
The spectrum of CD30+ T cell lymphoproliferative disorders in the skin
Introduction
Primary cutaneous CD30+ T cell lymphoproliferative disorders (pcCD30+ T cell LPDs) are the second most common type of cutaneous T cell lymphomas (CTCL), after mycosis fungoides (MF), accounting for 30% of all primary cutaneous lymphomas (1,2). Their diagnostic hallmark is CD30 expression by the atypical lymphocytes, in the same typical membranous and golgi pattern observed in systemic anaplastic large cell lymphoma (sALCL) (3). pcCD30+ T cell LPDs encompass a spectrum of diseases, including lymphomatoid papulosis (LyP), primary cutaneous anaplastic large cell lymphoma (pcALCL), and borderline lesions. LyP and pcALCL overlap substantially histopathologically and immunophenotypically, but often differ dramatically in their clinical presentation and progression. They also share some histological and immunophenotypic features with other CD30-expressing cutaneous lymphomas, including transformed MF and systemic CD30+ lymphomas. The treatment and prognosis of cutaneous CD30+ T cell LPDs differ significantly from those of systemic lymphomas, emphasizing the importance of a clinical pathological correlation and thorough workup to ensure an accurate diagnosis (4). Due to the advent of CD30 directed therapy, CD30 expression has also become a useful marker to guide treatment. As evident by the favorable prognosis of pcALCL and spontaneous regression of LyP, pcCD30+ T cell LPDs highlight the inconsistency between the high-grade morphology of the tumor cells and their paradoxical biological behavior, a discrepancy that remains unexplained. This review describes the structure and function of CD30, the clinical and histological features of pcCD30+ T cell LPDs, other CD30+ diseases, and briefly the outcomes of CD30 directed treatment.
CD30 structure and function
CD30 was first identified in 1982 in Hodgkin Reed-Sternberg (HRS) cells (5,6). The CD30 antigen, also known as Ki-1 antigen or TNFRSF8, is a 120kDa transmembrane glycoprotein receptor belonging to the tumor necrosis factor receptor (TNFR) superfamily (7,8). Similar to other TNFR molecules, CD30 has six extracellular cysteine-rich repeats, which form a scaffold of disulfide bonds in an extended confirmation. The cytoplasmic tail contains serine/threonine amino acids that bind TNF receptor-associated factor (TRAF) and TRAF binding proteins after phosphorylation (9-11). The cytoplasmic domain mediates activation of mitogen-activated protein (MAP) kinases, such as extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK) and p38, and nuclear factor kappa-B (NF-kB) via TRAFs (11,12). In some cells, CD30 signaling can induce pro-apoptotic stimuli via TRAF2 degradation and activation of TNFR1 mediated apoptosis (13). A soluble (sCD30) form can be detected in the serum of patients with autoimmune diseases or CD30+ malignancies, and represents a cleavage byproduct of the CD30 extracellular region (14,15). The ligand for CD30 (CD30L or CD153) is a 26kDa transmembrane protein that is expressed in resting and activated B cells, activated T cells, monocytes, granulocytes and natural killer cells (8,16,17).
The expression of CD30 is restricted to activated T, B and NK cells in healthy individuals. Its exact function is unknown and no human disease has been linked to CD30 or CD30L gene mutations (18,19). CD30 has a broad spectrum of biological effects, sometimes varying and conflicting according to conditions, which include stimulation of cytokine secretion, regulation of inflammation, induction of apoptosis, and promotion of cell survival and proliferation. CD30/CD30L signaling is involved in B cell class-switch DNA recombination and antibody production, and survival and effector functions of T cells (20). CD30 may also play a role in self-tolerance and autoimmune diseases, along with Th1 and Th2 responses and associated diseases (21-23). CD30/CD30L interaction regulates CD4+ T cell mediated graft versus host disease (GVHD) and suppression of T regulatory cell mediated allograft rejection (24,25).
CD30 expression has been observed in virus-infected cells, including those infected by Epstein-Barr virus (EBV), hepatitis C virus (HCV), human immunodeficiency virus (HIV), and human T-lymphotropic virus (HTLV) (26-28). CD30 expression in non-hematopoietic malignancies has also been reported, such as embryonal carcinomas, seminomas, mesotheliomas, and thyroid carcinomas (28). In hematologic malignancies, CD30 is strongly expressed by HRS cells in classical Hodgkin lymphoma (cHL) and by the neoplastic T cells of sALCL, regardless of the presence of anaplastic lymphoma kinase (ALK)-1 gene rearrangements. CD30 expression is variably detected in other lymphomas, including subsets of diffuse large B cell lymphoma (DLBCL), primary mediastinal B cell lymphoma (PMBCL), and peripheral T cell lymphoma (PTCL). Expression of CD30 can also be detected in enteropathy associated T cell lymphomas (EATL), HTLV-1 associated adult T cell leukemia/lymphomas (ATLL), extranodal NK/T cell lymphomas (ENKTL), primary effusion lymphomas (PEL), systemic mastocytosis, and mast cell leukemia (29-33).
In both cHL and ALCL tumor cells, the expression of CD30 is regulated by the JunB transcription factor (34,35), but epigenetically regulation of CD30 gene transcription has also been observed (36). The effects of CD30 signaling in tumor cells are dependent on the cell type and stimulus. In ALCL, CD30 signaling induces growth arrest and modest levels of cell death (37,38). Although there are many similarities between the neoplastic cells of sALCL and primary cutaneous CD30+ LPDs, they differ clinically. This may be explained by activation of distinct signaling pathways, and differences in the microenvironment and cellular infiltrate in each condition (29).
CD30+ T cell LPD spectrum
LyP, pcALCL, and borderline lesions comprise a spectrum of CD30+ T cell LPDs with clinical, histological and immunophenotypic variations. This spectrum is demonstrated by the spontaneous regression of LyP, along with its progression to a malignant lymphoma. In addition, reports of the concurrent presence or evolution of pcALCL and MF to LyP with shared TCR clonality further support the plasticity of CD30+ T cell LPDs (39-42).
The pathogenesis underlying the spectrum of CD30+ T cell LPDs is still under debate. Mutations in TGF-beta receptors result in resistance to growth inhibition by TGF-beta and progression of LyP to lymphoma (43,44). Evidence of genetic instabilities in microsatellites and chromosomes suggests progression of LyP to pcALCL, which is consistent with the multistep model of tumor progression (45). The presence of Dual specificity protein phosphatase 22 (DUSP22) rearrangements in both LyP and pcALCL provides further genetic basis to support a clinical and histological spectrum of CD30+ T cell LPDs. The expression of transcription factor, special AT-rich sequence-binding protein-1 (SATB1), in LyP and pcALCL is controlled by DNA promoter methylation and favors a Th17 polarization, which may play a role in the pathogenesis of CD30+ T cell LPDs and response to treatment (46). Using a global array expression system to identify specific genes, progression of LyP to pcALCL was associated with upregulation of genes involved in cell proliferation, cell survival, and drug resistance; and downregulation in genes of cell adhesion, apoptosis, cell activation and cell cycle inhibitors and genomic integrity (47). Increased protein expression involved in cell signaling, ion transport, apoptosis, cell adhesion, and activation of NF-kB and TGF-beta was also noted (48).
CD30 is not only a marker but is also a prognostic factor and therapeutic target in CD30+ T cell LPDs. An exact diagnosis requires a thorough history and physical, focusing on systemic symptoms, skin lesions and lymph nodes. An accurate description of the skin lesions, including their distribution, morphology and behavior, is necessary to make the correct diagnosis. Despite their worrisome histological findings, the clinical presentations of LyP and pcALCL can guide treatment and prevent recurrences. Surveillance for secondary malignancies in LyP and extracutaneous spread of pcALCL is important due to a poorer prognosis in these situations.
LyP
LyP was first described in 1968 as a recurring self-healing eruption that was clinically benign but histologically malignant (49). LyP is the most common pcCD30+ T cell LPD, with an incidence of 1.2 to 1.9 per million persons in the United States (50). LyP presents more often in men than women and has an average onset of 35 to 45 years (50). The disease has been described in Caucasians but can occur in patients of Asians and African origin (51,52). Clinically, LyP is characterized by crops of red to violaceous papules and nodules typically 3–10 mm in size, and usually not larger than 2 cm. Lesions are in various stages due to recurrent and successive crops, and spontaneously regress typically within 1–3 months. Lesions range from a few to hundreds, and often involve the trunk and extremities (53). LyP is usually not associated with systemic symptoms, and half of all patients are asymptomatic. Some patients may experience pruritus and pain due to ulceration, crusting and central necrosis (54). Lesions resolve with post-inflammatory hypo- or hyperpigmented macules and scars. Spontaneous resolution of the lesions characteristically occurs between 2 to 8 weeks, and up to 4 months (55). Recurrent crops can develop over several months to decades (53).
