
Immune evasion and current immunotherapy strategies in mycosis fungoides (MF) and Sézary syndrome (SS)
Introduction
Cutaneous T-cell lymphomas (CTCL) are a family of primary extranodal lymphoid neoplasms that develop from the malignant transformation of post-thymic skin-homing T cells. Mycosis fungoides (MF) is the most common form of CTCL, characterized by an aberrant and excessive proliferation of CD4 T cells in the skin. Clinically, MF typically presents as cutaneous patches, plaques, or tumors, without extracutaneous involvement. In contrast, Sézary syndrome (SS) is a more advanced subtype of CTCL that manifests with erythroderma, generalized superficial lymphadenopathy, and a high burden of circulating malignant T cells (1). Although early-stage MF patients have an indolent course, those with advanced stages and SS patients have compromised survival (2,3).
None of the existing therapies for CTCL, with the possible exception of allogeneic hematopoietic stem cell transplantation (HSCT), has been shown to produce long-term durable remissions that could be defined as “cure”. Hence, the current goal of treatment is to induce remissions across the anatomic compartments affected by the disease (skin, blood, lymph nodes, and viscera), relieve disease-related symptoms, and halt stage progression. Recently, there has been a growing interest in treatments that leverage the immune system and enhance cell-mediated immunity in cancer patients. While certain immunomodulatory treatments have been used for decades in the treatment of MF/SS, newer agents have been introduced in recent years. Immunotherapy, defined by an enhancement of the anti-tumor immune response, encompasses a range of treatment modalities for MF and SS. With this review we will examine the clinically tested immunotherapies for the treatment of MF and SS, as well as ongoing research on potential new targets for treatment.
Anti-tumor immune response in CTCL
The concept of tumor immunogenicity was first postulated by Burnet in 1970, who proposed that malignant cells express traits recognized by innate and adaptive immune cells (4). MF is an immunogenic malignancy and there is evidence that it can elicit an antitumor immune response (5,6). While the specific immunogenic characteristics of malignant CD4 T cells in MF/SS remain to be characterized, the presence of high numbers of CD8 cytotoxic T cells along with dermal dendritic cells in early MF lesions is suggestive of an antitumor response (5,6). In fact, the number of tumor-infiltrating CD8 T cells in MF lesions correlates with a more favorable outcome (7). Furthermore, enhanced CD8 T cell activation and increased expression of natural killer (NK) cell antigens in the blood of patients with early CTCL imply a systemic anti-tumor response in early stages (8). In line with this, Mundy-Bosse et al. demonstrated that CTCL patients had significantly higher levels of NK-cell cytotoxicity compared with normal donors. However, the in vivo mechanisms regulating the numbers and function of circulating NK cells in patients with MF/SS are complex and the clinical implication can be counter intuitive. As such, Mundy-Bosse et al. found that increased numbers of highly functioning NK cells [defined by increased expression of perforin, granzyme B, and interferon (IFN)-γ] are associated with a worse prognosis. Overall, CD8 and NK cells can play important roles in the anti-tumor cytotoxic response to CTCL but higher NK cell activity may not correlate with better prognosis (9).
Immune evasion mechanisms in CTCL
Previous work has established that CTCL tumor cells, similar to other cancer cells, evade immune surveillance (10). Defects in components of the cellular immune system and reduced T-helper type 1 (TH1) activity are observed in MF/SS. Supporting a dampened TH1 phenotype is the reduced production of the pro-inflammatory cytokines IFN-α, IFN-γ, and interleukin (IL)-12 (11-13). In turn, an increased TH2 activity with elevated TH2 cytokines, IL-4, IL-5, and IL-10, which are known inhibitors of TH1-type cytokine production, has been demonstrated in SS/MF (14-16). A TH2 dominant immune environment reduces the TH1 driven anti-tumor CD8 cytotoxic response. Moreover, in a TH2 dominant environment, decreased numbers of dendritic cells and their products IL-12 and IFN-α occurs in MF/SS (17). All these changes contribute to the immune evasive conditions in CTCL.
Another hallmark of an immune evasive tumor microenvironment is the presence of hyporeactive or exhausted T cells (18). Expression of inhibitory molecules such as programmed death receptor-1 (PD-1) and cytotoxic T-lymphocyte associated protein 4 (CTLA-4) is a feature of exhausted T cells. Signaling through these inhibitory molecules can be blocked with the use of checkpoint inhibitors in cancer treatment (19). In CTCL, increased expressions of PD-1, PD-1 ligand, and CTLA-4 have been demonstrated in lesional skin of MF patients (20). However, since inhibitory markers may be expressed on both malignant CD4 and cytotoxic CD8 T cells, it becomes difficult to determine how they may influence the anti-tumor response (21,22). We will discuss this paradigm further as we examine the use of checkpoint inhibitors in CTCL. Finally, regulatory T cells (Treg) play a role in dampening the immune response and upon infiltration in tumors, can contribute to immune evasion in malignancies (23). However, as in the case of circulating NK cells, broad generalizations about the role of specific immune effector cells in MF/SS should be avoided, as an increased number of Tregs in cutaneous lesions from patients with MF has been associated with improved survival (24). In CTCL, an increased number of Tregs has been observed in early MF lesions, but both malignant and reactive T cells can display phenotypic attributes of Tregs—most commonly forkhead box P3 (FOXP3) expression—and, in late MF stages, increased number of Tregs can in fact be associated with immune evasion (25,26).
Given the significant perturbations of the systemic and localized immune response in patients with CTCL, manipulation of the anti-tumor immune response via immunotherapy is an attractive treatment strategy in CTCL. Below we discuss the various existing and emerging immunotherapies used in CTCL.
Monoclonal antibodies (mAbs) that target neoplastic T-cells
Several mAbs targeting antigens expressed on neoplastic T-cells are currently in use for the treatment of CTCL. These antibodies bind to surface proteins expressed by the majority of, or major subsets, of malignant T cells and kill or deplete them via antibody-dependent cellular cytotoxicity (ADCC) (27), antibody-dependent cellular phagocytosis (ADCP), or complement-dependent cytotoxicity (CDC) (28,29).
Generally, the variable regions of these antibodies bind to their corresponding antigenic epitopes on malignant T cells. The Fc segment of the antibody in turn binds the Fc receptor on macrophages, NK cells, and other effector cells or activates complement. NK cells and macrophages are brought into proximity of tumor cells during this process and exert cytotoxic or phagocytic function against tumor cells. Since ADCC, ADCP, and CDC are killing mechanisms mediated by immune cells, they fit within the broader definition of immunotherapy. However, these destructive mechanisms are employed by all mAbs used in cancer treatment regardless of antibody specificity (29). The mAbs commonly used in the treatment of MF/SS and the surface markers they target on T-cells are listed in Table 1 (30-36).

Full table
It should be noted that some of these mAbs are engineered to exert additional effector properties distinct from ADCC, ADCP, and CDC. For example, brentuximab vedotin is an antibody-drug conjugate (ADC) produced by conjugating the anti-CD30 mAb (cAC10) to the cytotoxic payload synthetic monomethyl auristatin E (MMAE) via a complex cathepsin-cleavable linker (37). Upon binding to CD30, brentuximab is internalized into lysosomes and MMAE release leads to cell cycle arrest and apoptosis (38). Other mAbs exert their antitumor effect via additional immune mediated alterations on the tumor microenvironment. For example, depletion of Treg cells by targeting C-C chemokine receptor type 4 (CCR4) with mogamulizumab has been proposed to induce a less immune evasive environment in CTCL (39).
