
Targeting the tumor microenvironment for pancreatic ductal adenocarcinoma therapy
Introduction
Pancreatic cancer is one of the most dangerous cancers, and it has a very low overall 5-year survival rate (8%) that has shown little improvement (1). There are 10 types of major neoplasms of the pancreas, and invasive ductal adenocarcinoma is common (2). Pancreatic ductal adenocarcinoma (PDAC) cells infiltrate into the tissue to make the tumor firm and metastasize to other organs (3).
Solid tumor cancer cells exhibit self-sufficiency in growth signals, which results in unlimited cell proliferation, the ability to obtain nutrients, resistance to apoptosis, insensitivity to the growth inhibitory pathway, and the capacity to invade and metastasize (4). PDAC has these characteristics as well. Through microscopy, PDAC cells were observed to have a close relationship with nerves, lymphatic spaces and small veins, indicating that PDAC is very likely to become a metastatic carcinoma. The tumor microenvironment (TME) of PDAC is highly immunosuppressive and has been associated with cell secretion disorders and interactions with nerves (5-7). The majority of the PDAC tumor volume is filled by stroma/desmoplastic reaction, and the heterogeneous stroma consists of fibroblasts, myofibroblasts, immune cells, pancreatic stellate cells (PaSCs), extracellular matrix (ECM), and soluble proteins, such as cytokines, growth factors and blood vessels (8,9). The interaction of the tumor cells with factors in TME suppresses the immune reaction of the body to escape immunocyte apoptosis. Due to the complex tumor microenvironment and high interstitial fluid pressure (IFP), PDAC has chemoresistance and radioresistance, which creates major challenges for therapy (10). The interactions between components in TME and the tumor cells results in tumor development, metastasis and immune escape. In addition to poor therapy effects, there is no early detection test for PDAC, and many patients lack recognizable symptoms or signs of the disease. Therefore, patients are continuously considered to be at high-risk after being diagnosed with PDAC.
Fibroblasts
Desmoplasia is an important feature of the PDAC tumor microenvironment but can be an obstacle for therapeutic agents; fibroblasts are considered to be “murderers” that result in desmoplasia. Cancer cells expend a large amount of energy to recruit, proliferate and activate fibroblasts; then, as a reward, the activated fibroblasts deposit ECM and secrete numerous factors that affect tumor development (11) (Figure 1). An important origin of cancer-associated fibroblasts (CAFs) is from the Notch signaling pathway that activates PaSCs (12).
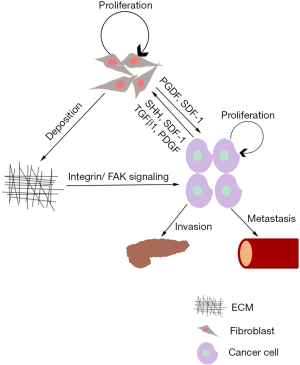
Several types of fibroblasts are deposited in the ECM, which may explain the poor therapy results of targeting CAFs. Two major types of PDAC FAP+ fibroblasts exist: periglandular αSMAhigh myofibroblastic CAFs (myCAFs), which likely restrain tumor growth; and diffusely distributed αSMAlow interleukin-6 (IL-6)-positive inflammatory CAFs, which promote tumor growth by secreting ECM proteins and cytokines such as IL-6 and IL-11 (13,14). Based on the fibroblast subtype, inhibiting IL-6R to reduce STAT3 activation slows tumor development (15). Although there are two types of CAFs and their functions seem to be opposed, CAFs were demonstrated to be a promoting tumor factor in the TME. The PDAC cell interactions with stromal fibroblasts increase hyaluronic acid production, causing an obvious increase in the migration of PDAC cells (16). New research has reported that the Wnt-nonproducing subtype, a kind of PDAC cell, required Wnt from CAFs (17).
CAFs stop CD8+ and CD4+ T cells, NK cells and Tregs to juxtatumoral compartments to exert normal physiological function (18-20). CAFs secrete CXCL12 to keep CXCR4+ T cells away from tumors and secrete CCL5, CCL2 and CCL17 to recruit monocytes and Tregs, which results in immunosuppression (21). Due to the presence of fibroblasts and other stellate cells, tumors have high intratumor IFP and matricellular tension (MCT) (22), both of which promote tumor progression, reduce vasculature to cut off therapeutic agents and induces tissue hypoxia (23). Subsequently, through autophagy-mediated degradation and a reduction in protein synthesis in the PaSCs, hypoxia reduces the expression of lumican, an inhibitor of tumor progression that is located in the TME (24).
During tumor metastasis, the fibroblasts, along with other cells, affect the microenvironment of the target organ. During the early stage of PDAC liver metastasis, metastasis-associated macrophages (MAMs), a kind of inflammatory monocyte, secrete granulin and activate resident hepatic stellate cells that turn into myofibroblasts, which secrete periostin to result in a fibrotic microenvironment that promotes metastatic tumor growth (25). This finding may explain why myofibroblasts appear when metastases only comprise 6–7 cells in the cell population within a metastatic lesion (26). Research has shown that PDAC cells that are treated with CAF-conditioned media have an increased risk of metastasis; the reason for this increase is the loss of metastasis suppressor 1 (27).