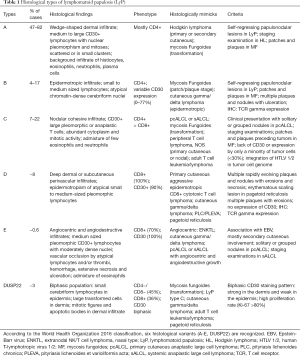
Six histological subtypes (A-E and DUSP22) are recognized in the 2016 World Health Organization (WHO) classification (Table 1) and can be present concurrently in the same patient (56). Type A is the most common and accounts for 75% of all LyP cases. The skin biopsies show a wedge-shaped dermal infiltrate of medium to large sized pleomorphic and anaplastic lymphoid cells. These cells are scattered or in small clusters, in an admixture background of neutrophils, eosinophils, histiocytes and plasma cells, similar to cHL (4,57). Type B has an epidermotropic infiltrate of small to medium sized lymphocytes with cerebriform nuclei, resembling MF. Type C displays a nodular cohesive infiltrate of large atypical lymphoid cells with few inflammatory cells, resembling ALCL (56). Type D shows epidermotropic infiltrates of CD8+ small to medium sized atypical lymphocytes with a deep dermal perivascular component, similar to primary cutaneous aggressive CD8+ cytotoxic T cell lymphoma (58-60). Type E contains angiocentric and angiodestructive infiltrates of medium sized pleomorphic atypical lymphocytes with moderately dense nuclei. Due to vascular damage and occlusions, lesions in Type E are rapidly evolving necrotic ulcerations up to 4 cm in size, which clinically resemble ENKTL and cutaneous gamma/delta lymphoma (61,62). Type B-D LyPs are distinguished from their malignant histological counterparts (MF, pcALCL, and ENKTL) based on the clinical appearance and behavior of lesions. LyP with DUSP22 translocations are characterized by the presence of a biphasic population of lymphocytes: small cerebriform cells in the epidermis, and large transformed cells in the dermis. CD30 also has a biphasic staining pattern: strong in the dermis and weak in the epidermis (63). A recently proposed subtype of follicular LyP contains perifollicular infiltrates with folliculotropism of CD30+ atypical lymphocytes (64). Follicular mucinosis with collections of neutrophils or eosinophils in the hair follicles can result in pustular lesions (64-66).
Full table
Immunohistochemistry is routinely used to further characterize the cellular infiltrate. Although CD30 is the hallmark of LyP, type B can be CD30-negative or have variable CD30 expression (57). The majority of LyP cases are CD4+ and CD45RO+. However, types D and E are CD8+. LyP with DUSP22 is more commonly CD4-/CD8-, with some cases that are CD8+. In addition to clinical presentation, CD45RO positivity helps differentiate type D from aggressive CD8+ CTCL, which is typically CD45RO negative (56). Variable loss of pan T cell antigens (CD2, CD3, CD5, and CD7) can be observed, with CD7 most often being absent. Expression of cytotoxic molecules, such as TIA-1 and granzyme B, has been observed in most LyP cases (67). The majority of LyP cases express the alpha/beta T cell receptor (TCR), but expression of the gamma/delta TCR has been found in type D. Despite these differences among the LyP subtypes, the clinical course and prognosis of the disease is not affected by the immunophenotype of the infiltrate (68).
Monoclonal rearrangements of TCR beta or gamma genes are detected in 22–100% of cases, depending on tissue fixation, modality, primers used, and the number of atypical lymphocytes in the specimen (69). TCR clonality data in LyP are therefore inconsistent. For example, one study demonstrated that the CD30+ cells had a monoclonal TCR, and the CD30 negative cells were polyclonal (70). However, another study showed polyclonal CD30+ large atypical cells (71). The chromosomal translocation t(6;7)(p25.3;q32.3) involving the DUSP22/IRF4 locus is found in a small proportion of LyP cases and other CD30+ lymphomas (72). The translocation t(2;5)(p23;q35) which fuses the ALK gene to the nucleophosmin (NPM) gene is not found in LyP (73).
Detection of high levels of expression of TRAF1 and MUM1 has been proposed as a way to differentiate LyP from pcALCL (74,75). The T cell activation marker CD134 (OX40) is strongly expressed in a proportion of patients with LyP, but not in MF or benign lymphocytic infiltrations (76).
The pathogenesis of LyP is not well understood. TGF-beta receptor mutations with a loss of TGF-beta mediated growth inhibition may contribute to progression. Interaction between CD30 and CD30L may play a role in the regression of LyP lesions (77). Increased apoptosis via pro-apoptotic proteins Fas and Bax may also contribute to the spontaneous resolution of LyP lesions (78,79). No animal model exists.
Due to its excellent prognosis, LyP can generally be managed with a “watch and wait” strategy (80). Accepted indications to treat include diffuse or progressive lesions that are symptomatic or with significant scarring or pigmentary changes causing disfigurement. The treatment goal is to prevent new outbreaks and shorten the time for resolution of lesions. Lesions few in number are often managed with potent topical steroids, while phototherapy or low dose oral methotrexate are first line for more extensive lesions (81). Recurrences occur in over 40% of patients treated with methotrexate. Cytotoxic chemotherapy has been associated with a rapid relapse in most LyP patients during or after discontinuation (81). Brentuximab vedotin (BV), an antibody-drug conjugate that targets CD30, had an overall response rate of 73% and complete response rate of 35% in patients with refractory LyP, and provides sustained responsiveness after only a few infusions (82,83).
LyP can be present for years but is not associated with any disease-specific mortality (4). A subset of patients can develop a second lymphoid malignancy prior to, concurrent with, or after the development of LyP. The incidence of secondary hematological malignancies in two large retrospective series was between 40–60% (84,85). The vast majority (>80%) of these secondary hematological malignancies are MF or pcALCL (84). HL is the most common systemic lymphoma reported in these patients, occurring in 3.5–7% of cases (84). Other B cell lymphomas have rarely been reported, and the direct relationship, if any, between aggressive B cell lymphomas and the indolent Lyp is not well known. LyP types B and C had higher rates of these secondary malignancies, while types A and D had a lower risk (84). Expression of the actin filament bundling protein Fascin, age older than 50 years, involvement of the head, higher degree of recurrences, and a positive TCR clone have been linked to the development of LyP associated lymphomas which are in most cases clonally related (86-88).
pcALCL
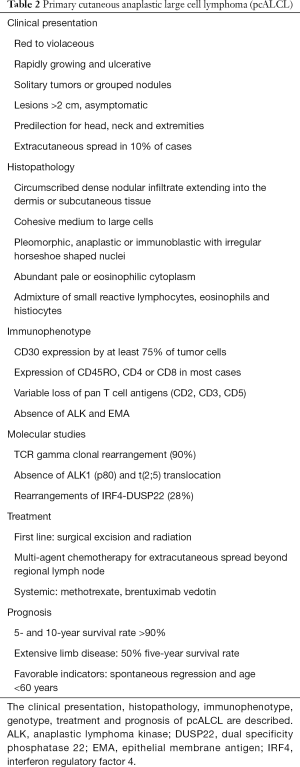
pcALCL is characterized by large T cells with prominent nuclear pleomorphism and positive CD30 in more than 75% of tumor cells (Table 2) (1,2). Due to its distinct clinical course, genetic features, and prognosis, pcALCL is considered and classified as a separate entity from sALCL (89). As in LyP, the highly atypical morphology of the CD30+ tumor cells and the often-rapid growth of the skin lesions is at odd with the favorable prognosis and lack of systemic dissemination in pcALCL. Patients with pcALCL are diagnosed at an older age (60 years) compared to LyP, and males are more often affected than females (3:1) (4).
Full table
pcALCL typically presents as rapidly growing, red to violaceous, solitary tumors or clusters of nodules. The lesions are typically larger than 2 cm, and often ulcerate (1,2). Multifocal disease, or 2 or more lesions at different anatomic sites, is present in ~20% of patients (4). The lesions are generally asymptomatic and are not associated with systemic symptoms such as fevers, chills, fatigue, night sweats or weight loss. Extracutaneous dissemination occurs in about 10% of cases, and most commonly involves regional lymph nodes (90). Spontaneous regressions have been reported in 10–42% of lesions (4,81). However, recurrences are common, particularly without therapeutic intervention. pcALCL is a common type of CTCL in individuals with HIV or organ transplant recipients (91,92).
