
Breast cancer prognostic staging and internal mammary lymph node metastases: a brief overview
Introduction
The incidence of cancer worldwide continues to increase, and it remains the second leading cause of death (1). Of the 17.5 million cancer cases worldwide in 2015, breast cancer was the most common in women (2.4 million cases) and was the leading cause of cancer deaths (523,000) in women (1). As such, the importance of effectively and efficiently communicating disease characteristics and prognosis remains essential to patients, clinicians, and researchers. Breast cancer staging often provides this foundation for communication and serves as a framework for therapeutic and research discussions. The American Joint Committee on Cancer’s (AJCC) Breast Cancer Staging Manual was recently updated (8th edition) to now include tumor biology in addition to the traditional anatomic factors (2). Given these significant changes, multiple studies have sought to investigate the implications of the new guidelines for various populations. In particular, Joo et al. recently evaluated the prognostic value of these guidelines among patients with internal mammary lymph node (IMN) metastases (3). As such, breast cancer staging, IMN metastases, and related literature are briefly reviewed.
Breast cancer staging overview
Breast cancer staging was initially created as a tool for conveying the predicted “life history” of a tumor (4). It provided standard nomenclature for communication and assisted with breast cancer prognostication. When it was developed, the mainstay of breast cancer treatment was radical surgery and radiation. As diagnostic techniques, systemic therapies, and our understanding of breast cancer have all substantially improved, the treatment of breast cancer has changed dramatically, and thus, the staging guidelines have been critically refined. Today, a patient’s breast cancer stage is assessed at diagnosis (clinical stage) and at the time of surgery (pathological stage). It concisely summarizes the disease by incorporating the location and extent of disease, as well as tumor biology.
The current guidelines include two staging systems—the anatomic stage and the prognostic stage (2). Anatomic staging is based solely on traditional anatomic factors, including the primary tumor size (T), nodal status (N), and distant metastasis (M). The prognostic stage builds on the anatomic stage and includes the TNM variables, as well as tumor grade, estrogen receptor (ER) status, progesterone receptor (PR) status, human epidermal growth factor receptor 2 (HER2) status, and when available, tumor multigene panel testing (i.e., Oncotype DX Recurrence Score). While the traditional anatomic factors (TNM) remain relevant (5), particularly for local-regional management, numerous studies have confirmed the prognostic significance of these biologic tumor factors as well, as they are often used for systemic treatment decisions. By incorporating all of these variables, the prognostic stage has been shown to be the most reliable and accurate source for the prognostication of patient outcomes (6,7).
However, the introduction of prognostic staging has resulted in significant stage changes for many patients. One such study of 411,372 patients in the National Cancer Data Base reported that 13% of patients were upstaged using the 8th edition criteria and 29.7% were downstaged, which refined survival estimates compared to the 7th edition criteria (8). Others have validated the latest staging guidelines and confirmed their relevance (6), including in locally advanced breast cancer (9). It is important to recognize that the staging guidelines were based on populations that largely received appropriate treatments (2), thus highlighting the continued importance of multidisciplinary care. With the incorporation of these new variables, staging can be used more effectively for patient-specific management, population-level assessments, and research purposes.
To determine the clinical stage, multiple diagnostic modalities are often employed. While the physical exam provides an initial clinical assessment of tumor size and nodal status, it is often supplemented with various imaging techniques. Standard breast imaging typically includes a mammogram and/or ultrasound, while a breast MRI may be used for select cases. Routine axillary imaging is controversial, but when considered, an axillary ultrasound may be obtained. Systemic staging is not routinely recommended (10), particularly for asymptomatic women with early stage breast cancer (11). Some clinicians may check bloodwork, such as a complete blood count (CBC) and comprehensive metabolic panel with liver function tests (CMP + LFTs). When additional imaging is performed, systemic staging may include a CT (computerized tomography) scan of the chest/abdomen/pelvis, nuclear medicine bone scan, and/or FDG-PET (18-fluoro-deoxyglucose positron emission tomography)/CT scan. For suspicious findings on imaging, biopsy should be considered of local-regional (breast and/or axilla) and/or distant sites (potential metastatic disease). If malignancy is confirmed, tumor histology, grade, and receptor status (ER, PR, and HER2) should be assessed (10).
Significance of prognostic staging variables
Early studies and more contemporary research have both demonstrated the prognostic significance of tumor size and nodal status (12,13). Similarly, tumor grade has been widely recognized as an important prognostic variable in breast cancer for many years, regardless of tumor size and/or nodal status (14), although it was not incorporated into the official staging guidelines until the introduction of the most recent AJCC staging manual (2). Tumor biomarkers were also identified as important determinants of prognosis early on, and two of the first biomarkers to be recognized were the estrogen and PRs (15,16). Subsequently, the HER2/neu oncogene was noted to be associated with prognosis as well (17). Given these findings, several studies have sought to evaluate the combination of anatomic extent of disease and tumor biology, compared to anatomic staging alone, and have demonstrated superior stratification of survival estimates when tumor biology was included (18). The latest research investigated the utility of tumor multigene panel testing, and it too has been shown to have prognostic significance in select breast cancer cohorts (19,20), thus leading to its incorporation into the latest staging guidelines (2). Taken together, the new prognostic staging includes a summary of the anatomic extent of disease and critical biologic tumor characteristics.
