Adjuvant therapy of stage III colon cancer
The decision for adjuvant therapy of colon cancer requires selection of acceptable treatment options as well as identification of the individuals who are both at risk for recurrence (prognosis) and likely to have a significant clinical benefit (prediction). In this article, we will identify currently established treatment regimens for stage III colon cancer and will discuss the status of prognostic and predictive markers. In general, we have significantly greater knowledge of clinicopathologic and genomic prognostic markers than predictive markers, but awareness of prognosis at least allows selection of patients at greatest risk (and presumably greatest benefit) from treatment. When reporting the results of clinical trials in this setting, it should be remembered that they are based on a highly selected group of patients who were eligible for clinical trials and treated with trial-specific regimens and follow-up. In the application of these results, it is likely that the closer the patient is to the population in the study, and the more they are treated per protocol, the more likely their benefit and toxicity will be similar to the trial results. Conversely, if the patient does not reflect the typical healthy 60 year old who enter trials and if the treatment deviates significantly from the protocol, the more likely it is that the therapy will be less effective and more toxic than the trial results. This is particularly true of the elderly, which will be discussed separately.
Biologic prognostic/predictive markers
Prognostic markers are associated with overall outcome of cancer patients independent of therapy. They may help to identify patients at high risk for tumor recurrence or metastasis; therefore, adjuvant therapy could improve the outcomes of these patients. On the other hand, predictive markers are those which may influence the choice of specific patient treatment, such as an intervention as adjuvant therapy, thus allowing decisions for therapy which are most likely to provide benefit to the individual patient.
Colon cancer is heterogeneous in clinical behavior and in the molecular mechanisms of pathogenesis. It is therefore necessary to identify prognostic and predictive markers to characterize risk factors for recurrence and to implement tailored therapeutic strategies. Until recently, the anatomic TNM classification (Table 1) remained the only validated prognostic tool in the adjuvant setting (Figure 1) (1). Clinical practice is still almost entirely based on the T and N staging system. To continuously improve the prognosis of colon cancer patients and the outcome of patients with recurrent risk by chemotherapy. The American Joint Committee of Cancer updates the staging systems every 6 years based on new evidence from clinic research and epidemiologic data. For example, T4 lesions have recently been subdivided as T4a (tumor penetrates to the surface of the visceral peritoneum) and T4b (tumor directly invades or is adherent to other organs or structures). Stage group II has been further subdivided into IIA (T3N0), IIB (T4aN0), and IIC (T4bN0) based on differential survival prognosis from the SEER analyses (Surveillance Epidemiology and End Results) (2). Meanwhile, a myriad of candidate molecular biomarkers has been reported for prognosis and for prediction of therapy in recent years. However, rigorous assessment in large patient datasets by multivariable analysis and subsequent implementation and validation in clinical practice is still needed.
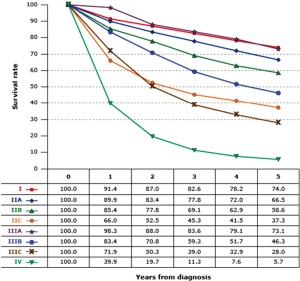
Colon patients with lymph node involvement (stage III) are commonly recommended for adjuvant therapy based on randomized studies, data from SEER, and meta-analyses with improved overall disease-free survival (DFS) and overall survival (OS). However, in patients with cancer invading through muscularis propria but no lymph node involvement (stage II), general recommendations consider that adjuvant chemotherapy may not contribute enough benefit in overall survival except in those with high risk factors (T4 lesions, clinical presentation with bowel obstruction or perforation, <12 LN recovered in the specimen, and poor differentiation) (3). A more detailed discussion of prognostic markers and treatment of stage II disease is presented in a separate article in this special edition of CCO.
Studies have shown that many genetic alterations are prognostic or predictive markers in colorectal cancer (4). Loss of heterozygosity at chromosome 18q indicates a poor prognosis. Other alterations that have prognostic value are allelic loss at chromosomes 17p, 1p, 3p, 4p, 5q, or 8p; changes in the levels of certain gene products, including the DCC (deleted in colorectal cancer) protein, p53, and p27klp1; mutation of the ras and raf genes; and increased expression of genes involved in fluoropyrimidine metabolism, with variability between stage II and III disease.
High levels of microsatellite instability (MSI-H), with insertions or deletions of nucleotides within repeated sequences of DNA, due to defective repair of mismatched nucleotides (dMMR), are characteristic of the hereditary nonpolyposis colorectal cancer syndrome. (See the article by Lynch et al. in this issue) However, in most cases such tumors are sporadic (~15% of such tumors are MSI-H, more often in stage II disease), which is also considered a prognostic marker with fewer metastases and a better prognosis than microsatellite-stable (MSS) cancers (4-6).