The characteristic histopathological feature of pcALCL is a circumscribed, dense nodular infiltrate of large lymphoid cells extending into the dermis or subcutaneous tissue. The atypical large lymphocytes have anaplastic morphology with irregularly horseshoe-shaped nuclei, eosinophilic nucleoli, and abundant cytoplasm (1). Epidermotropism is typically absent except when ulceration is present. Non-anaplastic, either pleomorphic or immunoblastic-like, cells are seen in 20–25% of cases. Neutrophil-rich and angiocentric/angioinvasive variants of pcALCL have been described, without any impact on prognosis (93,94).
By definition, at least 75% of the large atypical cells in pcALCL express CD30 in a membranous or Golgi pattern (1). The tumor cells have an activated T cell phenotype (CD2, CD25, CD71, CD45RO, HLA-DR), and express CD4 with variable loss of CD2, CD3 and CD5 (89). Cytotoxic markers, such as granzyme B, TIA-1 and perforin, are expressed in half of pcALCL cases (95). Cutaneous lymphocyte antigen (CLA) is typically positive and epithelial membrane antigen (EMA), usually expressed in sALCL, is negative in pcALCL (4). Rare cases of a positive ALK1 (2p23 rearrangement) in pcALCL have been reported in children and often with ALK protein expression restricted to the cytoplasm (96,97), but any ALK positive pcALCL should raise a high suspicion for cutaneous manifestation of the systemic counterpart. Monoclonal TCR gene rearrangements are found in more than 90% of pcALCL cases (98). The most common genetic abnormality is the IRF4-DUSP22 rearrangement, which occur in 28% of pcALCL (72). However, these rearrangements have also been rarely found in sALCL and LyP. Polymorphisms in CD30 promoter microsatellite repressor element have been linked to an increased predisposition towards pcALCL (99).
Although many similarities exist between pcALCL and other CD30+ LPD, their diverse clinical features likely reflect significant biological differences. Higher expression of CCR10 and CCR8, skin homing chemokine receptors, in pcALCL may support the lower tendency of extracutaneous dissemination (100). TNF-alpha, TNF-related apoptosis-inducing ligand (TRAIL) and CD95L (FasL) defects in the apoptotic pathways may facilitate the development and progression of pcALCL (101,102). Similar to LyP, loss of TGF-beta and overexpression of JUNB have also been noted in pcALCL (34,43). Genetic alterations in IRF4, CD30 (TNFRSF8), TRAF1, FAS, TP53 have been identified and may play a role in the pathogenesis of pcALCL (100,102,103).
Surgical excision and local radiation are common first line treatments for solitary or small clusters of pcALCL lesions, with durable complete response rates approaching 100% (81). Low-dose methotrexate is the first line systemic treatment for widespread or extracutaneous involvement. BV has been used for multifocal, refractory, extracutaneous, or relapsed pcALCL. With BV, all patients with pcALCL had a complete response in a phase II trial, while 63% had complete reduction in their skin disease in a phase III trial, although the sample sizes were small (81,104). Multi-agent chemotherapy is used for extracutaneous spread beyond regional lymph nodes that did not respond to previous treatments.
The prognosis of pcALCL is favorable with a 5 and 10-year survival rate of over 90%, including cases with involvement of lymph nodes in a single draining basin (4,105). Draining lymph node involvement greater than 1 nodal basin has a 5-year survival rate of 76–96%, which drops to 50% with extensive limb disease (4,106). Skin-limited relapses occur in 39% of patients, with extracutaneous spread in 13% (90). Spontaneous regression and age under 60 years are favorable prognostic indicators; while extensive limb disease, large skin involvement and extracutaneous disease are unfavorable (106). Visceral metastases are uncommon and may occur any time between 2 months and 10 years from initial diagnosis (107).
Borderline lesions
Borderline lesions refer to cases without a definite distinction between LyP and pcALCL despite a thorough clinical and pathological correlation. The distinction can be particularly challenging in patients with a short history of multifocal papular or nodular lesions (1,81). Borderline lesions are also used to designate a rapidly growing nodule in a patient with LyP. It is often difficult to determine whether a nodule may be an atypically large, spontaneously-regressing LyP lesion or an initial lesion of pcALCL. Most borderline lesions are diagnosed based on their clinical behavior on follow up.
CD30 expression in other lymphoid malignancies
CD30 is present in other cutaneous and systemic lymphomas, along with non-neoplastic conditions. Due to the advent of anti-CD30 therapy, evaluation of CD30 expression in various disorders provides important information regarding management. Given their varying treatment options and prognosis, careful evaluation for the following CD30-expressing diseases must be performed.
MF
MF is the most common type of CTCL, comprising nearly 50% of all cases (1). In most patients, it is an indolent chronic neoplasm of skin-homing CD4+ T cells. MF has a wide range of clinical presentations, from erythematous scaly patches in sun-protected areas to slightly raised plaques or nodules and tumors. Histologically, LyP, MF and pcALCL can be difficult to distinguish. In MF, patients with large cell transformation (LCT) have had long-standing lesions with tumor development. The lesions in LyP are widespread and will spontaneously remit. Patients with pcALCL tend to be younger with less truncal involvement. Therefore, clinical features and findings on physical exam must be evaluated when making the diagnosis.
Variable expression of CD30 may be found in all stages of MF, particularly in LCT (1,108). LCT is defined as the presence of more than 25% of the atypical lymphocytes with a size four times more than a normal lymphocyte (or large cell nodules), and is usually associated with tumors (109,110). Large atypical cells express CD30 in 15–39% of cases. Cases of LyP and MF occurring in the same patient have been noted to be clonally related (40-42). When greater than 75% of the large cells are CD30+, MF can histologically resemble pcALCL (110). Posing a difficult diagnostic dilemma, transformed MF and pcALCL have no reliable differentiating histologic or molecular markers (111). Low galectin-3 expression and the presence of CDKN2A/2B deletions, which is independently associated with reduced survival, are promising markers for identification of transformed MF (112,113).
sALCL
ALCL is characterized by strong CD30 expression and variable loss of T cell markers, and can be classified into sALCL and pcALCL, which have distinct clinical features, genetic characteristics, therapies and prognosis. sALCL can have secondary cutaneous involvement, which is challenging to distinguish histologically from transformed MF and LyP. The clinical presentation is used to differentiate between these diseases. All cases of pcALCL should be evaluated for any evidence of sALCL.
sALCL accounts for 5% of all NHL in adults and can be divided based on ALK positivity according to WHO classification (1). ALK positive sALCL is a malignant neoplasm of peripheral T cells that express an ALK fusion protein and CD30 by definition. ALK+ ALCL occurs predominantly in a young population and ALK negative ALCL affects adults, with rapidly progressing adenopathy and B symptoms (114). Extranodal involvement of the skin, soft tissue, liver, lung, bone and bone marrow is present in 40–68% of ALK+ cases and 20% of ALK negative cases (115,116). The two subsets of sALCL are not distinguishable morphologically. Cells can range from small medium sized to anaplastic large cells, which efface the architecture of the lymph node. Expression of CD56, CD43, CD45, EMA or clusterin is less common in ALK negative ALCL and may help to differentiate features (116). 80% of ALK+ ALCL show a cytogenetic translocation t(2;5), which fuses ALK to the NPM gene (1). ALK+ ALCL has a better prognosis compared to ALK negative ALCL, with a 5-year survival rate of 70–80% (114). Recently, DUSP22-IRF4 and TP63 rearrangements have been identified in ALK negative ALCL (72,117). DUSP22 rearrangements have a good prognosis similar to ALK+ ALCL, while TP63 rearrangements have the worst prognosis (118).
PTCL
PTCL, not otherwise specified (PTCL NOS) is a nodal T cell lymphoma that is a diagnosis of exclusion. Therefore, it is the most common and heterogenous PTCL. Median age of diagnosis is 70 years old, and 65% of patients have stage IV disease on diagnosis. Pruritus, blood eosinophilia and hemophagocytic syndrome can occur (119). PTCL NOS contains a mixture of small and large atypical cells with clear cytoplasm and increased vascularization (120,121). T cell antigens are variably expressed with frequent loss of CD7 and rarely CD5. CD30 is positive in up to 50% of patients, but typically with a lower percentage of expression compared to ALCL (122). Chromosomal abnormalities have been described in PTCL NOS, including recurrent gains in 8q (MYC locus), and deletions in chromosomes 5q, 10q, 12q may be associated with a better prognosis (123,124).