Since the elimination of desmoplasia has been indicated be harmful to patients, new research is more focused on ECM reprogramming to find new effective therapeutic agents. Malik et al. reported that CAFs remodel cell-derived extracellular matrices (CDMs) by altering the underlying substrate stiffness and inhibit tumor growth in an extracellular signal-regulated kinase 2 (EKR2)-dependent manner (28). CAFs treated with physiological stiffness (~1.5 kPa) generate CDMs similar to normal fibroblasts, and the biomechanical manipulation generated on physiological stiffness CDMs leads to a decrease in the nuclear translocation of pERK1/2 in KRAS-mutated pancreatic cells.
The immune system
Immune cells may affect the composition of pancreatic stroma to affect the progression of PDAC (29). Recent research reports that prominent overall leukocyte infiltration is associated with increased survival (30).
The TME and immune system have a close relationship, and a number of interactions between the TME and immune system affect tumor development (Figure 2) (31-36). The T cell immunocytes in particular can recognize the non-self and then remove the invaders. The process of normal cells turning into cancer cells is an example of the self to non-self progression, and T cells can kill the cancer cells. Once the effector T cell activation reaches a threshold, Tregs become activated and release TGF-β and IL-10 immunosuppressive cytokines to negatively regulate T cell function (37). To develop and proliferate, the cancer cells break this balance between the inhibitors and activation level though abrogating coactivatory signals and augmenting coinhibitory signals, which ultimately heightens anergy and exhaustion (38).
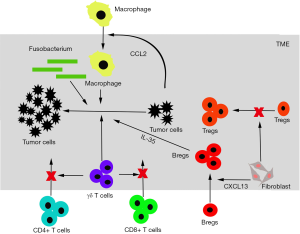
In a healthy body, the action of T cells through major histocompatibility complex (MHC) engagement is regulated by stimulating signals and inhibitory signals, which are referred to as immune checkpoints. To breakdown and benefit from this regulation, tumor cells expand the coinhibitory signal and abolish the coactivatory signal (38).
Research has focused on cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and programmed cell death protein 1 (PD-1), two factors that function on immune checkpoint inhibitors. The CLTA-4 pathway is activated by tumor cells to downregulate the CD28 costimulatory receptor, an important ligand for transmitting secondary signals for T cell activation and increasing the threshold of T cell activation, resulting in immunosuppression (39). Regulated by FUBP1, IRF1, cytokines, etc., the cancer cells upregulate PD-1, which binds to PD-1 receptors on T cells to cause a loss of T cell function, cell anergy, or cell death (40-43). In addition to CTLA-4 and PD-1, new targets have been identified. Bruton tyrosine kinase (BTK), a key B-cell and macrophage kinase, affects B-cell and macrophage-mediated T cell suppression in pancreatic adenocarcinomas, inhibits T cell antitumor function and improves the PDAC response to chemotherapy (44). The inhibitor of receptor tyrosine kinase Axl can be established as a preclinical mechanistic target to improve the overall survival rate (45). Dickkopf-3 (DKK3) is a potential therapeutic target as well (46).
Interleukins (ILs) are secreted by many kinds of cells that participate in immune regulation, hematopoiesis and inflammation. IL-6 is one of IL family members produced by pancreatic satellite cells (PSCs) and PDAC cells and is associated with tumor growth, survival, metastasis, chemoresistance, etc. (47,48). IL-6 can activate MDSCs via the JAK/STAT3 pathway, and a high MDSC level indicates a high risk of a poor patient outcome (49). The STAT3 pathway and NRF2 pathway, which are induced by STAT3, promote epithelial-mesenchymal transition (EMT) in PDAC (39,50). The IL-6/JAK/STAT3/NREF2 pathway benefits tumor development and thus targeting this pathway may inhibit tumor growth and metastasis to prolong patient survival. After EMT marker expression, IL-6 may be associated with a number of activation factors for the macrophage phenotype switch (51).
Neural invasion and neuroendocrinology
Neural invasion may depend on specific cancer cells and their interaction with the neural stroma (52). Neural invasion is a pathohistological hallmark of PDAC, and the observed incidence in PDAC is as high as 100% (53,54). The nerves that infiltrate the pancreatic tumor tissue interact with the TME to play an important role in carcinogenesis, tumor development and metastasis.
The complex tumor microenvironment has numerous factors that affect tumor development, and neurotransmitters must be included. For normal pancreatic beta-cells, dopamine increases proliferation, decreases apoptosis by extending intracellular cAMP content and prevents beta-cell dedifferentiation (55). However, in tumor tissue, dopamine receptors are activated and inhibit cancer cell growth and migration at both the cell level and tumor level in mice with xenografts (56). Some research reports that nitric oxide (NO) suppresses pancreatic cell apoptosis, and the overproduction of NO reduces insulin secretion (57,58). Pancreatic cancer cells express a high level of inducible NO synthase due to the overproduction of NO, and a high level of NO is significantly associated with poor survival (59). NO may be a therapeutic target and predictor of prognosis in the early stages.