Microsatellite status has been suggested as both a prognostic (most proven) and predictive marker of fluoropyrimidines-based therapy, and therefore has potential clinical implications in the adjuvant therapeutic strategy. It has been observed from retrospective studies that patients with MSI (dMMR) tumors have better overall survival. At the same time, studies report that patients with MSI may not benefit from adjuvant chemotherapy with 5-FU alone (7-9). However, the addition of oxaliplatin to 5-FU improves the RFS in patients with MSI-H tumors in comparison with 5-FU alone in stage III colon cancer patients (10). For stage III colon cancer after adjuvant fluorouracil-based therapy, it has been suggested that retention of 18q alleles in microsatellite-stable cancers and mutation of the gene for the type II receptor for TGFb 1 in cancer with MSI-H have a favorable prognosis (11). The prognostic significance of 18qLOH seems less important from more recent studies than previous assumed (12,13).
SMAD4 is an important transcriptional mediator in the TGF-β signaling pathway and has been implicated in colon cancer development in preclinical studies. Loss or low expression of SMAD4 has been associated with poor prognosis in colorectal cancer patients in small series (14).
Integrated analysis of molecular and clinical prognostic factors in stage II/III colon cancer was assessed based on the prospective collected colectomy specimens from 1,564 patients who were accrued to the PETACC3 study, a randomized phase III trial with a total 3,278 patients to assess the activity of irinotecan to 5-FU/LV in the adjuvant treatment of stage II/III colon cancer (15). FFPE (formalin-fixed paraffin embedded) tissue samples were tested for Kras and Braf mutations, 18qLOH, MSI, and SMAD4 protein expression. The results indicate MSI-H status and SMAD4 focal loss of expression are independent prognostic factors: MSI-H patients have better RFS (HR=0.54, P=0.003) and OS (HR=0.43, P=0.001); those with SMAD4 loss have worse RFS (HR=1.47, P<0.001) and OS (HR=1.58, P<0.001). This study indicated that T3N1 tumors with MSI-H and retained SMAD4 expression had outcomes similar to stage II disease. A German study studying expression of caspase-8, caspase-9, and apoptosis protease activating factor-1 (APAF-1), caspase-8 and -9 were significantly associated with poor survival. Caspase-9 may be an independent prognosticator in colon carcinoma. However, these data have to be confirmed prospectively, including results of some studies indicating different genomic profiles between right- and left-sided tumors (16).
Because of the heterogeneity and complexity for both prognostic and predictive outcomes, gene expression profiles (GEPs), signatures of gene expression—in which the expression levels of multiple genes are combined in a defined manner to provide a score or classification—have been developed for determine prognosis and guiding treatment decisions. There are many GEP assays that have been developed, and some are commercially available using proprietary approaches to measure RNA via PCR (reverse-transcriptase polymerase chain reactions) or gene chip microarray system with different gene signatures (ColoPrint®, an 18 gene expression profile; Oncotype DX® of seven prognostic and five reference genes; ColonPRS® of 163 genes, GeneFX® of 5 genes and OncoDefender-CRC of a 634 probe-set signature). Either FFPE tissue or fresh tissue or fresh frozen tissue are used for assays (17-20). While both great clinical interest and active research in GEP assays have been shown, with increasing commercial use and the potential advantages of using such assays, their ultimate clinical utility is still largely unproven. Notably, there is no overlap in the genes comprising these commercial available assays. They are marked as laboratory-developed tests (LDT), and none of them has specific FDA approval for stage II/III colon cancer. It is important to have gene expression signature assays showing improvement of net health outcomes from prospective studies. These include changes of the classifications based on GEP results, identification of patients at low risk of recurrence who can avoid adjuvant chemotherapy safely, and other patients who may benefit from adjuvant chemotherapy or a particular adjuvant chemotherapy with improved survival. A recent multi-center prospective study tried to characterize the impact of ‘recurrence score’ results from OncoTypeDX® on medical oncologists’ recommendations regarding adjuvant chemotherapy in T3, Mismatch Repair-proficient (MMR-P) stage II colon cancer patients, and noted that test was associated with a 45% change in treatment recommendations (21). In a report of stage II and III colon cancer from NSABP C-07, a predefined high recurrence score (RS) group (26% of patients) had a higher recurrence risk than a low RS group (39% of patients): HR=2.11, P<0.001. Cox model 5 yr. recurrence risks (95% CI) in FU-treated patients by RS group (low, int, high) were: st II 9% (6-13%), 13% (8-17%), 18% (12-25%); st IIIA/B 21% (16-26%), 29% (24-34%), 38% (30-46%); st IIIC 40% (32-48%), 51% (43-59%), 64% (55-74%). RS did not have significant interaction with stage (P=0.90) or age (P=0.76). The relative benefit of oxaliplatin was similar across range of RS (interaction P=0.48); the absolute benefit of oxaliplatin increased with higher RS (22). Although most patients with stage III colon cancer should be considered for adjuvant therapy, GEPs may be considered to further define risk in IIIA patients for observation alone, single-agent fluoropyrimidines or combination therapy.
