
A step ahead on CTHRC1, and not just reinventing the wheel!
The association of CTHRC1 with tumor progression is demonstrated in various human cancers, including hepatocellular carcinoma, gastric cancer, pancreatic cancer, GIST and colorectal cancer (1-3). In a meta-analysis done by Wang et al., the results revealed correlation of CTHRC1 expression with TNM stage in gastric cancer, breast cancer, and hepatocellular carcinoma (4). It indicated CTHRC1’s role as a biomarker for prognosis of cancer patients by showing that patients with higher CTHRC1 expression had decreased overall survival (OS) and disease-free survival (DFS) which was statistically significant (4).
In the current study done by Ni et al., role of CTHRC1 expression in predicting the prognosis of patients with colorectal cancer patients has been confirmed. Statistically significant association of high expression of CTHRC1 with advanced stage (P=0.016) and tumor budding (P=0.022) was demonstrated (5).
Ni et al. also showed high expression of CTHRC1 in tumor buds which also suggests a possible role of CTHRC1 in epithelial-mesenchymal transition (EMT) and cancer metastasis. Tumor budding which is considered to be the morphologic manifestation of EMT is considered to be an early event in the metastatic process of human cancers. Its role in prognosticating the colorectal cancer is well established (6,7). However, depending upon the study, interobserver variability in reporting tumor budding has ranged from moderate to very good, and it is recognised that difference is greater when pathologist inexperienced in tumor budding are included. It’s difficult to interpret tumor budding in mucinous carcinomas and in adenocarcinoma with inflammatory infiltrate. Also, it is unclear whether tumor budding is best evaluated as a continuous variable or with a tiered system (6). Within the confines of the above limitations of tumor budding assessment, CTHRC1 can serve as a better prognostic biomarker.
CTHRC1 has been linked with EMT which is considered to be modulated by the transforming growth factor (TGF-β) pathway. This study demonstrated that increased expression of CTHRC1 level in TGF-β treated cells. CTHRC1 knockdown had partially attenuated the migration and invasion induced by TGF-β by possibly hampering Smad2/3 signaling (which mediates the regulatory effect of TGF-β on EMT) and leads to partial recovery of effect of TGF-β on epithelial markers (E-cadherin and α-catenin) and mesenchymal markers (fibronectin and vimentin).
Previously, CTHRC1 has been shown to activate various other signaling pathways (like Wnt/PCP pathway, Src and Erk signaling) which are interlinked with TGF-β pathway (8,9).
Many studies has shown that acquisition of EMT features is associated with development of resistance to chemotherapy which could lead to recurrence and metastasis after standard chemotherapeutic treatment (10,11).
It has been previously postulated and supported by evidence from preclinical studies that interaction among cancer cells in the tumor microenvironment can induce EMT by auto- and/or paracrine secretion of mediators such as growth factors, cytokines, and ECM proteins (11) (Figure 1). In one study done by Erikson et al. on melanoma cells, CTHRC1 was found to be expressed by not only the melanoma cells but also by activated stromal fibroblasts and blood vessel endothelial cells (12). Few studies also suggest that cancer-associated fibroblasts may be supplied from cancer cells undergoing EMT as cells undergoing EMT acquires a fibroblastoid mesenchymal appearance (13,14).
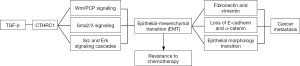
These findings further validate the crucial role of tumor micro-environment is involved in tumor progression and supports the Stephen Paget’s original “seed and soil” hypothesis (15).
In conclusion, although multiple steps involved in the biology of metastasis still require further elucidation, study done by Ni et al. surely fills some gap in our understanding.
This finding will help to develop better risk assessment tool to further individualize the treatment planning. Nevertheless, the full impact of CTHRC1 expression on cancer is still not entirely inferred and its role as a predictive marker of response to chemotherapy needs to be further explored. Also, pivotal role of CTHRC1 in human cancer progression makes it an attractive new target for therapy.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Kharaishvili G, Cizkova M, Bouchalova K, et al. Collagen triple helix repeat containing 1 protein, periostin and versican in primary and metastatic breast cancer: an immunohistochemical study. J Clin Pathol 2011;64:977-82. [Crossref] [PubMed]
- Association of Specific Genotypes in Metastatic Suppressor HTPAP with Tumor Metastasis and Clinical Prognosis in Hepatocellular Carcinoma|Cancer Research [Internet]. [cited 2019 Jan 25]. Available online: http://cancerres.aacrjournals.org/content/71/9/3278
- Wang P, Wang YC, Chen XY, et al. CTHRC1 is upregulated by promoter demethylation and transforming growth factor-β1 and may be associated with metastasis in human gastric cancer. Cancer Sci 2012;103:1327-33. [Crossref] [PubMed]
- Wang SF, Yin Z, Yin JJ, et al. Clinical significance of CTHRC1 protein expression in human cancers: a meta-analysis. Genet Mol Res 2016;15. [PubMed]
- Ni S, Ren F, Xu M, et al. CTHRC1 overexpression predicts poor survival and enhances epithelial-mesenchymal transition in colorectal cancer. Cancer Med 2018;7:5643-54. [Crossref] [PubMed]
- Cho SJ, Kakar S. Tumor Budding in Colorectal Carcinoma: Translating a Morphologic Score Into Clinically Meaningful Results. Arch Pathol Lab Med 2018;142:952-7. [Crossref] [PubMed]
- Graham RP, Vierkant RA, Tillmans LS, et al. Tumor Budding in Colorectal Carcinoma: Confirmation of Prognostic Significance and Histologic Cutoff in a Population-based Cohort. Am J Surg Pathol 2015;39:1340-6. [Crossref] [PubMed]
- Yamamoto S, Nishimura O, Misaki K, et al. Cthrc1 selectively activates the planar cell polarity pathway of Wnt signaling by stabilizing the Wnt-receptor complex. Dev Cell 2008;15:23-36. [Crossref] [PubMed]
- Kim HC, Kim YS, Oh HW, et al. Collagen Triple Helix Repeat Containing 1 (CTHRC1) acts via ERK-dependent induction of MMP9 to promote invasion of colorectal cancer cells. Oncotarget 2014;5:519-29. [Crossref] [PubMed]
- Adam L, Zhong M, Choi W, et al. miR-200 expression regulates epithelial-to-mesenchymal transition in bladder cancer cells and reverses resistance to epidermal growth factor receptor therapy. Clin Cancer Res 2009;15:5060-72. [Crossref] [PubMed]
- Iwatsuki M, Mimori K, Yokobori T, et al. Epithelial–mesenchymal transition in cancer development and its clinical significance. Cancer Sci 2010;101:293-9. [Crossref] [PubMed]
- Eriksson J, Le Joncour V, Nummela P, et al. Gene expression analyses of primary melanomas reveal CTHRC1 as an important player in melanoma progression. Oncotarget 2016;7:15065-92. [Crossref] [PubMed]
- Lewis MP, Lygoe KA, Nystrom ML, et al. Tumour-derived TGF-beta1 modulates myofibroblast differentiation and promotes HGF/SF-dependent invasion of squamous carcinoma cells. Br J Cancer 2004;90:822-32. [Crossref] [PubMed]
- Radisky DC, Kenny PA, Bissell MJ. Fibrosis and cancer: do myofibroblasts come also from epithelial cells via EMT? J Cell Biochem 2007;101:830-9. [Crossref] [PubMed]
- Fidler IJ, Poste G. The “seed and soil” hypothesis revisited. Lancet Oncol 2008;9:808. [Crossref] [PubMed]