Serotonin (5-HT) is a neurotransmitter in the central nervous system, and it also acts as a hormone in the peripheral region. 5-HT cannot cross the blood-brain barrier so 95% of 5-HT is directly produced in the periphery. In healthy bodies, 5-HT regulates pancreatic beta-cell autocrine function, promotes gluconeogenesis, suppresses glucose uptake, regulates adipose and affects other organs (60). In PDAC, 5-HT uptake promoted activation of the small GTPase Ras-related C3 botulinum toxin substrate 1 (Rac1), which is required for the transdifferentiation of acinar cells into acinar-to-ductal metaplasia (ADM), a key determinant in PDAC development (61). The level of 5-HT increases in PDAC tissue, and 5-HT promotes proliferation, prevents cell apoptosis, increases levels of metabolic enzymes involved in glycolysis, and increases participation in the phosphate pentose pathway and hexosamine biosynthesis pathway, activates PI3K-Akt-mTOR signaling and affects the Warburg effect by increasing protein levels of MYC and HIF1A (62). Gamma-aminobutyric acid (GABA) is a major neurotransmitter in the central nervous system and is widely distributed in the peripheral region; GABRP, a receptor of GABA, has been reported to have a close relationship with PDAC. GABRP interacts with macrophages. GABRP induces macrophages into PDAC tissue, and the deletion of macrophages mechanistically abrogates the oncogenic function of GABRP as GABRP interacts with KCNN4 to induce Ca2+ entry, which leads to activation of the NF-κB signaling pathway and ultimately facilitates macrophage infiltration by inducing CXCL5 and CCL20 expression (63).
Hormones adjust cell metabolism, growth, and differentiation, and these physiological process changes are hallmarks of cancer. Chronic psychological stress may promote tumor development, and norepinephrine (NE) is the main hormone in the response to stress. Researchers have reported that NE may promote the biological behavior of malignant PDAC through the Notch-1 pathway (64). Angiotensin has not been reported to directly affect PDAC cells; however, the expression of angiotensin-converting enzyme 2 (ACE2) decreases in PDAC tissue, and a reduction of ACE2 expression promotes pancreatic cancer cell proliferation (65). Furthermore, chronic systemic angiotensin inhibitor use in primary PDAC is associated with longer overall survival independent of chemotherapy due to the reduction of potentially malignant cancer cells and stimulation of the immune system (66). To break PDAC chemoresistance, research has attempted to use melatonin to inhibit the activation of NF-κB and increase melatonin expression by sorafenib to block the PDGFR-β/STAT3 pathway because the melatonin receptor mediates STAT3 (67,68).
P2RY2 is a purinergic receptor activated by ATP and belongs to the G-protein coupled receptor family. Investigations have revealed that inhibiting P2RY2 impaired cell growth and delayed tumor development because of its roles in reprogramming PDAC metabolism (69).
Insulin is an important hormone secreted by the pancreas. Increases in insulin and glucose concentration promote fibrosing response and cell proliferation in type 2 diabetes, which may be associated with PDAC (70). Apart from regulating glycometabolism, insulin with the insulin-like growth factor (IGF) family also promotes cell proliferation. Insulin/IGF signaling (IIS) drives cell proliferation via the Yorkie/YAP pathway, and YAP can be stimulated by insulin receptor and G protein-coupled receptor (GPCR) crosstalk through PI3K and PKD in PDAC (71,72). In addition to directly affecting PDAC cells, insulin also affects downstream factors, such as hormone-sensitive lipase, which regulates pancreatic cancer development (73).
Hormones and immunocytes can affect tumor development together. Pancreatic cancer cells secrete high levels of adrenomedullin (ADM), and CD11b+ myelomonocytic cells express all AMD receptor components, through which myelomonocytic cells enhance migration and invasion activities through the MAPK, PI3K/Akt and eNOS signaling pathways, as well as the expression and activity of MMP-2. Furthermore, AMD increases the expression of VCAM-1 and ICAM-1 to promote the adhesion and trans-endothelial migration of myelomonocytic cells in endothelial cells, and as we all know, myelomonocytic cells are related to tumor development (74).
Therapy
Because of the difficulties in diagnosing PDAC, only 15% to 20% of PDAC patients can be treated with resection procedures (75). Furthermore, the results of chemotherapy and radiotherapy are poor; therefore, new therapy options are necessary.
To treat PDAC, immunotherapy has been developed to target immune molecules, such as IL-2, to blocking immune checkpoints (76,77). The targeting of immune molecules (PD-1 and PD-L1) to disrupt immune checkpoints and treat tumors has been applied to metastatic melanoma, and this strategy is on track to replace chemotherapy and become a mainstream treatment (78,79). However, in PDAC, the treatment is not as straightforward as the treatment of melanoma. The CAFs that compose the ECM have suppressive immune functions and must be eliminated before a T cell response can ensue. CAFs secrete CCL2 and CXCL2 to recruit myeloid cells and synergistically suppress the immune response; clinical research has started targeting these chemokines and/or its receptors (80).