IFN
Mechanism of action
IFN are natural cytokines with antiviral, cytostatic, and immunomodulatory activity that are used in the treatment of several forms of malignancies, including MF/SS. Three types of IFN (alpha, beta, and gamma) are known, with IFN-α and IFN-β grouped together as Type I and INF-γ constituting a distinct type II IFN (40). Both IFN-α and IFN-γ have been used to treat CTCL with IFN-α being the most widely used type of IFN (41).
IFN-α treatment is believed to work by inducing a change in the immune evasive environment of MF/SS. Specifically, IFN-α administration shifts the TH2 dominant milieu towards a TH1 driven cytotoxic response. IFN-α inhibits IL-4 and IL-5 cytokine production (42,43). A shift to TH1 cytokines will further activate the anti-tumor response mediated by CD8 T-cells and NK cells (41).
IFN-γ is less commonly used to treat MF/SS, though in principle IFN-γ exerts similar effects as IFN-α, in shifting the TH1/TH2 paradigm in MF/SS. IFN-γ is a potent simulator of macrophages and dendritic cells and enhances the cytotoxicity mediated by CD8 T cells and activation of NK cells (44).
Monotherapy IFN-α
Bunn et al. initially studied the use of maximal dosage recombinant IFN-α (50×106 U/m2 body surface area) in 20 patients with MF/SS stages II–IVB (45,46). In this prospective study, partial response (PR) (>50% decrease in measurable lesions for at least 4 weeks) was observed in 45% of patients and minor response (MR) (objective remission in some lesions but stable or progressive lesions in other areas) was seen in 25%. There were no patients with complete remission (CR) and 30% had no response (NR). Of the 45% of patients that experienced PR, 89% experienced objective extracutaneous improvements as well, including lymph node size reduction and decrease in circulating malignant cells. Overall, IFN-α demonstrated a 45% objective response rate (ORR), defined as any response in lesions for at least 4 weeks. However, IFN-α adverse effects, including an influenza like syndrome, fatigue, anorexia and depression, required dose reductions to at least 50% of the initial dose in all patients in the first three months of treatment. More importantly, after lowering the IFN-α dosage, ten patients experienced relapses and required dose re-escalations (45,46).
Several small and large scale studies that looked at the efficacy of single agent IFN-α at the maximum tolerated dose (MTD) in early and advanced stage MF/SS are summarized in Table 2 (45-52). Combining the data from these studies, overall 183 patients received IFN-α. Of these, 37% achieved a PR and 24% a CR. Higher response rates were observed in patients with early stage disease, no prior systemic therapy, and treatment with higher doses of IFN-α. Overall, adverse events, including influenza like syndromes, leukopenia and reversible hepatotoxicity were significant. In a large, carefully characterized retrospective Australian cohort, 68 MF/SS patients treated with IFN-α had a longer time to next treatment (TTNT) when compared with other systemic therapies (TTNT of 8.7 months for IFN-α, 3.9 months for chemotherapy, and 4.5 months for histone deacetylase inhibitors) (53).
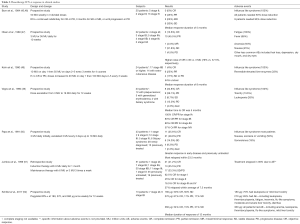
Full table
We conclude that while IFN-α is appropriately listed by the National Comprehensive Cancer Network (NCCN) guidelines as a standard therapy for CTCL, its safety profile limits its use in many patients, as evidenced by the frequency of dose reductions in single agent studies listed in Table 2. It should also be noted that the response criteria have changed dramatically since many of the earlier studies were conducted and published. Therefore, the CR, PR, and ORR rates reported here may not be directly comparable with those of newer studies.
Pegylated IFN-α
The addition of polyethylene glycol to standard IFN-α has led to the introduction of pegylated IFN-α to treatment modalities in MF/SS. This newer form has a lower rate of absorption and reduced clearance as compared with standard IFN-α (41).
In a small dose-escalation study by Schiller et al. evaluating pegylated IFN-α efficacy, 13 MF/SS patients were treated with 180, 270 or 360 µg/week for 12 weeks (52). Major response rates, defined as either a complete or partial remission, were observed in 50%, 83%, and 66% in the 180, 270 and 360 µg, respectively. The median response duration of 12 months was longer than the 5-month median response duration reported by Bunn’s initial study with standard IFN-α dosing. More than a third (38%) of the patients in the Schiller study required dose reductions or dose holdings due to adverse events, with the highest number of patients requiring dose changes in the 270-µg cohort (n=4). Most commonly reported side effects included fatigue, leukopenia and hepatic toxicity, similar to the side effects observed in standard dose IFN-α studies.
Although Schiller’s dose escalation study highlights pegylated IFN-α’s more tolerable profile and suggests higher response rates, when compared with standard IFN-α, larger studies are necessary to confirm these results. A larger MF/SS patient population is necessary to evaluate comparisons between pegylated and standard IFN-α.
Combination therapies with IFN-α
Combination therapy IFN-α has been introduced in an effort to augment standard IFN-α’s response rate and duration of response, as well as to avoid dose-limiting toxicities. It is a treatment regimen that consists of administering low doses of standard IFN-α with already established treatment modalities for MF/SS patients, such as with psoralen and ultraviolet A (PUVA) radiation and with retinoids (54-57). Phase I and Phase II trials were initiated to investigate PUVA and IFN-α effectiveness for early and late stage MF/SS patients (56,57). Complete responses were obtained in 62% to 80% of the patients with a median duration of response greater than 13 months. Long-term prospective studies have established prolonged efficacy and tolerability with IFN-α and PUVA, with a CR of 84% (55). Studies on IFN-α and PUVA have catalyzed the development of IFN-α inclusion in other treatment regimens, including low dose bexarotene, a retinoid used for early stage MF (58).
IFN-α’s augmenting properties expand its application in MF/SS. While its use in monotherapy was questionable due to dose limiting toxicities and short duration of responses, its efficacy as an augmenting agent prove more promising. Additional studies need to be conducted to examine its benefit in combination with the new emerging therapies.
IFN-γ
IFN-γ has been studied on a smaller scale in MF/SS. In one prospective study conducted by Kaplan and colleagues, 16 patients received IFN-γ. Thirty-one percent of patients had an objective PR with one of these patients having previously relapsed with IFN-α treatment (59). and no CR were observed. A subsequent small dose-escalating prospective study in 2004 showed was conducted for IFN-γ in CTCL (60). From five total MF/SS patients, one showed CR and others demonstrated PR. Additional studies on a larger scale are needed to establish a comparison between IFN-α and IFN-γ effect on MF/SS patients.
IL-12
Mechanism of action
IL-12 is a TH1 promoting cytokine, predominantly expressed by monocytes, that is a potent inducer of IFN-γ. IL-12 enhances NK cell activity and cytotoxic T cell responses in CTCL (61). As discussed before, a TH2 dominant cytokine milieu and reduced production of TH1-promoting cytokines such as IFN-γ and IL-12 are hallmarks of immune evasion in MF/SS (11,62). Preclinical studies demonstrated augmented cell lysis and tumor cell cytotoxicity after culturing cells from SS patients with IL-12, supporting this cytokine’s role in anti-tumor immune function (61). Therefore, therapeutic IL-12 administration has been proposed and clinically used in MF/SS to shift the cytokine balance towards a TH1-dominant microenvironment, more favorable to anti-tumor response.