The clinical course of PTCL NOS is aggressive with frequent early relapses, and overall outcome is poor with a 5-year survival of 20–30% (119). EBV positivity, TP63 rearrangements and expression of cytotoxic molecules have a poorer prognosis, while the lymphoepithelioid variant may have a better outcome (117,120,125,126). The presence of CD30 and variable expression of CD15 makes differentiation from ALCL and cHL difficult. Despite the lack of reliable differentiating features of PTCL NOS from other CD30-expresing diseases, its poor prognosis warrants consideration.
Extranodal NK/T cell lymphoma, nasal type (ENKTL)
ENKTL is a rare neoplasm of EBV infected NK or T cells. It predominantly affects middle-aged men in Asia, Mexico, South America, and rarely Western countries. It presents as tumors or destructive lesions in the nose, maxillary sinuses, and palate with dissemination to the skin, GI tract, and testis (1,127). ENKTL ranges from monomorphic small medium sized to large cells, and has frequent angioinvasion, anglocentrism, or necrosis (1). The neoplastic cells are CD2 positive, CD56 positive, CD7 positive, CD5 negative, express cytoplasmic CD3, and have an activated cytotoxic (TIA-1, granzyme B, and perforin) profile (128,129). CD30 has been reported to be positive in 20–50%, and up to 70%, of patients with ENKTL (122,130,131). Most cases of ENKTL are derived from NK cells, which do not express the TCR complex. However, up to 38% of cases are derived from clonal T cells with gamma/delta and alpha/beta, TCR expression (1).
The overall survival rate for localized disease is 50%, and 9–15.6% for advanced stage ENKTL (132). Significant indicators of a poor prognosis include primary non-upper aerodigestive tract site, high stage, bone marrow involvement, lack of radiotherapy, Ki67 >40% and CD25 expression (133). A recent meta-analysis concluded that CD30 is significantly associated with a better prognosis in ENKTL, especially for patients with regional lymphoma involvement (134). Survival of patients with cutaneous ENKTL is poor with a median survival of 7 to 13 months (135). With a poor prognosis and lack of definitive findings on pathology, a vigilant clinical evaluation for ENKTL is essential with CD30 positive skin lesions.
Angioimmunoblastic T cell lymphoma (AITL)
AITL is a neoplasm derived from follicular helper T cells, and is one of the most common PTCLs worldwide (1). It is more prevalent in Europe than North America or Asia and typically affects the elderly, with a median age of 59–65 years (1). AITL is clinically characterized by B symptoms, skin rash, generalized lymphadenopathy, and hypergammaglobulinemia due to a prominent immune reaction (136,137). Bone marrow involvement is seen in up to 70% of cases, signifies a higher tumor burden, and is associated with a greater incidence of B symptoms, hepatosplenomegaly and circulating tumor cells (138). Up to 50% of patients have a rash during the course of the disease (139). Other skin findings include nodules, plaques, purpura and urticarial (140). Autoimmune manifestations can also occur, and the majority of patients present with advanced disease (139).
AITL is characterized by a diffuse polymorphous infiltrate of neoplastic T cells, small lymphocytes, histiocytes, immunoblasts, eosinophils and plasma cells. This infiltrate effaces normal lymph node architecture with near absence of germinal centers, and is associated with high endothelial venule proliferation and follicular dendritic cell hyperplasia (1). The neoplastic cells express several markers of follicular helper T cells, including CXCL13, PD-1, ICOS, Bcl6, SAP, CD200, cMAF, and frequently CD10 (approximately 60% of cases) (141,142). AITL has a heterogeneous expression of CD30, which may be due to the coexistence of CD30+ B cells and T cells (143). TCR are present in up to 80% of the neoplastic cells. Nearly all patients with AITL have B cell clonality and EBV integration, and a subset develop an EBV associated B cell neoplasm (144). By cytogenetics, trisomy 3, 5 and 21, gain of X and loss of 6q chromosomes are detected in up to 90% of AITL cases. Overall prognosis of AITL is poor with a median survival of less than 3 years (1). Age >60 years, performance status >2, extranodal sites >1, B symptoms, platelet count <150,000 differentiate a high risk group with a 5-year overall survival of 24% (145). TET2 mutation is associated with an advanced stage disease and a shorter progression free survival (146).
Other CD30-expressing diseases
In addition to the conditions described above, several other less common CTCL and cutaneous B cell lymphomas can express CD30. Neoplastic cells in breast implant-associated ALCL, HL and B cell NHL have occasional or regular CD30 expression with variable intensity on flow cytometry (147-149). Drug induced reactive lymphoid hyperplasia, insect bites and scabies are exogenous causes of CD30+ lymphocytic diseases (150). Inflammatory conditions such as pityriasis lichenoides and atopic dermatitis can be associated with CD30 positivity (151). CD30 is expressed not only in lymphocytes, but also by mast cells, endothelium, and endometrium. Cases with CD30 expression have been described in embryonal carcinoma, nasopharyngeal carcinomas, mastocytosis and angiosarcoma (152,153).
Conclusions
CD30 expression in the context of cutaneous T cell LPD has a favorable prognosis. However, CD30 positivity can also be detected in other cutaneous and systemic lymphomas, highlighting the importance of an accurate clinical diagnosis. Additionally, CD30 is commonly expressed in benign dermatological conditions warranting close clinical consideration and follow-up. CD30 not only provides prognostic information, but also serves as a therapeutic target. In studies that addressed the correlation between tumor CD30 expression and clinical response to BV, responses to anti-CD30 therapy were observed even in patients with low or no expression of CD30. The exact underlying mechanism is unknown but is thought to be a bystander effect due to the release of the MMAE toxin payload of BV from dying CD30-positive cells (154). Further studies are necessary to understand the mechanism of action of anti-CD30 therapy in the spectrum of cutaneous CD30+ T cell LPDs. Identification of a biomarker to differentiate patients who will respond to anti-CD30 medications will provide a more personalized and effective treatment regimen.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood 2005;105:3768-85. [Crossref] [PubMed]
- Kempf W, Willemze R, Jaffe ES, et al. CD30+ T-cell lymphoproliferative disorders. In: LeBoit P, Burg G, Weedon D, Sarasin A, eds. World Health Organization Classification of Tumours Pathology and Genetics of Skin Tumours. Lyon: IARC Press, 2006:179-81.
