, DeMatteo RP, et al. Analysis of the extent of resection for adenocarcinoma of the gallbladder. Ann Surg Oncol 2009;16:806-16. [Crossref] [PubMed]
- Patkar S, Ostwal V, Ramaswamy A, et al. Emerging role of multimodality treatment in gall bladder cancer: Outcomes following 510 consecutive resections in a tertiary referral center. J Surg Oncol 2018;117:372-9. [Crossref] [PubMed]
- Horgan AM, Amir E, Walter T, et al. Adjuvant therapy in the treatment of biliary tract cancer: a systematic review and meta-analysis. J Clin Oncol 2012;30:1934-40. [Crossref] [PubMed]
- Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 2010;362:1273-81. [Crossref] [PubMed]
- Edeline J, Benabdelghani M, Bertaut A, et al. Gemcitabine and Oxaliplatin Chemotherapy or Surveillance in Resected Biliary Tract Cancer (PRODIGE 12-ACCORD 18-UNICANCER GI): A Randomized Phase III Study. J Clin Oncol 2019;37:658-67. [Crossref] [PubMed]
- Primrose JN, Fox RP, Palmer DH, et al. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): a randomised, controlled, multicentre, phase 3 study. Lancet Oncol 2019;20:663-73. [Crossref] [PubMed]
- Shroff RT, Kennedy EB, Bachini M, et al. Adjuvant Therapy for Resected Biliary Tract Cancer: ASCO Clinical Practice Guideline. J Clin Oncol 2019;37:1015-27. [Crossref] [PubMed]
- Nakachi K, Konishi M, Ikeda M, et al. A randomized Phase III trial of adjuvant S-1 therapy vs. observation alone in resected biliary tract cancer: Japan Clinical Oncology Group Study (JCOG1202, ASCOT). Jpn J Clin Oncol 2018;48:392-5. [Crossref] [PubMed]
- Stein A, Arnold D, Bridgewater J, et al. Adjuvant chemotherapy with gemcitabine and cisplatin compared to observation after curative intent resection of cholangiocarcinoma and muscle invasive gallbladder carcinoma (ACTICCA-1 trial) - a randomized, multidisciplinary, multinational phase III trial. BMC Cancer 2015;15:564. [Crossref] [PubMed]
- Ben-Josef E, Guthrie KA, El-Khoueiry AB, et al. SWOG S0809: A Phase II Intergroup Trial of Adjuvant Capecitabine and Gemcitabine Followed by Radiotherapy and Concurrent Capecitabine in Extrahepatic Cholangiocarcinoma and Gallbladder Carcinoma. J Clin Oncol 2015;33:2617-22. [Crossref] [PubMed]