Clinical studies
A small scale (N=10) phase I trial using 50 to 300 ng/kg IL-12 for MF/SS, stages T1 to T4, was initiated to estimate IL-12 anti-tumor activity in vivo (63). Results showed improvement in the skin surface area affected by the disease, with 40% of plaque stage patients reaching CR and 40% attaining PR within 7–8 weeks. Conclusions about the effect of IL-12 in patients with SS could not be drawn due to the fact that 2 of the 3 SS patients withdrew from treatment. The one remaining patient experienced a PR at week 13. Two tumor stage patients were included in the study and received intralesional IL-12 treatments. While they both experienced resolution of the tumors injected with escalating doses of IL-12, new lesions continued to develop elsewhere. Overall, 20% of the patients showed CR, 20% showed PR, and remaining patients demonstrated NR, MR or local response. Biopsies and subsequent immunohistological analysis of patients with CR or PR showed a shift towards a TH1 environment post-treatment. Most adverse events experienced by the patients were mild and of short duration and included fatigue, headache and myalgias.
Although this study was too small to reliably assess efficacy, the biopsy results showing an increase in CD8 T cells suggest that IL-12 supplementation may play a role in augmenting the immune system’s anti-tumor effects. An ongoing phase II trial (NCT02542124) examining the effects of IL-12 with total skin electron beam therapy (TSEBT), a highly effective therapy option for MF, is enrolling patients (64). Early results indicate that of five enrolled patients, one has achieved CR and two achieved PR. Continuation of the study with a larger patient enrollment is necessary to determine correlation with disease stage and show comparisons between TSEBT with IL-12 and TSEBT monotherapy. IL-12’s clinical use for patients remains limited as it has not yet been approved for MF/SS and its efficacy is being evaluated in clinical trials.
Extracorporeal photochemotherapy (ECP)
Mechanism of action
ECP is a therapeutic modality by which a patient’s blood is drawn and centrifuged to separate the white blood cells (WBC) from the red blood cells and plasma. Methoxsalen (8-methoxypsoralen; 8-MOP) is added to the WBCs which are then exposed to UVA, leading to photoactivation of methoxsalen and formation of DNA adducts. Treated and untreated blood components are then reinfused to the patient (65). Photoactivated methoxsalen enters the malignant leukocytes and intercalates with DNA strands to induce apoptosis (66). In addition, ECP is proposed to possess immunomodulatory effects that have been referred to as “transimmunization” (67). Briefly, ECP leads to activation of monocytes and their differentiation into dendritic cells. Upon reinfusion, dendritic cells phagocytose apoptotic lymphocytes and present antigen peptides. Furthermore, an immune response shifting the TH1/TH2 environment has been described (67). However, the immunomodulatory effect of ECP can be complex due to its stimulatory effect on Treg differentiation and anti-inflammatory cytokine production (68).
Clinical studies
The efficacy of ECP in CTCL was first shown by Edelson et al., with encouraging results in advanced stage MF/SS (69). Administering the therapy on two consecutive days every 4 weeks resulted in a 73% response rate in patients with resistant MF/SS with an average 64% improvement of cutaneous involvement. Moreover, 83% of erythrodermic patients experienced improvement of erythroderma. Further studies have demonstrated beneficial responses in MF/SS patients, either with ECP monotherapy or in combination with other treatment modalities such as IFN-α (70-76). Zic et al. compiled data from large series of over 400 CTCL patients of all stages treated with ECP and calculated an ORR of 55.7% and a 17.6% CR rate (65). In SS patients, peripheral blood flow cytometry showed clearance of detectable circulating Sézary cells and elimination of the malignant clones (74).
ECP gained United States Food and Drug Administration (FDA) approval in 1988. CTCL recommendation guidelines suggest ECP use for SS, erythrodermic MF and late stage MF (IIB to IV) that are refractory to other treatments (77). Its use in early-stage MF remains controversial. Additionally, randomized controlled trials comparing ECP efficacy with other established treatments for MF/SS have not yet been reported.
Toll-like receptor agonism
Mechanism of action
Toll like receptors (TLR) are primarily expressed on dendritic cells and macrophages and function as part of the innate immune system to recognize pathogen-associated molecular patterns (PAMPs) during infections and stress (78). Once bound to ligand, they stimulate antigen presentation, increase cell proliferation, and upregulate stimulatory molecule expression. Together, these functions enhance T cell activation and augment cell cytotoxicity against inflammation and tumors (79). Several TLRs are targeted in MF/SS, including TLR7 and TLR8 which bind viral RNA particles and TLR9 that binds bacterial and viral DNAs.
Imiquimod mechanism of action
In humans, TLR7 is solely expressed on a subset of dendritic cells called plasmacytoid dendritic cells (pDCs) (80). Targeting TLR7 with a synthetic ligand, imiquimod, induces production of pro-inflammatory cytokines by pDCs. Specifically, increased production of IFN-α by pDCs results in an increase in TH1 response and a shift towards an anti-tumor immune response.
Imiquimod clinical studies
Although evidence from large clinical studies is lacking, several case reports and case series demonstrate efficacy for imiquimod in treatment of MF/SS, findings summarized in Table 3 (81-89). Results compiled from all studies show 24 treated MF patients, predominantly early stage, with 71% achieving CR and 17% having stable disease. The most common adverse events were skin irritation that self-resolved. In patients with CRs, there was a higher chance of developing severe skin reactions, such as ulcers or erosions that required dose reductions or even temporarily withholding treatment. While these results are promising, imiquimod is not currently approved for use in MF/SS. Larger trials are needed to assess the efficacy of imiquimod in MF/SS.

Full table
Resiquimod mechanism of action
Similar to imiquimod, resiquimod belongs to the imidazoquinolone family. Unlike imiquimod, however, resiquimod modifies the immune response by binding to both TLR7 and TLR8. Although both receptors are structurally similar, they differ in their signaling and therefore, lead to the release of different cytokines.
Gorden and colleagues studied the effect of cytokine release when TLR7 and TLR7/8 were stimulated with their respective agonists (90). TLR7 induction led to a 5 to 10 times greater release of IFN-α whereas TLR7/8 stimulation led to an additional ten times greater production of TNF-α and IL-12. As discussed above, TLR7 engagement by its ligands primarily activates pDCs, causing the predominant IFN-α release associated with imiquimod therapy. However, TLR7/8 agonist stimulates a wider subset of monocyte derived dendritic cells and promotes a wider range of cytokine production, including IFN-α, TNF-α and IL-12.
Although IFN-α, TNF-α and IL-12 all work to augment the immune response, they each function differently. We previously discussed IFN-α and IL-12 function in detail. Briefly, TNF-α is an acute phase reactant that promotes the inflammatory response and inhibits tumorigenesis (91).