- Benharroch D, Meguerian-Bedoyan Z, Lamant L, et al. ALK-positive lymphoma: a single disease with a broad spectrum of morphology. Blood 1998;91:2076-84. [PubMed]
- Bekkenk MW, Geelen FA, vanVoorst Vader PC, et al. Primary and secondary cutaneous CD30+ lymphoproliferative disorders: a report from the Dutch Cutaneous Lymphoma Group on the long-term follow-up data of 219 patients and guidelines for diagnosis and treatment. Blood 2000;95:3653-61. [PubMed]
- Schwab U, Stein H, Gerdes J, et al. Production of a monoclonal antibody specific for Hodgkin and Sternberg-Reed cells of Hodgkin's disease and a subset of normal lymphoid cells. Nature 1982;299:65-7. [Crossref] [PubMed]
- Stein H, Gerdes J, Schwab U, et al. Identification of Hodgkin and Sternberg-reed cells as a unique cell type derived from a newly-detected small-cell population. Int J Cancer 1982;30:445-59. [Crossref] [PubMed]
- Dürkop H, Latza U, Hummel M, et al. Molecular cloning and expression of a new member of the nerve growth factor receptor family that is characteristic for Hodgkin's disease. Cell 1992;68:421-7. [Crossref] [PubMed]
- Smith CA, Gruss HJ, Davis T, et al. CD30 antigen, a marker for Hodgkin's lymphoma, is a receptor whose ligand defines an emerging family of cytokines with homology to TNF. Cell 1993;73:1349-60. [Crossref] [PubMed]
- Dong L, Hulsmeyer M, Durkop H, et al. Human CD30: structural implications from epitope mapping and modeling studies. J Mol Recognit 2003;16:28-36. [Crossref] [PubMed]
- Mukai Y, Nakamura T, Yoshikawa M, et al. Solution of the structure of the TNF-TNFR2 complex. Sci Signal 2010;3:ra83. [Crossref] [PubMed]
- Duckett CS, Gedrich RW, Gilfillan MC, et al. Induction of nuclear factor kappaB by the CD30 receptor is mediated by TRAF1 and TRAF2. Mol Cell Biol 1997;17:1535-42. [Crossref] [PubMed]
- Harlin H, Podack E, Boothby M, et al. TCR-independent CD30 signaling selectively induces IL-13 production via a TNF receptor-associated factor/p38 mitogen-activated protein kinase-dependent mechanism. J Immunol 2002;169:2451-9. [Crossref] [PubMed]
- Duckett CS, Thompson CB. CD30-dependent degradation of TRAF2: implications for negative regulation of TRAF signaling and the control of cell survival. Genes Dev 1997;11:2810-21. [Crossref] [PubMed]
- Younes A, Aggarwall BB. Clinical implications of the tumor necrosis factor family in benign and malignant hematologic disorders. Cancer 2003;98:458-67. [Crossref] [PubMed]
- Josimovic-Alasevic O, Durkop H, Schwarting R, et al. Ki-1 (CD30) antigen is released by Ki-1-positive tumor cells in vitro and in vivo. I. Partial characterization of soluble Ki-1 antigen and detection of the antigen in cell culture supernatants and in serum by an enzyme-linked immunosorbent assay. Eur J Immunol 1989;19:157-62. [Crossref] [PubMed]
- Younes A, Consoli U, Zhao S, et al. CD30 ligand is expressed on resting normal and malignant human B lymphocytes. Br J Haematol 1996;93:569-71. [Crossref] [PubMed]
- Gruss HJ, Boiani N, Williams DE, et al. Pleiotropic effects of the CD30 ligand on CD30-expressing cells and lymphoma cell lines. Blood 1994;83:2045-56. [PubMed]
- Falini B, Pileri S, Pizzolo G, et al. CD30 (Ki-1) molecule: a new cytokine receptor of the tumor necrosis factor receptor superfamily as a tool for diagnosis and immunotherapy. Blood 1995;85:1-14. [PubMed]
- Ellis TM, Simms PE, Slivnick DJ, et al. CD30 is a signal-transducing molecule that defines a subset of human activated CD45RO+ T cells. J Immunol 1993;151:2380-9. [PubMed]
- Bowen MA, Lee RK, Miragliotta G, et al. Structure and expression of murine CD30 and its role in cytokine production. J Immunol 1996;156:442-9. [PubMed]
- Kurts C, Carbone FR, Krummel MF, et al. Signalling through CD30 protects against autoimmune diabetes mediated by CD8 T cells. Nature 1999;398:341-4. [Crossref] [PubMed]
- Gaspal FM, Kim MY, McConnell FM, et al. Mice deficient in OX40 and CD30 signals lack memory antibody responses because of deficient CD4 T cell memory. J Immunol 2005;174:3891-6. [Crossref] [PubMed]
- Gerli R, Lunardi C, Vinante F, et al. Role of CD30+ T cells in rheumatoid arthritis: a counter-regulatory paradigm for Th1-driven diseases. Trends Immunol 2001;22:72-7. [Crossref] [PubMed]
- Blazar BR, Levy RB, Mak TW, et al. CD30/CD30 ligand (CD153) interaction regulates CD4+ T cell-mediated graft versus-host disease. J Immunol 2004;173:2933-41. [Crossref] [PubMed]
- Dai Z, Li Q, Wang Y, et al. CD4+ CD25+ regulatory T cells suppress allograft rejection mediated by memory CD8+ T cells via a CD30-dependent mechanism. J Clin Invest 2004;113:310-7. [Crossref] [PubMed]
- Abbondanzo SL, Sato N, Straus SE, et al. Acute infectious mononucleosis: CD30 (Ki-1) antigen expression and histologic correlations. Am J Clin Pathol 1990;93:698-702. [Crossref] [PubMed]
- Herbst H, Stein H. Tumor viruses in CD30-positive anaplastic large cell lymphomas. Leuk Lymphoma 1993;9:321-8. [Crossref] [PubMed]
- Dürkop H, Foss HD, Eitelbach F, et al. Expression of the CD30 antigen in non-lymphoid tissues and cells. J Pathol 2000;190:613-8. [Crossref] [PubMed]
- de Leval L, Gaulard P. CD30+ lymphoproliferative disorders. Haematologica 2010;95:1627-30. [Crossref] [PubMed]
- Gruss HJ, DaSilva N, Hu ZB, et al. Expression and regulation of CD30 ligand and CD30 in human leukemia-lymphoma cell lines. Leukemia 1994;8:2083-94. [PubMed]
- Gattei V, Degan M, Gloghini A, et al. CD30 ligand is frequently expressed in human hematopoietic malignancies of myeloid and lymphoid origin. Blood 1997;89:2048-59. [PubMed]
- Blatt K, Cerny-Reiterer S, Schwaab J, et al. Identification of the Ki-1 antigen (CD30) as a novel therapeutic target in systemic mastocytosis. Blood 2015;126:2832-41. [Crossref] [PubMed]
- Sotlar K, Cerny-Reiterer S, Petat-Dutter K, et al. Aberrant expression of CD30 in neoplastic mast cells in high-grade mastocytosis. Mod Pathol 2011;24:585-95. [Crossref] [PubMed]
- Watanabe M, Ogawa Y, Ito K, et al. AP-1 mediated relief of repressive activity of the CD30 promoter microsatellite in Hodgkin and Reed-Sternberg cells. Am J Pathol 2003;163:633-41. [Crossref] [PubMed]
- Hsu FY, Johnston PB, Burke KA, et al. The expression of CD30 in anaplastic large cell lymphoma is regulated by nucleophosmin-anaplastic lymphoma kinase-mediated JunB level in a cell type-specific manner. Cancer Res 2006;66:9002-8. [Crossref] [PubMed]
- Watanabe M, Ogawa Y, Itoh K, et al. Hypomethylation of CD30 CpG islands with aberrant JunB expression drives CD30 induction in Hodgkin lymphoma and anaplastic large cell lymphoma. Lab Invest 2008;88:48-57. [Crossref] [PubMed]
- Mir SS, Richter BW, Duckett CS. Differential effects of CD30 activation in anaplastic large cell lymphoma and Hodgkin disease cells. Blood 2000;96:4307-12. [PubMed]
- Hirsch B, Hummel M, Bentink S, et al. CD30-induced signaling is absent in Hodgkin's cells but present in anaplastic large cell lymphoma cells. Am J Pathol 2008;172:510-20. [Crossref] [PubMed]
- Aoki M, Niimi Y, Takezaki S, et al. CD30+ lymphoproliferative disorder: primary cutaneous anaplastic large cell lymphoma followed by lymphomatoid papulosis. Br J Dermatol 2001;145:123-6. [Crossref] [PubMed]
- de la Garza Bravo MM, Patel KP, et al. Shared clonality in distinctive lesions of lymphomatoid papulosis and mycosis fungoides occurring in the same patients suggests a common origin. Hum Pathol 2015;46:558-69. [Crossref] [PubMed]
- Wood GS, Crooks CF, Uluer AZ. Lymphomatoid papulosis and associated cutaneous lymphoproliferative disorders exhibit a common clonal origin. J Invest Dermatol 1995;105:51-5. [Crossref] [PubMed]