Vaccine immunotherapy for PDAC is currently being investigated, and this method has two steps that are designed to treat PDAC. The low number of lymphocytes and high number of immunosuppressive cells are responsible for the poor immune response of PDAC (81). The first step aims to accumulate lymphocytes, which secrete interferon gamma and other immune elements that induce tumor cells and immune cells to express a high level of PD-L1/PD-1 (82,83). The second step aims to inhibit the PD-L1/PD-1 signaling pathway to increase lymphocyte proliferation and function (41). The vaccine therapy method is expected to increase the number of immune cells and enhance their function to prevent tumor growth.
Because of the desmoplastic changes of the ECM, the treatment of PDAC has become a huge challenge; the components of the PDAC ECM, including CAFs, collagen, proteoglycan, and hyaluronan, are targeted for treatment. Some labs have begun trying to stop the detrimental collaboration of CAFs and tumor cells through drugs, and the use of 4-methylumbelliferone attempted to solve the hyaluronan problem (84,85). In this clinic, studies have tested the safety and efficacy of the Nab-paclitaxel-gemcitabine method, and because this method has low toxicity, it is recommended as a first-line therapy for patients with metastatic disease (86,87).
The TME stromal factors lead to PDAC being more complicated, immunosuppressive and resistant to drugs. Recent research has focused not only on the factors themselves but also on the downstream or upstream factors. KRAS, one of the most potent of all human oncogenes, regretfully cannot be effectively targeted as a drug because of its smooth surface (88,89). To target KRAS, microRNA was designed to decrease KRAS expression at the RNA level (90). The TGF-β, TGF-βR and TGF-β pathway activation protein SMAD commonly has mutations in pancreatic cancer. To target the TGF-β pathway, siRNA and negatively regular protein SnoN were tested for their ability to decrease cell proliferation as a possible targeted therapy for TGF-β (91,92). SHH binds to the PTCH1 receptor and regulates the Smoothened protein (SMO) and its downstream pathways; treating tumors with inhibitors of the SMO receptor in combination with gemcitabine decreases desmoplasia and collagen deposition in TME and enhances the gemcitabine concentration, resulting in increased overall survival in mice (93). However, the results of the SMO antagonist in clinical research disappointedly demonstrated no significant results (94).
Apart from therapy methods, many medicines and therapy methods have been investigated to conquer this deadly cancer. Tamoxifen, a candidate for PDAC therapy, remodels TME and reduces the tumor cell survival rate by hypoxia-inducible factor-1 alpha (95). A series of antibodies for immune cell inhibitors that target the immunosuppressive TME of PDAC have been tested (96). Urolithin A, which targets the PI3K/AKT/mTOR pathway, has been investigated as a potential therapeutic agent in recent research (97).
Discussion
Due to its complex microenvironment, PDAC suppresses the immune system and resists radiotherapy and chemotherapy, causing cognitive difficulty in PDAC patients. The soluble proteins and cells that surround the tumor cells stop drugs and immunocytes from reaching the cancer cells, which results in difficulties for therapy and a low overall 5-year survival rate. Breaking this obstacle has become the first step to treating pancreatic cancer, but recent clinical research has not found an effective way to approach and kill pancreatic cancer cells. The studies that targeted CAFs and PD-1 immune inhibitors have shown the effort applied in advancing pancreatic cancer therapy, but it is not enough. More factors and mechanisms need to be found to support the basic knowledge behind clinical work, and more drug and therapy methods need to be applied to identify basic scientific problems.
Acknowledgements
State Key Laboratory of Oncogenes and Related Genes, Ren Ji Hospital, Shanghai Jiao Tong University of Medicine, Shanghai Jiao Tong University.
Funding: This work was supported by the grants from National Natural Science Foundation of China (81871923, 81802890, 81872242, 81502382, 81500461 and 81761138045), State Key Laboratory of Oncogenes and Related Genes (No. 91-17-24) and Shanghai health and family planning commission foundation (201740105).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7-30. [Crossref] [PubMed]
- Wolfgang CL, Herman JM, Laheru DA, et al. Recent progress in pancreatic cancer. CA Cancer J Clin 2013;63:318. [Crossref] [PubMed]
- Hruban RH, Pitman MB, Klimstra DS. Tumors of the pancreas. In: AFIP Atlas of Tumor Pathology. Fourth Series, Fascicle 6. Washington, DC: American Registry of Pathology/Armed Forces Institute of Pathology; 2007.