Resiquimod clinical studies
Topical resiquimod is a potent TLR7 and TLR8 agonist that is currently being studied in MF/SS patients. A phase I trial, enrolling 12 patients with stages IA to IIA MF, demonstrated promising results for stimulation of the antitumor cytotoxic response and subsequent lesion improvement (92). Patients enrolled were predominantly of early-stage MF with 83% having stage IB. CR was observed in 17% of the patients and PR was attained in 75% of patients. Furthermore, elevated dosage correlated with better response. Only minor adverse effects, such as local skin irritation, were reported by some patients. The trial demonstrated the clearance of untreated lesions as well as treated lesions, a response that had not yet been reported with other topical therapies.
Resiquimod treatment led to either reduction of the monoclonal T cell clone in skin biopsies (90%) or complete elimination of the clone (30%). An expansion of TNF-α and IFN-γ, cytokines mediating the CD8 and NK cell cytotoxic response were observed in lesional skin and correlated positively with treatment response. Overall, these findings provide evidence that resiquimod induction of TLR7 and TLR8 activity augments adaptive immune function.
Although only a small cohort of patients with predominantly early-stage MF were included in the phase I trial of resiquimod, the results show encouraging CR and PR rates. Additionally, this topical medication possibly induces a systemic response and improvement in even untreated lesions. A larger phase II trial is currently ongoing to assess the duration and efficacy of this systemic response (93).
TLR9 agonist mechanism of action
TLR9 is an intracellular receptor expressed on numerous immune cells and is activated by unmethylated CpG sequences found in bacterial or viral DNA. Ligand binding to TLR9 induces pro-inflammatory cytokine production such as type I IFNs and IL-12 (94).
TLR9 agonist clinical studies
In a phase 1/2 study by Kim et al. 15 MF patients received in situ intratumoral vaccination with TLR9 agonist CpG oligodeoxynucleotides combined with localized radiation (95). The hypothesis was that local radiation rendered tumor antigens more available to pDCs, which were in turn primed by TLR9 agonism. The overall response rate was 35.7% with an average time to response of 7.9 weeks and median response duration of 7 weeks. While intratumoral vaccination with TLR9 ligand presents a potential new immunotherapy for MF, larger studies are lacking to comprehensively determine its efficacy.
PD-1 blockade
Mechanism of action
The PD-1 (CD279) is a 268 amino acids transmembrane receptor protein encoded by the PDCD1 gene on chromosome 2q37.3, a member of the extended CD28/CTLA-4 family of T-cell co-regulators. PD-1 is expressed by activated T cells, B cells and myeloid cells and promotes immune tolerance by transducing immune-inhibitory signals (96). Under normal conditions, most circulating T cells do not express PD-1. However, T cells can be induced to express PD-1 upon activation. The inhibitory signals relayed through PD-1 oppose uncontrolled activation of primed T cells through inhibition of T-cell proliferation, cytokine production, and cytolytic function (97). In chronic viral infections, primed cytotoxic T cells express high levels of PD-1 on their surface, become hyporeactive, and are rendered unable to exert antiviral effector functions (98). Similarly, hyporeactive or “exhausted” CD8 T cells within tumors express high levels of PD-1 on their surface and become inefficient in eliminating tumor cells (99).
The two primary ligands that interact with PD-1 are programmed death-ligand 1 (PD-L1, also known as B7-H1 and CD274) and programmed death-ligand 2 (PD-L2, also known as B7-DC and CD273). The PD-1 pathway, consisting of the inhibitory receptor PD-1 on T cells and its ligands on antigen-presenting cells or tumor cells is a major mechanism of tumor immune evasion (100). In solid tumors, malignant cells can escape immune surveillance by expressing PD-L1 and inducing immune-inhibitory effects on tumor infiltrating T cells that express PD-1. Use of PD-1 inhibitors to block this interaction promotes anti-tumor immune response and has produced outstanding clinical results in cancer treatment (99).
In T cell malignancies, however, targeting PD-1 is a more complex proposition as malignant T cells may also express PD-1. Theoretically, PD-1 blockade can remove the inhibitory activity of PD-1 signaling in malignant T cells. In fact, it has been shown in a mouse model of T-cell lymphoma that the activity of PD-1 enhances levels of the tumor suppressor PTEN and attenuates signaling by the kinases AKT and PKC in pre-malignant T cells. Thus, the inhibitory PD-1 immune checkpoint receptor may function as a tumor suppressor in T cell lymphomas (101).
Several groups investigated the expression of PD-1 and its ligands in MF and SS. Samimi et al. detected PD-1 expression on both malignant (CD4+, CD26−) and non-malignant (CD4+, CD26+) T cells collected from blood of SS patients (22). PD-1 expression was significantly higher on circulating CD4 T cells from SS patients compared with either MF or healthy patients. Kantekure and colleagues retrieved MF tissue specimens and examined PD-1 and PD-L1 in patch, plaque and tumor stages (102). While lymphocytes in early patch and plaque stages showed elevated PD-1 expression, PD-1 expression was observed less frequently in tumor stages. However, the reported data did not distinguish PD-1 expression on malignant versus non-malignant T cells. PD-L1 tissue expression was found on all stages and was found to be increasing as tumor progressed. Overall, previous reports show evidence of expression for both PD-1 and its ligands but little is known about the extent of PD-1 activity in malignant versus non-malignant T cells in MF/SS.
Clinical studies
PD-1 blockade may be achieved through the use of anti-PD-1 mAbs such as pembrolizumab and nivolumab. In a phase I study to determine the efficacy of nivolumab in refractory hematologic malignancies, 13 MF patients and two patients with SS were included in the total of 81 subjects (103). In the MF group, two patients experienced PR (15%) while 0 had CR and 9 had SD (69%). Only one of the two SS patients had SD while the other patient showed progression of disease. Commonly reported adverse events included fatigue, skin reactions (rash and pruritus), and pneumonitis, with most events being graded as mild or moderate.
A phase II trial examining pembrolizumab (MK3475) efficacy in refractory MF/SS was conducted via the Cancer Immunotherapy Trials Network (CITN) (104). Khodadoust and colleagues enrolled 24 patients with higher stages of MF/SS in this trial, with 96% being stage IIB or above. Results showed 1 CR, 8 PR (33%), and nine patients with SD (38%). Median duration of response was 32 weeks. The most commonly reported adverse event was a skin flare reaction, with six patients experiencing this event. More serious adverse events, including pneumonitis and diarrhea, were observed in only two patients. As a result of this study, pembrolizumab is now listed in the NCCN guidelines compendium as a treatment option for CTCL and appears to be an acceptable treatment option for MF/SS. Future studies are being conducted combining pembrolizumab and other immunotherapies including IFN-γ.
In summary, PD-1 inhibitors can play a role in the treatment of a subset of patients with MF/SS given the ORR of 38% in the recent phase II trial. However, future work is needed to identify the characteristics of responder versus non-responders and to determine how PD-1 blockade affects malignant versus non-malignant T cells in MF/SS.
CTLA-4 blockade
Mechanism of action
CTLA-4 is another member of the costimulatory family of molecules for T cells and a homolog of CD28 (105). CTLA-4 is expressed on activated T cells and binds to its ligand B7 with much higher binding affinity compared to CD28. While binding of CD28 to B7 produces a stimulatory signal in T cells, the competitive binding of CTLA-4 to B7 produces inhibitory signals that counteract the stimulatory signals from CD28:B7 binding (106). Blockade of CTLA-4 inhibitory signals in T cells has emerged as an effective form of cancer immunotherapy to activate tumor reactive T cells.