- Basarab T, Fraser-Andrews EA, Orchard G, et al. Lymphomatoid papulosis in association with mycosis fungoides: a study of 15 cases. Br J Dermatol 1998;139:630-8. [PubMed]
- Schiemann WP, Pfeifer WM, Levi E, et al. A deletion in the gene for transforming growth factor beta type I receptor abolishes growth regulation by transforming growth factor beta in a cutaneous T-cell lymphoma. Blood 1999;94:2854-61. [PubMed]
- Kadin ME, Levi E, Kempf W. Progression of lymphomatoid papulosis to systemic lymphoma is associated with escape from growth inhibition by transforming growth factor-beta and CD30 ligand. Ann N Y Acad Sci 2001;941:59-68. [Crossref] [PubMed]
- Rübben A, Kempf W, Kadin ME, et al. Multilineage progression of genetically unstable tumor subclones in cutaneous T-cell lymphoma. Exp Dermatol 2004;13:472-83. [Crossref] [PubMed]
- Sun J, Yi S, Qiu L, et al. SATB1 Defines a Subtype of Cutaneous CD30+ Lymphoproliferative Disorders Associated with a T-Helper 17 Cytokine Profile. J Invest Dermatol 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Li S, Ross DT, Kadin ME, et al. Comparative genome-scale analysis of gene expression profiles in T cell lymphoma cells during malignant progression using a complementary DNA microarray. Am J Pathol 2001;158:1231-7. [Crossref] [PubMed]
- Kadin ME. Pathobiology of CD30+ cutaneous T-cell lymphomas. J Cutan Pathol 2006;33 Suppl 1:10-7. [Crossref] [PubMed]
- Macaulay WL. Lymphomatoid papulosis: A continuing self-healing eruption, clinically benign-histologically malignant. Arch Dermatol 1968;97:23-30. [Crossref] [PubMed]
- Wang HH, Lach L, Kadin ME. Epidemiology of lymphomatoid papulosis. Cancer 1992;70:2951-7. [Crossref] [PubMed]
- Angra K, Kennedy LJ, Rodney IJ. Lymphomatoid papulosis in a young adult of African descent. JAAD Case Rep 2015;1:212-4. [Crossref] [PubMed]
- Uchiyama A, Motegi SI, Ishikawa O. Angioinvasive lymphomatoid papulosis (type E): a first Japanese case. Eur J Dermatol 2016;26:507-8. [PubMed]
- Sauder MB, O'Malley JT, LeBoeuf NR. CD30+ Lymphoproliferative Disorders of the Skin. Hematol Oncol Clin North Am 2017;31:317-34. [Crossref] [PubMed]
- Wieser I, Tetzlaff MT, Torres Cabala CA, et al. Primary cutaneous CD30(+) lymphoproliferative disorders. J Dtsch Dermatol Ges 2016;14:767-82. [PubMed]
- LeBoeuf NR, McDermott S, Harris NL. Case records of the Massachusetts General Hospital. Case 5-2015. A 69-year-old woman with recurrent skin lesions after treatment for lymphoma. N Engl J Med 2015;372:650-9. [Crossref] [PubMed]
- Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016;127:2375-90. [Crossref] [PubMed]
- El Shabrawi-Caelen L, Kerl H, Cerroni L. Lymphomatoid papulosis: reappraisal of clinicopathologic presentation and classification into subtypes A, B, and C. Arch Dermatol 2004;140:441-7. [Crossref] [PubMed]
- Saggini A, Gulia A, Argenyi Z, et al. A variant of lymphomatoid papulosis simulating primary cutaneous aggressive epidermotropic CD8+ cytotoxic T-cell lymphoma. Description of 9 cases. Am J Surg Pathol 2010;34:1168-75. [Crossref] [PubMed]
- Cardoso J, Duhra P, Thway Y, et al. Lymphomatoid papulosis typed: a newly described variant easily confused with cutaneous aggressive CD8-positive cytotoxic T-cell lymphoma. Am J Dermatopathol 2012;34:762-5. [Crossref] [PubMed]
- Bertolotti A, Pham-Ledard AL, Vergier B, et al. Lymphomatoid papulosis type D: an aggressive histology for an indolent disease. Br J Dermatol 2013;169:1157-9. [Crossref] [PubMed]
- Kempf W, Kazakov DV, Scharer L, et al. Angioinvasive lymphomatoid papulosis: a new variant simulating aggressive lymphomas. Am J Surg Pathol 2013;37:1-13. [Crossref] [PubMed]
- Sharaf MA, Romanelli P, Kirsner R, et al. Angioinvasive lymphomatoid papulosis: another case of a newly described variant. Am J Dermatopathol 2014;36:e75-7. [Crossref] [PubMed]
- Karai LJ, Kadin ME, Hsi ED, et al. Chromosomal rearrangements of 6p25.3 define a new subtype of lymphomatoid papulosis. Am J Surg Pathol 2013;37:1173-81. [Crossref] [PubMed]
- Ross NA, Truong H, Keller MS, et al. Follicular lymphomatoid papulosis: an eosinophilic-rich follicular subtype masquerading as folliculitis clinically and histologically. Am J Dermatopathol 2016;38:e1-10. [Crossref] [PubMed]
- Requena L, Sanchez M, Coca S, et al. Follicular lymphomatoid papulosis. Am J Dermatopathol 1990;12:67-75. [Crossref] [PubMed]
- Ba W, Yang Y, Zhang Z, et al. Lymphomatoid papulosis with folliculotropism, eccrinotropism and neurotropism. J Cutan Pathol 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Bekkenk MW, Kluin PM, Jansen PM, et al. Lymphomatoid papulosis with a natural killer-cell phenotype. Br J Dermatol 2001;145:318-22. [Crossref] [PubMed]
- Morimura S, Sugaya M, Tamaki Z, et al. Lymphomatoid papulosis showing gamma delta T-cell phenotype. Acta Derm Venereol 2011;91:712-3. [Crossref] [PubMed]
- Greisser J, Palmedo G, Sander C, et al. Detection of clonal rearrangement of T-cell receptor genes in the diagnosis of primary cutaneous CD30 lymphoproliferative disorders. J Cutan Pathol 2006;33:711-5. [Crossref] [PubMed]
- Steinhoff M, Hummel M, Anagnostopoulos I, et al. Single-cell analysis of CD30+ cells in lymphomatoid papulosis demonstrates a common clonal T-cell origin. Blood 2002;100:578-84. [Crossref] [PubMed]
- Gellrich S, Wernicke M, Wilks A, et al. The cell infiltrate in lymphomatoid papulosis comprises a mixture of polyclonal large atypical cells (CD30-positive) and smaller monoclonal Tcells (CD30-negative). J Invest Dermatol 2004;122:859-61. [Crossref] [PubMed]
- Wada DA, Law ME, His ED, et al. Specificity of IRF4 translocations for primary cutaneous anaplastic large cell lymphoma: a multicenter study of 204 skin biopsies. Mod Pathol 2011;24:596-605. [Crossref] [PubMed]
- Herbst H, Sander C, Tronnier M, et al. Absence of anaplastic lymphoma kinase (ALK) and Epstein-Barr virus gene products in primary cutaneous anaplastic large cell lymphoma and lymphomatoid papulosis. Br J Dermatol 1997;137:680-6. [Crossref] [PubMed]
- Assaf C, Hirsch B, Wagner F, et al. Differential expression of TRAF1 aids in the distinction of cutaneous CD30-positive lymphoproliferations. J Invest Dermatol 2007;127:1898-904. [Crossref] [PubMed]
- Kempf W, Kutzner H, Cozzio A, et al. MUM1 expression in cutaneous CD30+ lymphoproliferative disorders: a valuable tool for the distinction between lymphomatoid papulosis and primary cutaneous anaplastic large-cell lymphoma. Br J Dermatol 2008;158:1280-7. [Crossref] [PubMed]
- Gniadecki R, Rossen K. Expression of T-cell activation marker CD134 (OX40) in lymphomatoid papulosis. Br J Dermatol 2003;148:885-91. [Crossref] [PubMed]
- Mori M, Manuelli C, Pimpinelli N, et al. CD30-CD30 ligand interaction in primary cutaneous CD30+ T-cell lymphomas: a clue to the pathophysiology of clinical regression. Blood 1999;94:3077-83. [PubMed]
- Granados S, Hwang ST. Roles for CD30 in the Biology and treatment of CD30+ lymphoproliferative diseases. J Invest Dermatol 2004;122:1345-7. [Crossref] [PubMed]
- Greisser J, Doebbeling U, Roos M, et al. Apoptosis in CD30-positive lymphoproliferative disorders of the skin. Exp Dermatol 2005;14:380-5. [Crossref] [PubMed]
- Kadin ME. Current management of primary cutaneous CD30+ T-cell lymphoproliferative disorders. Oncology (Williston Park) 2009;23:1158. [PubMed]
- Kempf W, Pfaltz K, Vermeer MH, et al. EORTC, ISCL, and USCLC consensus recommendations for the treatment of primary cutaneous CD30-positive lymphoproliferative disorders: lymphomatoid papulosis and primary cutaneous anaplastic large-cell lymphoma. Blood 2011;118:4024. [Crossref] [PubMed]
- Duvic M, Tetzlaff MT, Gangar P, et al. Results of a Phase II Trial of Brentuximab Vedotin for CD30+ Cutaneous T-Cell Lymphoma and Lymphomatoid Papulosis. J Clin Oncol 2015;33:3759-65. [Crossref] [PubMed]