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646-74. [Crossref] [PubMed]
- Reccia I, Kumar J, Habib N, et al. The use of radiofrequency ablation in pancreatic cancer in the midst of the dawn of immuno-oncology. Med Oncol 2018;35:151. [Crossref] [PubMed]
- Koliopanos A, Avgerinos C, Paraskeva C, et al. Molecular aspects of carcinogenesis in pancreatic cancer. Hepatobiliary Pancreat Dis Int 2008;7:345-56. [PubMed]
- He D, Manzoni A, Florentin D, et al. Biologic effect of neurogenesis in pancreatic cancer. Hum Pathol 2016;52:182. [Crossref] [PubMed]
- Pandol S, Edderkaoui M, Gukovsky I, et al. Desmoplasia of pancreatic ductal adenocarcinoma. Clin Gastroenterol Hepatol 2009;7:S44-7. [Crossref] [PubMed]
- Lunardi S, Muschel RJ, Brunner TB. The stromal compartments in pancreatic cancer: are there any therapeutic targets? Cancer Lett 2014;343:147-55. [Crossref] [PubMed]
- Provenzano PP, Cuevas C, Chang AE, et al. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell 2012;21:418-29. [Crossref] [PubMed]
- Cannon A, Thompson C, Hall B, et al. Desmoplasia in pancreatic ductal adenocarcinoma: insight into pathological function and therapeutic potential. Genes Cancer 2018;9:78-86. [PubMed]
- Song H, Zhang Y. Regulation of pancreatic stellate cell activation by Notch3. BMC Cancer 2018;18:36. [Crossref] [PubMed]
- Boj SF, Hwang CI, Baker LA, et al. Organoid Models of Human and Mouse Ductal Pancreatic Cancer. Cell 2015;160:324-38. [Crossref] [PubMed]
- Öhlund D, Handly-Santana A, Biffi G, et al. Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J Exp Med 2017;214:579-96. [PubMed]
- Long KB, Tooker G, Tooker E, et al. IL-6 receptor blockade enhances chemotherapy efficacy in pancreatic ductal adenocarcinoma. Mol Cancer Ther 2017;16:1898-908. [Crossref] [PubMed]
- Cheng XB, Sato N, Kohi S, et al. 4-Methylumbelliferone inhibits enhanced hyaluronan synthesis and cell migration in pancreatic cancer cells in response to tumor-stromal interactions. Oncol Lett 2018;15:6297-301. [PubMed]
- Seino T, Kawasaki S, Shimokawa M, et al. Human Pancreatic Tumor Organoids Reveal Loss of Stem Cell Niche Factor Dependence during Disease Progression. Cell Stem Cell 2018;22:454-467.e6. [Crossref] [PubMed]
- Ene-Obong A, Clear AJ, Watt J, et al. Activated pancreatic stellate cells sequester CD8+ T cells to reduce their infiltration of the juxtatumoral compartment of pancreatic ductal adenocarcinoma. Gastroenterology 2013;145:1121-32. [Crossref] [PubMed]
- Hartmann N, Giese NA, Giese T, et al. Prevailing role of contact guidance in intrastromal T-cell trapping in human pancreatic cancer. Clin Cancer Res 2014;20:3422-33. [Crossref] [PubMed]
- Armstrong T, Packham G, Murphy LB, et al. Type I collagen promotes the malignant phenotype of pancreatic ductal adenocarcinoma. Clin Cancer Res 2004;10:7427-37. [Crossref] [PubMed]
- Feig C, Jones JO, Kraman M, et al. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc Natl Acad Sci U S A 2013;110:20212-7. [Crossref] [PubMed]
- Laklai H, Miroshnikova YA, Pickup MW, et al. Genotype tunes pancreatic ductal adenocarcinoma tissue tension to induce matricellular fibrosis and tumor progression.
- Jacobetz MA, Chan DS, Neesse A, et al. Hyaluronan impairs vascular function and drug delivery in a mouse model of pancreatic cancer. Gut 2013;62:112-20. [Crossref] [PubMed]
- Li X, Lee Y, Kang Y, et al. Hypoxia-induced autophagy of stellate cells inhibits expression and secretion of lumican into microenvironment of pancreatic ductal adenocarcinoma. Cell Death Differ 2019;26:382-93. [Crossref] [PubMed]
- Nielsen SR, Quaranta V, Linford A, et al. Corrigendum: Macrophage-secreted granulin supports pancreatic cancer metastasis by inducing liver fibrosis. Nat Cell Biol 2016;18:822. [Crossref] [PubMed]
- Aiello NM, Bajor DL, Norgard RJ, et al. Metastatic progression is associated with dynamic changes in the local microenvironment. Nat Commun 2016;7:12819. [Crossref] [PubMed]
- Zeleniak AE, Huang W, Brinkman MK, et al. Loss of MTSS1 results in increased metastatic potential in pancreatic cancer. Oncotarget 2017;8:16473-87. [Crossref] [PubMed]
- Malik R, Luong T, Cao X, et al. Rigidity controls human desmoplastic matrix anisotropy to enable pancreatic cancer cell spread via extracellular signal-regulated kinase 2. Matrix Biology 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Mahajan UM, Langhoff E, Goni E, et al. Immune Cell and Stromal Signature Associated With Progression-free Survival of Patients With Resected Pancreatic Ductal Adenocarcinoma. Gastroenterology 2018;155:1625-39.e2. [Crossref] [PubMed]
- Gentles AJ, Newman AM, Liu CL, et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat Med 2015;21:938-45. [Crossref] [PubMed]