Wong et al. observed an abnormal in vitro up-regulation of CTLA-4 in peripheral blood T cells isolated from patients with MF (107). This upregulation was mediated by the TH2 transcription factor GATA3, which was overexpressed by the neoplastic T-cells in MF/SS, suggests that CTLA-4 contributes to the immunosuppression in MF/SS (108).
Clinical studies
Ipilimumab, a CTLA-4 blocking antibody, is used as an effective form of immunotherapy in solid tumors such as melanoma. However, its efficacy in CTCL has yet to be determined. In a single case report, a 44-year-old male with MF and melanoma, exhibited a complete resolution of MF cutaneous lesions after treatment with ipilimumab for advanced melanoma (109).
CD47
Mechanism of action
CD47 is a glycoprotein expressed on many normal cells and binds to its ligand, signal regulatory protein alpha (SIRPα), present on macrophages, dendritic cells and monocytes to inhibit macrophage phagocytic function (110). Upregulation of CD47 contributes to tumor immune evasion by preventing tumor cell elimination (111).
Targeting this pathway has been a major focus of immunotherapy for cancer, including breast cancer, Hodgkin lymphoma and ovarian cancer (112,113). Furthermore, CD47 blockade combined with rituximab has shown promising results for non-Hodgkin lymphoma in a phase 1b study (114). Its application in MF/SS has only recently been investigated
Clinical studies
TTI-62 is a CD47 antagonist that is currently in a phase I trial for MF/SS patients. Preliminary results from this trial show enrollment of 10 MF/SS patients (115). Lesion improvement was assessed using a composite assessment of index lesion severity (CAILS). Rapid reduction of CAILS and lesion improvement was observed in 90% of the patients. More importantly, in patients with circulating Sézary cells, all patients experienced reduction in circulating cell number after only one tumor injection. No toxicities required dose adjustments were reported.
Despite its use in other hematologic and solid cancers and its preliminary success in MF/SS, the specific mechanisms of CD47 blockade remain unclear. Future preclinical studies are essential to better understand this mechanism and its efficacy in MF/SS clinical settings.
Discussion
Recent breakthrough information about how malignant T cells in MF/SS evade the immune system has led to the development of therapies that aim at counteracting these mechanisms. Specifically, immunotherapies that enhance the immune system’s anti-tumor responses are increasingly used in MF/SS. Studies and clinical trials have reported different success rates depending on the mechanism leading to the enhancement of CD8 T cell, macrophage, and NK cell function. While numerous studies have been conducted with older therapies, such as IFN-α and ECP, the evidence for clinical benefit of the newer therapies is starting to be gathered and looks very promising.
This review has highlighted the complex role of the immune system in controlling tumor cell growth in CTCL and the numerous pathways that are exploited by malignant cells to evade the anti-tumor immune response. We also survey the clinical benefits that have already been obtained with the introduction of new immunotherapies and highlight the promise of newer treatment strategies focused on modulating immune responses in CTCL.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare
References
- Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016;127:2375-90. [Crossref] [PubMed]
- Jawed SI, Myskowski PL, Horwitz S, et al. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome): part II. Prognosis, management, and future directions. J Am Acad Dermatol 2014;70:223.e1-17; quiz 240-2.
- Wong HK, Mishra A, Hake T, et al. Evolving insights in the pathogenesis and therapy of cutaneous T-cell lymphoma (mycosis fungoides and Sezary syndrome). Br J Haematol 2011;155:150-66. [Crossref] [PubMed]
- Burnet FM. The concept of immunological surveillance. Prog Exp Tumor Res 1970;13:1-27. [PubMed]
- Vermeer MH, van Doorn R, Dukers D, et al. CD8+ T cells in cutaneous T-cell lymphoma: expression of cytotoxic proteins, Fas Ligand, and killing inhibitory receptors and their relationship with clinical behavior. J Clin Oncol 2001;19:4322-9. [Crossref] [PubMed]
- Goteri G, Filosa A, Mannello B, et al. Density of neoplastic lymphoid infiltrate, CD8+ T cells, and CD1a+ dendritic cells in mycosis fungoides. J Clin Pathol 2003;56:453-8. [Crossref] [PubMed]
- Hoppe RT, Medeiros LJ, Warnke RA, et al. CD8-positive tumor-infiltrating lymphocytes influence the long-term survival of patients with mycosis fungoides. J Am Acad Dermatol 1995;32:448-53. [Crossref] [PubMed]
- Asadullah K, Friedrich M, Docke WD, et al. Enhanced expression of T-cell activation and natural killer cell antigens indicates systemic anti-tumor response in early primary cutaneous T-cell lymphoma. J Invest Dermatol 1997;108:743-7. [Crossref] [PubMed]
- Mundy-Bosse B, Denlinger N, McLaughlin E, et al. Highly cytotoxic natural killer cells are associated with poor prognosis in patients with cutaneous T-cell lymphoma. Blood Adv 2018;2:1818-27. [Crossref] [PubMed]
- Chong BF, Wilson AJ, Gibson HM, et al. Immune function abnormalities in peripheral blood mononuclear cell cytokine expression differentiates stages of cutaneous T-cell lymphoma/mycosis fungoides. Clin Cancer Res 2008;14:646-53. [Crossref] [PubMed]
- Rook AH, Vowels BR, Jaworsky C, et al. The immunopathogenesis of cutaneous T-cell lymphoma. Abnormal cytokine production by Sezary T cells. Arch Dermatol 1993;129:486-9. [Crossref] [PubMed]
- Rook AH, Kubin M, Cassin M, et al. IL-12 reverses cytokine and immune abnormalities in Sezary syndrome. J Immunol 1995;154:1491-8. [PubMed]
- Wood NL, Kitces EN, Blaylock WK. Depressed lymphokine activated killer cell activity in mycosis fungoides. A possible marker for aggressive disease. Arch Dermatol 1990;126:907-13. [Crossref] [PubMed]
- Vowels BR, Lessin SR, Cassin M, et al. Th2 cytokine mRNA expression in skin in cutaneous T-cell lymphoma. J Invest Dermatol 1994;103:669-73. [Crossref] [PubMed]