IMN overview
The IMNs are considered a first-echelon nodal drainage site in breast cancer, similar to the axillary lymphatic system. On average, there are 6 IMNs, which are located just lateral to the sternum (behind the costal cartilage) near the internal mammary (IM) vessels and within the 1st–4th intercostal spaces (21). IMNs may be visualized on ultrasound, magnetic resonance imaging (MRI), CT scan, FDG-PET scan, and/or lymphoscintigraphy. When sentinel lymph node mapping is performed, 10–30% of patients have visible lymphatic drainage via the IMNs (depending on the injection site). If an IMN is suspicious for metastasis on imaging, fine needle aspiration (FNA) or resection may be considered for histologic diagnosis (21).
For women with breast cancer, IMN involvement is more common in those with more advanced disease, medially-located tumors, and axillary nodal metastases (22,23). For patients with positive axillary nodes, an estimated 28–52% may also have IMN metastases, while 5–17% of patients with negative axillary nodes may have IMN involvement (24). Although identification of IMN metastases may change the disease stage for select patients (Table 1), indications for biopsy and/or resection remain controversial (23). In addition, women with IMN metastases have been shown to have a worse overall prognosis; however, resection of IMN nodes in particular has not been shown to improve outcomes in numerous studies (23).
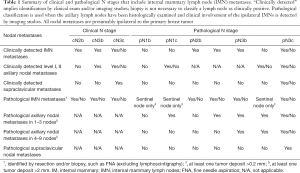
Full table
Currently, the NCCN guidelines recommend radiation therapy of the IMNs when delivering regional nodal irradiation (10). Studies have demonstrated reduced regional recurrences and distant metastases with the addition of nodal irradiation, particularly for those with axillary nodal metastases in ≥4 nodes (24). However, IMN irradiation is not generally recommended for patients with negative axillary nodes (10). When considering IMN irradiation, it is important to also consider the potential associated risks, such as pulmonary and cardiac toxicities (23,24).
IMN staging
With the recent changes to the AJCC breast cancer staging guidelines (2), several studies have sought to re-evaluate the prognostic significance of various tumor and biologic variables. Joo et al. recently performed a single institution retrospective review to specifically assess the prognostic value of the new guidelines for patients with IMN metastases (3). Prior to this, they published a review of 70 women with suspicious IMNs either by size and morphology or FDG avidity (cN2b or cN3b; all underwent FNA biopsy), who were treated with neoadjuvant chemotherapy, surgery, and radiation (25). After stratifying by the results of the FNA biopsy (positive or negative/failed), they demonstrated that patients with FNA + IMNs (57%, N=40) had worse treatment outcomes compared to those with clinically diagnosed IMN metastases and negative FNA (25). On multivariate analysis, FNA + IMN was not significantly associated with overall survival or progression-free survival (25). However, it is unclear how these results may have been impacted by tumor burden in the IMNs and the selection bias inherent in retrospectively identifying patients who under IMN biopsy.
Using this same cohort of 70 patients, Joo et al. more recently sought to investigate the impact of IMN metastases on breast cancer staging using the new guidelines, with the additional exclusion of patients with isolated IMN metastases (and negative axillary nodes); the final cohort included 66 patients with cN3b disease (3). Based on anatomic staging, all patients were classified as overall stage IIIC, while prognostic staging downstaged 61% of patients by stratifying them into 3 groups (2 patients downstaged to IIIA and 38 to IIIB; 26 patients remained IIIC). Based on the prognostic staging stratification, significant differences in survival were consistently noted. Thus, the authors concluded that the new staging guidelines provided more accurate prognostic estimates than anatomic staging alone, likely due to the inclusion of tumor burden and biology (3). Although this was a small retrospective study, these findings are consistent with those of other studies (6,9) and further validate the utility and accuracy of the prognostic staging guidelines.
Conclusions
The latest AJCC breast cancer staging guidelines now combine the anatomic extent of disease (traditional TNM factors) with tumor biology (tumor grade, molecular biomarkers, and multigene panel testing) to provide superior prognostic estimates, which will ultimately improve patient care and advance research efforts. In addition, it will continue to serve as a universal tool for consistent and efficient communication among clinicians and researchers worldwide. While IMN metastases were once thought to represent a universally dismal prognosis, the new staging guidelines stratify this population into multiple groups, thus allowing for more personalized prognostic estimates. As the biology of breast cancer is further elucidated, diagnostic techniques and therapeutic strategies will also continue to evolve, and thus, the staging guidelines will undoubtedly undergo further refinement.
Acknowledgments
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Fitzmaurice C, Allen C, Barber RM, et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol 2017;3:524-48. [Crossref] [PubMed]
- Hortobagyi GN, Connolly JL, D'Orsi CJ, et al. Chapter 48: Breast. In: Edge SB, Byrd DR, Compton CC, et al. editors. AJCC Cancer Staging Manual. 8th edition. New York: Springer, 2017.