- Daley D, Zambirinis CP, Seifert L, et al. γδ T Cells Support Pancreatic Oncogenesis by Restraining αβ T Cell Activation. Cell 2016;166:1485-99.e15. [Crossref] [PubMed]
- Nicholl MB, Ledgewood CL, Chen X, et al. IL-35 promotes pancreas cancer growth through enhancement of proliferation and inhibition of apoptosis: Evidence for a role as an autocrine growth factor. Cytokine 2014;70:126-33. [Crossref] [PubMed]
- Pylayeva-Gupta Y, Das S, Handler JS, et al. IL-35 producing B cells promote the development of pancreatic neoplasia. Cancer Discov 2016;6:247-55. [Crossref] [PubMed]
- Özdemir BC, Pentcheva-Hoang T, Carstens JL, et al. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell 2014;25:719-34. [Crossref] [PubMed]
- Sanford DE, Belt BA, Panni RZ, et al. Inflammatory monocyte mobilization decreases patient survival in pancreatic cancer: a role for targeting the CCL2/CCR2 axis. Clin Cancer Res 2013;19:3404-15. [Crossref] [PubMed]
- Mitsuhashi K, Nosho K, Sukawa Y, et al. Association of Fusobacterium species in pancreatic cancer tissues with molecular features and prognosis. Oncotarget 2015;6:7209-20. [Crossref] [PubMed]
- Tanaka A, Sakaguchi S. Regulatory T cells in cancer immunotherapy. Cell Res 2017;27:109-18. [Crossref] [PubMed]
- Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell 2015;27:450. [Crossref] [PubMed]
- Chen L. Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat Rev Immunol 2004;4:336-47. [Crossref] [PubMed]
- Flies DB, Chen L. The new B7s: playing a pivotal role in tumor immunity. J Immunother 2007;30:251-60. [Crossref] [PubMed]
- Soares KC, Rucki AA, Wu AA, et al. PD-1/PD-L1 blockade together with vaccine therapy facilitates effector T-cell infiltration into pancreatic tumors. J Immunother 2015;38:1-11. [Crossref] [PubMed]
- Ebine K, Kumar K, Pham TN, et al. Interplay between interferon regulatory factor 1 and BRD4 in the regulation of PD-L1 in pancreatic stellate cells. Sci Rep 2018;8:13225. [Crossref] [PubMed]
- Fan P, Ma J, Jin X. Far upstream element-binding protein 1 is up-regulated in pancreatic cancer and modulates immune response by increasing programmed death ligand 1. Biochem Biophys Res Commun 2018;505:830-6. [Crossref] [PubMed]
- Gunderson AJ, Kaneda MM, Tsujikawa T, et al. Bruton Tyrosine Kinase-Dependent Immune Cell Cross-talk Drives Pancreas Cancer. Cancer Discov 2016;6:270-85. [Crossref] [PubMed]
- Ludwig KF, Du W, Sorrelle NB, et al. Small-Molecule Inhibition of Axl Targets Tumor Immune Suppression and Enhances Chemotherapy in Pancreatic Cancer. Cancer Res 2018;78:246-55. [Crossref] [PubMed]
- Zhou L, Husted H, Moore T, et al. Suppression of stromal-derived Dickkopf-3 (DKK3) inhibits tumor progression and prolongs survival in pancreatic ductal adenocarcinoma. Sci Transl Med 2018.10. [PubMed]
- Zhang H, Wu H, Guan J, et al. Paracrine SDF-1α signaling mediates the effects of PSCs on GEM chemoresistance through an IL-6 autocrine loop in pancreatic cancer cells. Oncotarget 2015;6:3085-97. [PubMed]
- Gabitass RF, Annels NE, Stocken DD, et al. Elevated myeloid-derived suppressor cells in pancreatic, esophageal and gastric cancer are an independent prognostic factor and are associated with significant elevation of the Th2 cytokine interleukin-13. Cancer Immunol Immunother 2011;60:1419-30. [Crossref] [PubMed]
- Mace TA, Bloomston M, Lesinski GB. Pancreatic cancer-associated stellate cells: A viable target for reducing immunosuppression in the tumor microenvironment. Oncoimmunology 2013;2:e24891. [Crossref] [PubMed]
- Hamada S, Masamune A, Yoshida N, et al. IL-6/STAT3 Plays a Regulatory Role in the Interaction Between Pancreatic Stellate Cells and Cancer Cells. Dig Dis Sci 2016;61:1561-71. [Crossref] [PubMed]
- Edderkaoui M, Xu S, Chheda C, et al. HDAC3 mediates smoking-induced pancreatic cancer. Oncotarget 2016;7:7747-60. [Crossref] [PubMed]
- Cavel O, Shomron O, Shabtay A, et al. Endoneurial macrophages induce perineural invasion of pancreatic cancer cells by secretion of GDNF and activation of RET tyrosine kinase receptor. Cancer Res 2012;72:5733-43. [Crossref] [PubMed]
- Bockman DE, Büchler M, Beger HG. Interaction of pancreatic ductal carcinoma with nerves leads to nerve damage. Gastroenterology 1994;107:219-30. [Crossref] [PubMed]
- Liebl F, Demir IE, Mayer K, et al. The impact of neural invasion severity in gastrointestinal malignancies: a clinicopathological study. Ann Surg 2014;260:900-7; discussion 907-8. [Crossref] [PubMed]
- Sakano D, Choi S, Kataoka M, et al. Dopamine D2 Receptor-Mediated Regulation of Pancreatic β Cell Mass. Stem Cell Reports 2016;7:95-109. [Crossref] [PubMed]