- Vowels BR, Rook AH, Cassin M, et al. Expression of interleukin-4 and interleukin-5 mRNA in developing cutaneous late-phase reactions. J Allergy Clin Immunol 1995;96:92-6. [Crossref] [PubMed]
- Lessin SR, Vowels BR, Rook AH. Th2 cytokine profile in cutaneous T-cell lymphoma. J Invest Dermatol 1995;105:855-6. [Crossref] [PubMed]
- Wysocka M, Zaki MH, French LE, et al. Sezary syndrome patients demonstrate a defect in dendritic cell populations: effects of CD40 ligand and treatment with GM-CSF on dendritic cell numbers and the production of cytokines. Blood 2002;100:3287-94. [Crossref] [PubMed]
- Zarour HM. Reversing T-cell Dysfunction and Exhaustion in Cancer. Clin Cancer Res 2016;22:1856-64. [Crossref] [PubMed]
- Ilcus C, Bagacean C, Tempescul A, et al. Immune checkpoint blockade: the role of PD-1-PD-L axis in lymphoid malignancies. Onco Targets Ther 2017;10:2349-63. [Crossref] [PubMed]
- Querfeld C, Leung S, Myskowski PL, et al. Primary T Cells from Cutaneous T-cell Lymphoma Skin Explants Display an Exhausted Immune Checkpoint Profile. Cancer Immunol Res 2018;6:900-9. [Crossref] [PubMed]
- Cetinozman F, Jansen PM, Vermeer MH, et al. Differential expression of programmed death-1 (PD-1) in Sezary syndrome and mycosis fungoides. Arch Dermatol 2012;148:1379-85. [Crossref] [PubMed]
- Samimi S, Benoit B, Evans K, et al. Increased programmed death-1 expression on CD4+ T cells in cutaneous T-cell lymphoma: implications for immune suppression. Arch Dermatol 2010;146:1382-8. [Crossref] [PubMed]
- Speiser DE, Ho PC, Verdeil G. Regulatory circuits of T cell function in cancer. Nat Rev Immunol 2016;16:599-611. [Crossref] [PubMed]
- Gjerdrum LM, Woetmann A, Odum N, et al. FOXP3+ regulatory T cells in cutaneous T-cell lymphomas: association with disease stage and survival. Leukemia 2007;21:2512-8. [Crossref] [PubMed]
- Abraham RM, Zhang Q, Odum N, et al. The role of cytokine signaling in the pathogenesis of cutaneous T-cell lymphoma. Cancer Biol Ther 2011;12:1019-22. [Crossref] [PubMed]
- Krejsgaard T, Odum N, Geisler C, et al. Regulatory T cells and immunodeficiency in mycosis fungoides and Sezary syndrome. Leukemia 2012;26:424-32. [Crossref] [PubMed]
- Herbrand U. Anitbody-Dependent cellular phagocytosis: the mechanism of action that gets no respect A discussion about improving bioassay reproducibility. BioProcessing Journal 2016;15:26-9. [Crossref]
- Rogers LM, Veeramani S, Weiner GJ. Complement in monoclonal antibody therapy of cancer. Immunol Res 2014;59:203-10. [Crossref] [PubMed]
- Sliwkowski MX, Mellman I. Antibody therapeutics in cancer. Science 2013;341:1192-8. [Crossref] [PubMed]
- Duvic M, Evans M, Wang C. Mogamulizumab for the treatment of cutaneous T-cell lymphoma: recent advances and clinical potential. Ther Adv Hematol 2016;7:171-4. [Crossref] [PubMed]
- Sugaya M, Morimura S, Suga H, et al. CCR4 is expressed on infiltrating cells in lesional skin of early mycosis fungoides and atopic dermatitis. J Dermatol 2015;42:613-5. [Crossref] [PubMed]
- Ito A, Ishida T, Yano H, et al. Defucosylated anti-CCR4 monoclonal antibody exercises potent ADCC-mediated antitumor effect in the novel tumor-bearing humanized NOD/Shi-scid, IL-2Rgamma(null) mouse model. Cancer Immunol Immunother 2009;58:1195-206. [Crossref] [PubMed]
- Thonnart N, Caudron A, Legaz I, et al. KIR3DL2 is a coinhibitory receptor on Sezary syndrome malignant T cells that promotes resistance to activation-induced cell death. Blood 2014;124:3330-2. [Crossref] [PubMed]
- Schmitt C, Marie-Cardine A, Bensussan A. Therapeutic Antibodies to KIR3DL2 and Other Target Antigens on Cutaneous T-Cell Lymphomas. Front Immunol 2017;8:1010. [Crossref] [PubMed]
- Lundin J, Hagberg H, Repp R, et al. Phase 2 study of alemtuzumab (anti-CD52 monoclonal antibody) in patients with advanced mycosis fungoides/Sezary syndrome. Blood 2003;101:4267-72. [Crossref] [PubMed]
- Falini B, Pileri S, Pizzolo G, et al. CD30 (Ki-1) molecule: a new cytokine receptor of the tumor necrosis factor receptor superfamily as a tool for diagnosis and immunotherapy. Blood 1995;85:1-14. [PubMed]
- Francisco JA, Cerveny CG, Meyer DL, et al. cAC10-vcMMAE, an anti-CD30-monomethyl auristatin E conjugate with potent and selective antitumor activity. Blood 2003;102:1458-65. [Crossref] [PubMed]
- van de Donk NW, Dhimolea E. Brentuximab vedotin. MAbs 2012;4:458-65. [Crossref] [PubMed]
- Ni X, Langridge T, Duvic M. Depletion of regulatory T cells by targeting CC chemokine receptor type 4 with mogamulizumab. Oncoimmunology 2015;4:e1011524. [Crossref] [PubMed]
- Wang BX, Rahbar R, Fish EN. Interferon: current status and future prospects in cancer therapy. J Interferon Cytokine Res 2011;31:545-52. [Crossref] [PubMed]
- Spaccarelli N, Rook AH. The Use of Interferons in the Treatment of Cutaneous T-Cell Lymphoma. Dermatol Clin 2015;33:731-45. [Crossref] [PubMed]
- Axelrod PI, Lorber B, Vonderheid EC. Infections complicating mycosis fungoides and Sezary syndrome. JAMA 1992;267:1354-8. [Crossref] [PubMed]
- Suchin KR, Cassin M, Gottleib SL, et al. Increased interleukin 5 production in eosinophilic Sezary syndrome: regulation by interferon alfa and interleukin 12. J Am Acad Dermatol 2001;44:28-32. [Crossref] [PubMed]
- Olsen EA, Rook AH, Zic J, et al. Sezary syndrome: immunopathogenesis, literature review of therapeutic options, and recommendations for therapy by the United States Cutaneous Lymphoma Consortium (USCLC). J Am Acad Dermatol 2011;64:352-404. [Crossref] [PubMed]
- Bunn PA Jr, Foon KA, Ihde DC, et al. Recombinant leukocyte A interferon: an active agent in advanced cutaneous T-cell lymphomas. Ann Intern Med 1984;101:484-7. [Crossref] [PubMed]
- Bunn PA Jr, Ihde DC, Foon KA. The role of recombinant interferon alfa-2a in the therapy of cutaneous T-cell lymphomas. Cancer 1986;57:1689-95. [Crossref] [PubMed]
- Olsen EA, Rosen ST, Vollmer RT, et al. Interferon alfa-2a in the treatment of cutaneous T cell lymphoma. J Am Acad Dermatol 1989;20:395-407. [Crossref] [PubMed]
- Kohn EC, Steis RG, Sausville EA, et al. Phase II trial of intermittent high-dose recombinant interferon alfa-2a in mycosis fungoides and the Sezary syndrome. J Clin Oncol 1990;8:155-60. [Crossref] [PubMed]