- Lewis DJ, Talpur R, Huen AO, et al. Brentuximab Vedotin for Patients With Refractory Lymphomatoid Papulosis: An Analysis of Phase 2 Results. JAMA Dermatol 2017;153:1302-6. [Crossref] [PubMed]
- Wieser I, Oh CW, Talpur R, et al. Lymphomatoid papulosis: Treatment response and associated lymphomas in a study of 180 patients. J Am Acad Dermatol 2016;74:59-67. [Crossref] [PubMed]
- AbuHilal M, Walsh S, Shear N. Associated Hematolymphoid Malignancies in Patients With Lymphomatoid Papulosis: A Canadian Retrospective Study. J Cutan Med Surg 2017;21:507-12. [Crossref] [PubMed]
- de Souza A, el-Azhary RA, Camilleri MJ, et al. In search of prognostic indicators for lymphomatoid papulosis: a retrospective study of 123 patients. J Am Acad Dermatol 2012;66:928-37. [Crossref] [PubMed]
- Kempf W, Levi E, Kamarashev J, et al. Fascin expression in CD30-positive cutaneous lymphoproliferative disorders. J Cutan Pathol 2002;29:295-300. [Crossref] [PubMed]
- Cordel N, Tressieres B, D'Incan M, et al. Frequency and risk factors for associated lymphomas in patients with lymphomatoid papulosis. Oncologist 2016;21:76-83. [Crossref] [PubMed]
- Stein H, Foss HD, Durkop H, et al. CD30+ anaplastic large cell lymphoma: a review of its histopathologic, genetic, and clinical features. Blood 2000;96:3681-95. [PubMed]
- Liu HL, Hoppe RT, Kohler S, et al. CD30+ cutaneous lymphoproliferative disorders: the Stanford experience in lymphomatoid papulosis and primary cutaneous anaplastic large cell lymphoma. J Am Acad Dermatol 2003;49:1049. [Crossref] [PubMed]
- Kerschmann RL, Berger TG, Weiss LM, et al. Cutaneous presentations of lymphoma in human immunodeficiency virus disease. Predominance of Tcell lineage. Arch Dermatol 1995;131:1281-8. [Crossref] [PubMed]
- Yurtsever H, Kempf W, Laeng RH. Posttransplant CD30+ anaplastic large cell lymphoma with skin and lymph node involvement. Dermatology 2003;207:107-10. [Crossref] [PubMed]
- Kong YY, Dai B, Kong JC, et al. Neutrophil/eosinophil-rich type of primary cutaneous anaplastic large cell lymphoma: a clinicopathological, immunophenotypic and molecular study of nine cases. Histopathology 2009;55:189-96. [Crossref] [PubMed]
- Kempf W, Kazakov DV, Paredes BE, et al. Primary cutaneous anaplastic large cell lymphoma with angioinvasive features and cytotoxic phenotype: a rare lymphoma variant within the spectrum of CD30+ lymphoproliferative disorders. Dermatology 2013;227:346-52. [Crossref] [PubMed]
- Felgar RE, Macon WR, Kinney MC. at al. TIA-1 expression in lymphoid neoplasms. Identification of subsets with cytotoxic T lymphocyte or natural killer cell differentiation. Am J Pathol 1997;150:1893-900. [PubMed]
- Kadin ME, Pinkus JL, Pinkus GS, et al. Primary cutaneous ALCL with phosphorylated/activated cytoplasmic ALK and novel phenotype: ema/muc1+, cutaneous lymphocyte antigen negative. Am J Surg Pathol 2008;32:1421-6. [Crossref] [PubMed]
- Pulitzer M, Ogunrinade O, Lin O, et al. ALK-positive (2p23 rearranged) anaplastic large cel lymphoma with localization to the skin in a pediatric patient. J Cutan Pathol 2015;42:182-7. [Crossref] [PubMed]
- Macgrogan G, Vergier B, Dubus P, et al. CD30-positive cutaneous large cell lymphomas. A comparative study of clinicopathologic and molecular features of 16 cases. Am J Clin Pathol 1996;105:440-50. [Crossref] [PubMed]
- Franchina M, Kadin ME, Abraham LJ. Polymorphism of the CD30 promoter microsatellite repressive element is associated with development of primary cutaneous lymphoproliferative disorders. Cancer Epidemiol Biomarkers Prev 2005;14:1322-5. [Crossref] [PubMed]
- van Kester MS, Tensen CP, Vermeer MH, et al. Cutaneous anaplastic large cell lymphoma and peripheral T-cell lymphoma NOS show distinct chromosomal alterations and differential expression of chemokine receptors and apoptosis regulators. J Invest Dermatol 2010;130:563-75. [Crossref] [PubMed]
- Levi E, Wang Z, Petrogiannis-Haliotis T, Pfeifer WM, Kempf W, Drews R, Kadin ME. Distinct effects of CD30 and Fas signaling in cutaneous anaplastic lymphomas: a possible mechanism for disease progression. J Invest Dermatol 2000;115:1034-40. [Crossref] [PubMed]
- Zoi-Toli O, Vermeer MH, De Vries E, et al. Expression of Fas and Fas-lingand in primary cutaneous Tcell lymphoma (CTCL): association between lack of Fas expression and aggressive types of CTCL. Br J Dermatol 2000;143:313. [Crossref] [PubMed]
- Sánchez-Schmidt JM, Salgado R, et al. Primary cutaneous CD30+ anaplastic large-cell lymphomas show a heterogeneous genomic profile: an oligonucleotide array CGH approach. J Invest Dermatol 2011;131:269-71. [Crossref] [PubMed]
- Prince HM, Kim YH, Horwitz SM, et al. Brentuximab vedotin or physician's choice in CD30-positive cutaneous T-cell lymphoma (ALCANZA): an international, open-label, randomised, phase 3, multicentre trial. Lancet 2017;390:555-66. [Crossref] [PubMed]
- Booken N, Goerdt S, Klemke CD. Clinical spectrum of primary cutaneous CD30-positive anaplastic large cell lymphoma: an analysis of the Mannheim Cutaneous Lymphoma Registry. J Dtsch Dermatol Ges 2012;10:331-9. [PubMed]
- Woo DK, Jones CR, Vanoli-Storz MN, et al. Prognostic factors in primary cutaneous anaplastic large cell lymphoma: characterization of clinical subset with worse outcome. Arch Dermatol 2009;145:667-74. [Crossref] [PubMed]
- Benner MF, Willemze R. Applicability and prognostic value of the new TNM classification system in 135 patients with primary cutaneous anaplastic large cell lymphoma. Arch Dermatol 2009;145:1399-404. [Crossref] [PubMed]
- Barberio E, Thomas L, Skowron F, et al. Transformed mycosis fungoides: clinicopathological features and outcome. Br J Dermatol 2007;157:284-9. [Crossref] [PubMed]
- Benner MF, Jansen PM, Vermeer MH, et al. Prognostic factors in transformed mycosis fungoides: a retrospective analysis of 100 cases. Blood 2012;119:1643-9. [Crossref] [PubMed]
- Fauconneau A, Pham-Ledard A, Cappellen D, et al. Assessment of diagnostic criteria between primary cutaneous anaplastic large-cell lymphoma and CD30-rich transformed mycosis fungoides;a study of 66 cases. Br J Dermatol 2015;172:1547-54. [Crossref] [PubMed]
- Edinger JT, Clark BZ, Pucevich BE, et al. CD30 expression and proliferative fraction in nontransformed mycosis fungoides. Am J Surg Pathol 2009;33:1860-8. [Crossref] [PubMed]
- Mitteldorf C, Robson A, Tronnier M, et al. Galectin-3 Expression in Primary Cutaneous CD30-Positive Lymphoproliferative Disorders and Transformed Mycosis Fungoides. Dermatology 2015;231:164-70. [Crossref] [PubMed]
- Nicolae-Cristea AR, Benner MF, Zoutman WH, et al. Diagnostic and prognostic significance of CDKN2A/CDKN2B deletions in patients with transformed mycosis fungoides and primary cutaneous CD30-positive lymphoproliferative disease. Br J Dermatol 2015;172:784-8. [Crossref] [PubMed]
- Xing X, Feldman AL. Anaplastic large cell lymphomas: ALK positive, ALK negative, and primary cutaneous. Adv Anat Pathol 2015;22:29-49. [Crossref] [PubMed]
- Fraga M, Brousset P, Schlaifer D, et al. Bone marrow involvement in anaplastic large cell lymphoma. Immunohistochemical detection of minimal disease and its prognostic significance. Am J Clin Pathol 1995;103:82-9. [Crossref] [PubMed]
- Savage KJ, Harris NL, Vose JM, et al. ALK anaplastic large-cell lymphoma is clinically and immunophenotypically different from both ALK+ ALCL and peripheral T-cell lymphoma, not otherwise specified: Report from the International Peripheral T-Cell Lymphoma Project. Blood 2008;111:5496-504. [Crossref] [PubMed]