- Jandaghi P, Najafabadi HS, Bauer AS, et al. Expression of DRD2 is Increased in Human Pancreatic Ductal Adenocarcinoma and Inhibitors Slow Tumor Growth in Mice. Gastroenterology 2016;151:1218-31. [Crossref] [PubMed]
- Oleson BJ, Broniowska KA, Naatz A, et al. Nitric Oxide Suppresses β-Cell Apoptosis by Inhibiting the DNA Damage Response. Mol Cell Biol 2016;36:2067-77. [Crossref] [PubMed]
- Rouintan Z, Farrokhfall K, Karbalaei N, et al. Nitric Oxide Overproduction Reduces Insulin Secretion from Isolated Islets in Fetal Hypothyroid Rats. Horm Metab Res 2016;48:145-50. [Crossref] [PubMed]
- Wang J, He P, Gaida M, et al. Inducible nitric oxide synthase enhances disease aggressiveness in pancreatic cancer. Oncotarget 2016;7:52993-3004. [PubMed]
- El-Merahbi R, Löffler M, Mayer A, et al. The roles of peripheral serotonin in metabolic homeostasis. Febs Lett 2015;589:1728-34. [Crossref] [PubMed]
- Saponara E, Visentin M, Baschieri F, et al. Serotonin uptake is required for Rac1 activation in Kras-induced acinar-to-ductal metaplasia in the pancreas. J Pathol 2018;246:352-65. [Crossref] [PubMed]
- Jiang SH, Li J, Dong FY, et al. Increased Serotonin Signaling Contributes to the Warburg Effect in Pancreatic Tumor Cells under Metabolic Stress and Promotes Growth of Pancreatic Tumors in Mice. Gastroenterology 2017;153:277-291.e19. [Crossref] [PubMed]
- Jiang SH, Zhu LL, Zhang M, et al. GABRP regulates chemokine signalling, macrophage recruitment and tumour progression in pancreatic cancer through tuning KCNN4-mediated Ca2+ signalling in a GABA-independent manner. Gut 2019. [Epub ahead of print]. [Crossref] [PubMed]
- Qian W, Lv S, Li J, et al. Norepinephrine enhances cell viability and invasion, and inhibits apoptosis of pancreatic cancer cells in a Notch-1-dependent manner. Oncol Rep 2018;40:3015-23. [PubMed]
- Zhou L, Zhang R, Yao W, et al. Decreased expression of angiotensin-converting enzyme 2 in pancreatic ductal adenocarcinoma is associated with tumor progression. Tohoku J Exp Med 2009;217:123-31. [Crossref] [PubMed]
- Liu H, Naxerova K, Pinter M, et al. Use of Angiotensin System Inhibitors is Associated with Immune Activation and Longer Survival in Non-Metastatic Pancreatic Ductal Adenocarcinoma. Clin Cancer Res 2017;23:5959. [Crossref] [PubMed]
- Ju HQ, Li H, Tian T, et al. Melatonin overcomes gemcitabine resistance in pancreatic ductal adenocarcinoma by abrogating NF-κB activation. J Pineal Res 2016;60:27-38. [Crossref] [PubMed]
- Fang Z, Jung KH, Yan HH, et al. Melatonin Synergizes with Sorafenib to Suppress Pancreatic Cancer via Melatonin Receptor and PDGFR-β/STAT3 Pathway. Cell Physiol Biochem 2018;47:1751-68. [Crossref] [PubMed]
- Hu LP, Zhang XX, Jiang SH, et al. Targeting Purinergic Receptor P2Y2 Prevents the Growth of Pancreatic Ductal Adenocarcinoma by Inhibiting Cancer Cell Glycolysis. Clin Cancer Res 2019;25:1318-30. [Crossref] [PubMed]
- Yang J, Waldron RT, Su HY, et al. Insulin promotes proliferation and fibrosing responses in activated pancreatic stellate cells. Am J Physiol Gastrointest Liver Physiol 2016;311:G675-87. [Crossref] [PubMed]
- Straßburger K, Tiebe M, Pinna F, et al. Insulin/IGF signaling drives cell proliferation in part via Yorkie/YAP. Dev Biol 2012;367:187-96. [Crossref] [PubMed]
- Hao F, Xu Q, Zhao Y, et al. Insulin Receptor and GPCR Crosstalk Stimulates YAP via PI3K and PKD in Pancreatic Cancer Cells. Mol Cancer Res 2017;15:929-41. [Crossref] [PubMed]
- Xu M, Chang HH, Jung X, et al. Deficiency in hormone-sensitive lipase accelerates the development of pancreatic cancer in conditional KrasG12D mice. BMC Cancer 2018;18:797. [Crossref] [PubMed]
- Xu M, Qi F, Zhang S, et al. Adrenomedullin promotes the growth of pancreatic ductal adenocarcinoma through recruitment of myelomonocytic cells. Oncotarget 2016;7:55043-56. [PubMed]
- Konstantinidis IT, Warshaw AL, Allen JN, et al. Pancreatic ductal adenocarcinoma: is there a survival difference for R1 resections versus locally advanced unresectable tumors? What is a "true" R0 resection? Ann Surg 2013;257:731-6. [Crossref] [PubMed]
- Dougan M, Dranoff G. Immune therapy for cancer. Annu Rev Immunol 2009;27:83-117. [Crossref] [PubMed]
- Hodi FS, O'Day SJ, Mcdermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711-23. [Crossref] [PubMed]
- Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti–PD-1 antibody in cancer. N Engl J Med 2012;366:2443-54. [Crossref] [PubMed]
- Postow MA, Chesney J, Pavlick AC, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med 2015;372:2006-17. [Crossref] [PubMed]
- Dougan SK. The Pancreatic Cancer Microenvironment. (1540-336X (Electronic)).