- Vegna ML, Papa G, Defazio D, et al. Interferon alpha-2a in cutaneous T-cell lymphoma. Eur J Haematol Suppl 1990;52:32-5. [PubMed]
- Papa G, Tura S, Mandelli F, et al. Is interferon alpha in cutaneous T-cell lymphoma a treatment of choice? Br J Haematol 1991;79 Suppl 1:48-51. [Crossref] [PubMed]
- Jumbou O, N'Guyen JM, Tessier MH, et al. Long-term follow-up in 51 patients with mycosis fungoides and Sezary syndrome treated by interferon-alfa. Br J Dermatol 1999;140:427-31. [Crossref] [PubMed]
- Schiller M, Tsianakas A, Sterry W, et al. Dose-escalation study evaluating pegylated interferon alpha-2a in patients with cutaneous T-cell lymphoma. J Eur Acad Dermatol Venereol 2017;31:1841-7. [Crossref] [PubMed]
- Hughes CF, Khot A, McCormack C, et al. Lack of durable disease control with chemotherapy for mycosis fungoides and Sezary syndrome: a comparative study of systemic therapy. Blood 2015;125:71-81. [Crossref] [PubMed]
- Stadler R, Otte HG, Luger T, et al. Prospective randomized multicenter clinical trial on the use of interferon -2a plus acitretin versus interferon -2a plus PUVA in patients with cutaneous T-cell lymphoma stages I and II. Blood 1998;92:3578-81. [PubMed]
- Rupoli S, Goteri G, Pulini S, et al. Long-term experience with low-dose interferon-alpha and PUVA in the management of early mycosis fungoides. Eur J Haematol 2005;75:136-45. [Crossref] [PubMed]
- Kuzel TM, Gilyon K, Springer E, et al. Interferon alfa-2a combined with phototherapy in the treatment of cutaneous T-cell lymphoma. J Natl Cancer Inst 1990;82:203-7. [Crossref] [PubMed]
- Kuzel TM, Roenigk HH Jr, Samuelson E, et al. Effectiveness of interferon alfa-2a combined with phototherapy for mycosis fungoides and the Sezary syndrome. J Clin Oncol 1995;13:257-63. [Crossref] [PubMed]
- McGinnis KS, Junkins-Hopkins JM, Crawford G, et al. Low-dose oral bexarotene in combination with low-dose interferon alfa in the treatment of cutaneous T-cell lymphoma: clinical synergism and possible immunologic mechanisms. J Am Acad Dermatol 2004;50:375-9. [Crossref] [PubMed]
- Kaplan EH, Rosen ST, Norris DB, et al. Phase II study of recombinant human interferon gamma for treatment of cutaneous T-cell lymphoma. J Natl Cancer Inst 1990;82:208-12. [Crossref] [PubMed]
- Dummer R, Hassel JC, Fellenberg F, et al. Adenovirus-mediated intralesional interferon-gamma gene transfer induces tumor regressions in cutaneous lymphomas. Blood 2004;104:1631-8. [Crossref] [PubMed]
- Rook AH, Kubin M, Fox FE, et al. The potential therapeutic role of interleukin-12 in cutaneous T-cell lymphoma. Ann N Y Acad Sci 1996;795:310-8. [Crossref] [PubMed]
- Vowels BR, Cassin M, Vonderheid EC, et al. Aberrant cytokine production by Sezary syndrome patients: cytokine secretion pattern resembles murine Th2 cells. J Invest Dermatol 1992;99:90-4. [Crossref] [PubMed]
- Rook AH, Wood GS, Yoo EK, et al. Interleukin-12 therapy of cutaneous T-cell lymphoma induces lesion regression and cytotoxic T-cell responses. Blood 1999;94:902-8. [PubMed]
- Kim YH, Hoppe RT, Rook AH, et al. A Single-Arm PHASE 2A Study of NM-IL-12 (rHu-IL12) in Patients with Mycosis Fungoides-Type CTCL (MF) Undergoing Low-Dose TOTAL Skin Electron BEAM Therapy (LD-TSEBT). Blood 2016;128:4165.
- Zic JA. The treatment of cutaneous T-cell lymphoma with photopheresis. Dermatol Ther 2003;16:337-46. [Crossref] [PubMed]
- Alfred A, Taylor PC, Dignan F, et al. The role of extracorporeal photopheresis in the management of cutaneous T-cell lymphoma, graft-versus-host disease and organ transplant rejection: a consensus statement update from the UK Photopheresis Society. Br J Haematol 2017;177:287-310. [Crossref] [PubMed]
- Cho A, Jantschitsch C, Knobler R. Extracorporeal Photopheresis-An Overview. Front Med (Lausanne) 2018;5:236. [Crossref] [PubMed]
- Worel N, Leitner G. Clinical Results of Extracorporeal Photopheresis. Transfus Med Hemother 2012;39:254-62. [Crossref] [PubMed]
- Edelson R, Berger C, Gasparro F, et al. Treatment of cutaneous T-cell lymphoma by extracorporeal photochemotherapy. Preliminary results. N Engl J Med 1987;316:297-303. [Crossref] [PubMed]
- Heald PW, Perez MI, Christensen I, et al. Photopheresis therapy of cutaneous T-cell lymphoma: the Yale-New Haven Hospital experience. Yale J Biol Med 1989;62:629-38. [PubMed]
- Armus S, Keyes B, Cahill C, et al. Photopheresis for the treatment of cutaneous T cell lymphoma. J Am Acad Dermatol 1990;23:898-902. [Crossref] [PubMed]
- Prinz B, Behrens W, Holzle E, et al. Extracorporeal photopheresis for the treatment of cutaneous T-cell lymphoma--the Dusseldorf and Munich experience. Arch Dermatol Res 1995;287:621-6. [Crossref] [PubMed]
- Zic JA, Stricklin GP, Greer JP, et al. Long-term follow-up of patients with cutaneous T-cell lymphoma treated with extracorporeal photochemotherapy. J Am Acad Dermatol 1996;35:935-45. [Crossref] [PubMed]
- Gottlieb SL, Wolfe JT, Fox FE, et al. Treatment of cutaneous T-cell lymphoma with extracorporeal photopheresis monotherapy and in combination with recombinant interferon alfa: a 10-year experience at a single institution. J Am Acad Dermatol 1996;35:946-57. [Crossref] [PubMed]
- Duvic M, Hester JP, Lemak NA. Photopheresis therapy for cutaneous T-cell lymphoma. J Am Acad Dermatol 1996;35:573-9. [Crossref] [PubMed]
- Owsianowski M, Garbe C, Ramaker J, et al. Therapeutic experiences with extracorporeal photopheresis. Technical procedure, follow-up and clinical outcome in 31 skin diseases. Hautarzt 1996;47:114-23. [Crossref] [PubMed]
- Marques MB, Adamski J. Extracorporeal photopheresis: technique, established and novel indications. J Clin Apher 2014;29:228-34. [Crossref] [PubMed]
- Morrison C, Baer MR, Zandberg DP, et al. Effects of Toll-like receptor signals in T-cell neoplasms. Future Oncol 2011;7:309-20. [Crossref] [PubMed]
- Mempel M, Kalali BN, Ollert M, et al. Toll-like receptors in dermatology. Dermatol Clin 2007;25:531-40. viii. [Crossref] [PubMed]
- Gibson SJ, Lindh JM, Riter TR, et al. Plasmacytoid dendritic cells produce cytokines and mature in response to the TLR7 agonists, imiquimod and resiquimod. Cell Immunol 2002;218:74-86. [Crossref] [PubMed]