- Vasmatzis G, Johnson SH, Knudson RA, et al. Genome-wide analysis reveals recurrent structural abnormalities of TP63 and other p53-related genes in peripheral T-cell lymphomas. Blood 2012;120:2280-9. [Crossref] [PubMed]
- Parrilla Castellar ER, Jaffe ES, Said JW, et al. ALK-negative anaplastic large cell lymphoma is a genetically heterogeneous disease with widely disparate clinical outcomes. Blood 2014;124:1473-80. [Crossref] [PubMed]
- Gisselbrecht C, Gaulard P, Lepage E, et al. Prognostic significance of T-cell phenotype in aggressive non-Hodgkin’s lymphomas. Groupe d’Etudes des Lymphomes de l’Adulte (GELA). Blood 1998;92:76-82. [PubMed]
- Geissinger E, Odenwald T, Lee SS, et al. Nodal peripheral Tcell lymphomas and, in particular, their lymphoepithelioid (Lennert’s) variant are often derived from CD8(+) cytotoxic T-cells. Virchows Arch 2004;445:334-43. [Crossref] [PubMed]
- de Leval L, Savilo E, Longtine J, et al. Peripheral T-cell lymphoma with follicular involvement and a CD4+/bcl-6+ phenotype. Am J Surg Pathol 2001;25:395-400. [Crossref] [PubMed]
- Bossard C, Dobay MP, Parrens M, et al. Immunohistochemistry as a valuable tool to assess CD30 expression in peripheral T-cell lymphomas: high correlation with mRNA levels. Blood 2014;124:2983-6. [Crossref] [PubMed]
- Thorns C, Bastian B, Pinkel D, et al. Chromosomal aberrations in angioimmunoblastic T-cell lymphoma and peripheral T-cell lymphoma unspecified: A matrix-based CGH approach. Genes Chromosomes Cancer 2007;46:37-44. [Crossref] [PubMed]
- Zettl A, Rudiger T, Konrad MA, et al. Genomic profiling of peripheral T-cell lymphoma, unspecified, and anaplastic large T-cell lymphoma delineates novel recurrent chromosomal alterations. Am J Pathol 2004;164:1837-48. [Crossref] [PubMed]
- Dupuis J, Emile JF, Mounier N, et al. Prognostic significance of Epstein-Barr virus in nodal peripheral T-cell lymphoma, unspecified: a Groupe d'Etude des Lymphomes de l'Adulte (GELA) study. Blood. 2006;108:4163-9. [Crossref] [PubMed]
- Asano N, Suzuki R, Kagami Y, et al. Clinicopathologic and prognostic significance of cytotoxic molecule expression in nodal peripheral T-cell lymphoma, unspecified. Am J Surg Pathol 2005;29:1284-93. [Crossref] [PubMed]
- Cho KH, Choi WW, Youn CS, et al. Skin is the frequent site for involvement of peripheral T-cell and natural killer cell lymphomas in Korea. J Dermatol 2000;27:500-7. [Crossref] [PubMed]
- Emile JF, Boulland ML, Haioun C, et al. CD5- CD56+ T-cell receptor silent peripheral T-cell lymphomas are natural killer cell lymphomas. Blood 1996;87:1466-73. [PubMed]
- Mori N, Yatabe Y, Oka K, et al. Expression of perforin in nasal lymphoma. Am J Pathol 1996;149:699-705. [PubMed]
- Sabattini E, Pizzi M, Tabanelli V, et al. CD30 expression in peripheral T-cell lymphomas. Haematologica 2013;98:e81-2. [Crossref] [PubMed]
- Kawamoto K, Miyoshi H, Suzuki T, et al. Frequent expression of CD30 in extranodal NK/T-cell lymphoma: Potential therapeutic target for anti-CD30 antibody-based therapy. Hematol Oncol 2018;36:166-73. [Crossref] [PubMed]
- Au WY, Weisenburger DD, Intragumtornchai T, et al. Clinical differences between nasal and extranasal natural killer/T-cell lymphoma: a study of 136 cases from the International Peripheral T-Cell Lymphoma Project. Blood 2009;113:3931-7. [Crossref] [PubMed]
- Pongpruttipan T, Sukpanichnant S, Assanasen T, et al. Extranodal NK/T-cell lymphoma, nasal type, includes cases of natural killer cell and αβ, γδ, and αβ/γδ T-cell origin: a comprehensive clinicopathologic and phenotypic study. Am J Surg Pathol 2012;36:481-99. [Crossref] [PubMed]
- Chen Z, Guan P, Shan T, et al. CD30 expression and survival in extranodal NK/T-cell lymphoma: a systematic review and meta-analysis. Oncotarget 2018;9:16547-56. [PubMed]
- Yu JB, Zuo Z, Tang Y, et al. Extranodal nasal-type natural killer/ T-cell lymphoma of the skin: a clinicopathologic study of 16 cases in China. Hum Pathol 2009;40:807-16. [Crossref] [PubMed]
- Freter CE, Cossman J. Angioimmunoblastic lymphadenopathy with dysproteinemia. Semin Oncol 1993;20:627-35. [PubMed]
- Frizzera G, Moran EM, Rappaport H. Angio-immunoblastic lymphadenopathy. Diagnosis and clinical course. Am J Med 1975;59:803-18. [Crossref] [PubMed]
- Cho YU, Chi HS, Park CJ, et al. Distinct features of angioimmunoblastic T-cell lymphoma with bone marrow involvement. Am J Clin Pathol 2009;131:640-6. [Crossref] [PubMed]
- de Leval L, Gisselbrecht C, Gaulard P. Advances in the understanding and management of angioimmunoblastic T-cell lymphoma. Br J Haematol 2010;148:673-89. [Crossref] [PubMed]
- Martel P, Laroche L, Courville P, et al. Cutaneous involvement in patients with angioimmunoblastic lymphadenopathy with dysproteinemia: a clinical, immunohistological, and molecular analysis. Arch Dermatol 2000;136:881-6. [Crossref] [PubMed]
- Gaulard P, deLeval L. Follicular helper T cells: implications in neoplastic hematopathology. Semin Diagn Pathol 2011;28:202-13. [Crossref] [PubMed]
- Bisig B, Thielen C, Herens C, et al. c-Maf expression in angioimmunoblastic T-cell lymphoma reflects follicular helper T-cell derivation rather than oncogenesis. Histopathology 2012;60:371-6. [Crossref] [PubMed]
- Onaindia A, Martínez N, Montes-Moreno S, et al. CD30 Expression by B and T Cells: A Frequent Finding in Angioimmunoblastic T-Cell Lymphoma and Peripheral T-Cell Lymphoma-Not Otherwise Specified. Am J Surg Pathol 2016;40:378-85. [Crossref] [PubMed]
- Nicolae A, Pittaluga S, Venkataraman G, et al. Peripheral T-cell lymphomas of follicular T-helper cell derivation with Hodgkin/Reed-Sternberg cells of B-cell lineage: both EBV-positive and EBV-negative variants exist. Am J Surg Pathol 2013;37:816-26. [Crossref] [PubMed]
- Federico M, Rudiger T, Bellei M, et al. Clinicopathologic characteristics of angioimmunoblastic T-cell lymphoma: analysis of the International Peripheral T-Cell Lymphoma Project. J Clin Oncol 2013;31:240-6. [Crossref] [PubMed]
- Couronné L, Bastard C, Bernard OA. TET2 and DNMT3A mutations in human T- cell lymphoma. N Engl J Med 2012;366:95-6. [Crossref] [PubMed]
- Story SK, Schowalter MK, Geskin LJ. Breast implant-associated ALCL: a unique entity in the spectrum of CD30+ lymphoproliferative disorders. Oncologist 2013;18:301-7. [Crossref] [PubMed]
- Magro CM, Nash JW, Werling RW, et al. Primary cutaneous CD30+ large cell B-cell lymphoma: a series of 10 cases. Appl Immunohistochem Mol Morphol 2006;14:7-11. [Crossref] [PubMed]
- Hu S, Xu-Monette ZY, Balasubramanyam A, et al. CD30 expression defines a novel subgroup of diffuse large B-cell lymphoma with favorable prognosis and distinct gene expression signature: a report from the International DLBCL Rituximab-CHOP Consortium Program Study. Blood 2013;121:2715-24. [Crossref] [PubMed]
- Gallardo F, Barranco C, Toll A, et al. CD30 antigen expression in cutaneous inflammatory infiltrates of scabies: a dynamic immunophenotypic pattern that should be distinguished from lymphomatoid papulosis. J Cutan Pathol 2002;29:368-73. [Crossref] [PubMed]
- Bengtsson A, Holm L, Back O, et al. Elevated serum levels of soluble CD30 in patients with atopic dermatitis (AD). Clin Exp Immunol 1997;109:533-7. [Crossref] [PubMed]
- van Anrooij B, Kluin PM, Oude Elberink JN, et al. CD30 in systemic mastocytosis. Immunol Allergy Clin North Am 2014;34:341-55. [Crossref] [PubMed]
- Alimchandani M, Wang ZF, Miettinen M. CD30 expression in malignant vascular tumors and its diagnostic and clinical implications: a study of 146 cases. Appl Immunohistochem Mol Morphol 2014;22:358-62. [Crossref] [PubMed]
- Masuda S, Miyagawa S, Sougawa N, et al. CD30-targeting immunoconjugates and bystander effects. Nat Rev Clin Oncol 2015;12. [Crossref] [PubMed]