- Joo JH, Kim SS, Son BH, et al. Evaluation of the Prognostic Stage in the 8th Edition of the American Joint Committee on Cancer in Patients with Breast Cancer and Internal Mammary Lymph Node Metastasis. Anticancer Res 2018;38:5357-61.
- Manual for Staging of Cancer. 1st ed. Philadelphia, PA: Lippincott-Raven Publishers, 1977.
- Plichta JK, Campbell BM, Mittendorf EA, et al. Anatomy and Breast Cancer Staging: Is It Still Relevant? Surg Oncol Clin N Am 2018;27:51-67. [Crossref] [PubMed]
- Weiss A, Chavez-MacGregor M, Lichtensztajn DY, et al. Validation Study of the American Joint Committee on Cancer Eighth Edition Prognostic Stage Compared With the Anatomic Stage in Breast Cancer. JAMA Oncol 2018;4:203-9.
- Hortobagyi GN, Edge SB, Giuliano A. New and Important Changes in the TNM Staging System for Breast Cancer. Am Soc Clin Oncol Educ Book 2018.457-67. [Crossref] [PubMed]
- Plichta JK, Ren Y, Thomas SM, et al. Implications for Breast Cancer Restaging Based on the 8th Edition AJCC Staging Manual. Ann Surg 2018. [Epub ahead of print].
- Wang M, Chen H, Wu K, et al. Evaluation of the prognostic stage in the 8th edition of the American Joint Committee on Cancer in locally advanced breast cancer: An analysis based on SEER 18 database. Breast 2018;37:56-63.
- Gradishar WJ, Anderson BO, Aft R, et al. NCCN Guidelines: Breast Cancer, Version 1.20182018 3/20/2018. Available online: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf
- Moy L, Newell MS, Mahoney MC, et al. ACR Appropriateness Criteria Stage I Breast Cancer: Initial Workup and Surveillance for Local Recurrence and Distant Metastases in Asymptomatic Women. J Am Coll Radiol 2016;13:e43-52. [Crossref] [PubMed]
- Carter CL, Allen C, Henson DE. Relation of tumor size, lymph node status, and survival in 24,740 breast cancer cases. Cancer 1989;63:181-7. [Crossref] [PubMed]
- Hayes DF, Allred C, Anderson BO, et al. Chapter 32: Breast. In: Edge SB, Byrd DR, Compton CC, et al. editors. AJCC Cancer Staging Manual. 7th edition. New York: Springer, 2010.
- Henson DE, Ries L, Freedman LS, et al. Relationship among outcome, stage of disease, and histologic grade for 22,616 cases of breast cancer. The basis for a prognostic index. Cancer 1991;68:2142-9. [Crossref] [PubMed]
- Knight WA, Livingston RB, Gregory EJ, et al. Estrogen receptor as an independent prognostic factor for early recurrence in breast cancer. Cancer Res 1977;37:4669-71. [PubMed]
- Clark GM, McGuire WL, Hubay CA, et al. Progesterone Receptors as a Prognostic Factor in Stage II Breast Cancer. New England Journal of Medicine 1983;309:1343-7. [Crossref] [PubMed]
- Slamon DJ, Clark GM, Wong SG, et al. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 1987;235:177-82. [Crossref] [PubMed]
- Yi M, Mittendorf EA, Cormier JN, et al. Novel staging system for predicting disease-specific survival in patients with breast cancer treated with surgery as the first intervention: time to modify the current American Joint Committee on Cancer staging system. J Clin Oncol 2011;29:4654-61. [Crossref] [PubMed]
- Sparano JA, Gray RJ, Makower DF, et al. Prospective Validation of a 21-Gene Expression Assay in Breast Cancer. N Engl J Med 2015;373:2005-14. [Crossref] [PubMed]
- Cardoso F, van't Veer LJ, Bogaerts J, et al. 70-Gene Signature as an Aid to Treatment Decisions in Early-Stage Breast Cancer. N Engl J Med 2016;375:717-29. [Crossref] [PubMed]
- Urano M, Denewar FA, Murai T, et al. Internal mammary lymph node metastases in breast cancer: what should radiologists know? Jpn J Radiol 2018;36:629-40. [Crossref] [PubMed]
- Freedman GM, Fowble BL, Nicolaou N, et al. Should internal mammary lymph nodes in breast cancer be a target for the radiation oncologist? Int J Radiat Oncol Biol Phys 2000;46:805-14. [Crossref] [PubMed]
- Chen RC, Lin NU, Golshan M, et al. Internal mammary nodes in breast cancer: diagnosis and implications for patient management -- a systematic review. J Clin Oncol 2008;26:4981-9. [Crossref] [PubMed]
- Cong BB, Cao XS, Cao L, et al. Internal mammary lymph nodes radiotherapy of breast cancer in the era of individualized medicine. Oncotarget 2017;8:81583-90. [Crossref] [PubMed]
- Joo JH, Kim SS, Ahn SD, et al. Impact of pathologic diagnosis of internal mammary lymph node metastasis in clinical N2b and N3b breast cancer patients. Breast Cancer Res Treat 2017;166:511-8. [Crossref] [PubMed]