- Suchin KR, Junkins-Hopkins JM, Rook AH. Treatment of stage IA cutaneous T-Cell lymphoma with topical application of the immune response modifier imiquimod. Arch Dermatol 2002;138:1137-9. [Crossref] [PubMed]
- Dummer R, Urosevic M, Kempf W, et al. Imiquimod induces complete clearance of a PUVA-resistant plaque in mycosis fungoides. Dermatology 2003;207:116-8. [Crossref] [PubMed]
- Chong A, Loo WJ, Banney L, et al. Imiquimod 5% cream in the treatment of mycosis fungoides--a pilot study. J Dermatolog Treat 2004;15:118-9. [Crossref] [PubMed]
- Deeths MJ, Chapman JT, Dellavalle RP, et al. Treatment of patch and plaque stage mycosis fungoides with imiquimod 5% cream. J Am Acad Dermatol 2005;52:275-80. [Crossref] [PubMed]
- Chiam LY, Chan YC. Solitary plaque mycosis fungoides on the penis responding to topical imiquimod therapy. Br J Dermatol 2007;156:560-2. [Crossref] [PubMed]
- Coors EA, Schuler G, Von Den Driesch P. Topical imiquimod as treatment for different kinds of cutaneous lymphoma. Eur J Dermatol 2006;16:391-3. [PubMed]
- Martinez-Gonzalez MC, Verea-Hernando MM, Yebra-Pimentel MT, et al. Imiquimod in mycosis fungoides. Eur J Dermatol 2008;18:148-52. [PubMed]
- Gordon MC, Sluzevich JC, Jambusaria-Pahlajani A. Clearance of folliculotropic and tumor mycosis fungoides with topical 5% imiquimod. JAAD Case Rep 2015;1:348-50. [Crossref] [PubMed]
- Lewis DJ, Byekova YA, Emge DA, et al. Complete resolution of mycosis fungoides tumors with imiquimod 5% cream: a case series. J Dermatolog Treat 2017;28:567-9. [Crossref] [PubMed]
- Gorden KB, Gorski KS, Gibson SJ, et al. Synthetic TLR agonists reveal functional differences between human TLR7 and TLR8. J Immunol 2005;174:1259-68. [Crossref] [PubMed]
- Cantaert T, Baeten D, Tak PP, et al. Type I IFN and TNFalpha cross-regulation in immune-mediated inflammatory disease: basic concepts and clinical relevance. Arthritis Res Ther 2010;12:219. [Crossref] [PubMed]
- Rook AH, Gelfand JM, Wysocka M, et al. Topical resiquimod can induce disease regression and enhance T-cell effector functions in cutaneous T-cell lymphoma. Blood 2015;126:1452-61. [Crossref] [PubMed]
- Killock D. Haematological cancer: Resiquimod-a topical CTCL therapy. Nat Rev Clin Oncol 2015;12:563. [Crossref] [PubMed]
- Medzhitov R. TLR-mediated innate immune recognition. Semin Immunol 2007;19:1-2. [Crossref] [PubMed]
- Kim YH, Gratzinger D, Harrison C, et al. In situ vaccination against mycosis fungoides by intratumoral injection of a TLR9 agonist combined with radiation: a phase 1/2 study. Blood 2012;119:355-63. [Crossref] [PubMed]
- Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med 2000;192:1027-34. [Crossref] [PubMed]
- Riley JL. PD-1 signaling in primary T cells. Immunol Rev 2009;229:114-25. [Crossref] [PubMed]
- Kahan SM, Wherry EJ, Zajac AJ. T cell exhaustion during persistent viral infections. Virology 2015;479-480:180-93. [Crossref] [PubMed]
- Lee J, Ahn E, Kissick HT, et al. Reinvigorating Exhausted T Cells by Blockade of the PD-1 Pathway. For Immunopathol Dis Therap 2015;6:7-17. [Crossref] [PubMed]
- Zhao Y, Harrison DL, Song Y, et al. Antigen-Presenting Cell-Intrinsic PD-1 Neutralizes PD-L1 in cis to Attenuate PD-1 Signaling in T Cells. Cell Rep 2018;24:379-390.e6. [Crossref] [PubMed]
- Wartewig T, Kurgyis Z, Keppler S, et al. PD-1 is a haploinsufficient suppressor of T cell lymphomagenesis. Nature 2017;552:121-5. Erratum: PD-1 is a haploinsufficient suppressor of T cell lymphomagenesis. [Nature 2018].
- Kantekure K, Yang Y, Raghunath P, et al. Expression patterns of the immunosuppressive proteins PD-1/CD279 and PD-L1/CD274 at different stages of cutaneous T-cell lymphoma/mycosis fungoides. Am J Dermatopathol 2012;34:126-8. [Crossref] [PubMed]
- Lesokhin AM, Ansell SM, Armand P, et al. Nivolumab in Patients With Relapsed or Refractory Hematologic Malignancy: Preliminary Results of a Phase Ib Study. J Clin Oncol 2016;34:2698-704. [Crossref] [PubMed]
- Khodadoust M, Rook AH, Porcu P, et al. Pembrolizumab for Treatment of Relapsed/Refractory Mycosis Fungoides and Sezary Syndrome: Clinical Efficacy in a Citn Multicenter Phase 2 Study. Blood 2016;128:181.
- Thompson CB, Allison JP. The emerging role of CTLA-4 as an immune attenuator. Immunity 1997;7:445-50. [Crossref] [PubMed]
- Fallarino F, Fields PE, Gajewski TF. B7-1 engagement of cytotoxic T lymphocyte antigen 4 inhibits T cell activation in the absence of CD28. J Exp Med 1998;188:205-10. [Crossref] [PubMed]
- Wong HK, Wilson AJ, Gibson HM, et al. Increased expression of CTLA-4 in malignant T-cells from patients with mycosis fungoides -- cutaneous T cell lymphoma. J Invest Dermatol 2006;126:212-9. [Crossref] [PubMed]
- Gibson HM, Mishra A, Chan DV, et al. Impaired proteasome function activates GATA3 in T cells and upregulates CTLA-4: relevance for Sezary syndrome. J Invest Dermatol 2013;133:249-57. [Crossref] [PubMed]
- Bar-Sela G, Bergman R. Complete regression of mycosis fungoides after ipilimumab therapy for advanced melanoma. JAAD Case Rep 2015;1:99-100. [Crossref] [PubMed]
- Folkes AS, Feng M, Zain JM, et al. Targeting CD47 as a cancer therapeutic strategy: the cutaneous T-cell lymphoma experience. Curr Opin Oncol 2018;30:332-7. [PubMed]
- Huang Y, Ma Y, Gao P, et al. Targeting CD47: the achievements and concerns of current studies on cancer immunotherapy. J Thorac Dis 2017;9:E168-74. [Crossref] [PubMed]
- Chao MP, Alizadeh AA, Tang C, et al. Anti-CD47 antibody synergizes with rituximab to promote phagocytosis and eradicate non-Hodgkin lymphoma. Cell 2010;142:699-713. [Crossref] [PubMed]
- Willingham SB, Volkmer JP, Gentles AJ, et al. The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc Natl Acad Sci U S A 2012;109:6662-7. [Crossref] [PubMed]
- Advani R, Flinn I, Popplewell L, et al. CD47 Blockade by Hu5F9-G4 and Rituximab in Non-Hodgkin's Lymphoma. N Engl J Med 2018;379:1711-21. [Crossref] [PubMed]
- Querfeld C, Thompson JA, Taylor M, et al. A single direct intratumoral injection of TTI-621 (SIRPaFc) induces antitumor activity in patients with relapsed/refractory mycosis fungoides and sezary syndrome: preliminary findings employing an immune checkpoint inhibitor blocking the CD47 “do not eat” signal. ASH 2017: abstr 4076.