- Zheng L, Xue J, Jaffee EM, et al. Role of immune cells and immune-based therapies in pancreatitis and pancreatic ductal adenocarcinoma. Gastroenterology 2013;144:1230-40. [Crossref] [PubMed]
- Lutz ER, Wu AA, Bigelow E, et al. Immunotherapy converts nonimmunogenic pancreatic tumors into immunogenic foci of immune regulation. Cancer Immunol Res 2014;2:616-31. [Crossref] [PubMed]
- Topalian SL, Drake CG, Pardoll DM. Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol 2012;24:207-12. [Crossref] [PubMed]
- Wang L, Liu X, Zhou Q, et al. Terminating the criminal collaboration in pancreatic cancer: Nanoparticle-based synergistic therapy for overcoming fibroblast-induced drug resistance. Biomaterials 2017;144:105-18. [Crossref] [PubMed]
- Kudo D, Suto A, Hakamada K. The Development of a Novel Therapeutic Strategy to Target Hyaluronan in the Extracellular Matrix of Pancreatic Ductal Adenocarcinoma. Int J Mol Sci 2017.18. [PubMed]
- Von Hoff DD, Ramanathan RK, Borad MJ, et al. Gemcitabine plus nab-paclitaxel is an active regimen in patients with advanced pancreatic cancer: a phase I/II trial. J Clin Oncol 2011;29:4548-54. [Crossref] [PubMed]
- Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 2013;369:1691-703. [Crossref] [PubMed]
- Zeitouni D, Pylayeva-Gupta Y, Der CJ, et al. KRAS Mutant Pancreatic Cancer: No Lone Path to an Effective Treatment. Cancers (Basel) 2016.8. [PubMed]
- Vanhaesebroeck B, Guillermetguibert J, Graupera M, et al. The emerging mechanisms of isoform-specific PI3K signalling. Nat Rev Mol Cell Biol 2010;11:329-41. [Crossref] [PubMed]
- Ferino A, Miglietta G, Picco R, et al. MicroRNA therapeutics: design of single-stranded miR-216b mimics to target KRAS in pancreatic cancer cells. RNA Biol 2018;15:1273-85. [Crossref] [PubMed]
- Ellermeier J, Wei J, Duewell P, et al. Therapeutic efficacy of bifunctional siRNA combining TGF-β1 silencing with RIG-I activation in pancreatic cancer. Cancer Res 2013;73:1709-20. [Crossref] [PubMed]
- Liu C, Zhang H, Zang X, et al. The influence of SnoN gene silencing by siRNA on the cell proliferation and apoptosis of human pancreatic cancer cells. Diagn Pathol 2015;10:30. [Crossref] [PubMed]
- Jones S. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science 2016;321:1801-6. [Crossref] [PubMed]
- Kim EJ, Sahai V, Abel EV, et al. Pilot Clinical Trial of Hedgehog Pathway Inhibitor GDC-0449 (Vismodegib) in Combination with Gemcitabine in Patients with Metastatic Pancreatic Adenocarcinoma. Clin Cancer Res 2014;20:5937-45. [Crossref] [PubMed]
- Cortes E, Lachowski D, Robinson B, et al. Tamoxifen mechanically reprograms the tumor microenvironment via HIF-1A and reduces cancer cell survival. EMBO Rep 2019.20. [PubMed]
- Lee J, Kang TH, Yoo W, et al. An Antibody Designed to Improve Adoptive NK-Cell Therapy Inhibits Pancreatic Cancer Progression in a Murine Model. Cancer Immunol Res 2019;7:219-29. [PubMed]
- Totiger TM, Srinivasan S, Jala VR, et al. Urolithin A, a Novel Natural Compound to Target PI3K/AKT/mTOR Pathway in Pancreatic Cancer. Mol Cancer Ther 2019;18:301-11. [Crossref] [PubMed]