Adjuvant therapy for stage III colon cancer
Prior to the early 1990s, a series of underpowered studies using frequently ineffective schedules of the only available agent--5-fluorouracil (5-FU)—failed to show benefit of an adjuvant treatment after surgery for stage III colon cancer. Finally, a study by Moertel et al. using 5-FU plus the antihelminthic levamisole demonstrated benefit compared to surgery alone. INT-0035 confirmed these results in a larger population of stage III patients, showing fluorouracil plus levamisole reduced the recurrence rate by 40% (P<0.0001) and the death rate by 33% (P=0.0007). Levamisole alone reduced the recurrence rate by only 2% and the death rate by only 6% (23). This treatment rapidly became standard adjuvant therapy for most of the Western world. However, the role of levamisole was uncertain, and increased cytotoxicity with biochemical modulation of 5-FU by leucovorin raised questions about optimal therapy. To answer these questions, at least three large randomized trials were performed: INT-0089, an NCCTG trial and NSABP C-04 (24-26). The combined results of these studies demonstrated at least three conclusions. First, 6 months of therapy was at least as effective as 12 months. Second, levamisole was not a necessary component of therapy. Third, 5-FU plus leucovorin was at least as effective as a levamisole-containing regimen. Over time, a number of options became available for patients: bolus 5-FU/LV, bolus and infusional 5-FU/LV (LV5FU2) and capecitabine alone (X-ACT trial) (27,28). Each of these increased the 3 yr. disease-free survival for high-risk stage II/III colon cancer from approximately 60-63% with surgery alone to approximately 70-75% with adjuvant treatment.
This standard single-agent approach was challenged, especially in high-risk stage III patients, by the availability of two new effective cytotoxics, irinotecan and oxaliplatin, and two biologics, bevacizumab and cetuximab. It has been nearly a decade since the results of the MOSAIC trial (the Multicenter International Study of Oxaliplatin/5-Fluorouracil/Leucovorin in the Adjuvant Treatment of Colon Cancer) were reported (29), although earlier results of the trial presented in abstracts altered the standard combination adjuvant treatment of stage III to a bolus and infusional 5-FU/LV regimen (LV5FU2) plus oxaliplatin (FOLFOX4). 1,123 patients were randomly assigned to either 5-FU/LV or FOLFOX. After a median follow-up of 37.9 months, disease-free survival at three years (the primary endpoint) was 78.2 percent (95 percent CI, 75.6 to 80.7) in the group given LV5FU2 plus oxaliplatin and 72.9 percent (95 percent CI, 70.2 to 75.7) in the LV5FU2 group (P=0.002 by the stratified log-rank test). In a further update, 5-year DFS rates were 73.3% and 67.4% in the FOLFOX4 and LV5FU2 groups, respectively [hazard ratio (HR)=0.80; 95% CI, 0.68 to 0.93; P=0.003]. Six-year OS rates were 78.5% and 76.0% in the FOLFOX4 and LV5FU2 groups, respectively (HR=0.84; 95% CI, 0.71 to 1.00; P=0.046). 6-year OS rates stage III disease were 72.9% and 68.7%, respectively (HR=0.80; 95% CI, 0.65 to 0.97; P=0.023). No difference in OS was seen in the stage II population (30).
The results of the MOSAIC trial were largely upheld by the NSABP C-07 study, which compared weekly bolus 5-FU/LV to the same regimen with biweekly oxaliplatin (FLOX) (31). With 8 years median follow-up, OS was similar between treatment groups [hazard ratio (HR), 0.88; 95% CI, 0.75 to 1.02; P=0.08]. FLOX remained superior for DFS (HR, 0.82; 95% CI, 0.72 to 0.93; P=0.002). The effect of oxaliplatin on OS did not differ by stage of disease (interaction P=0.38 for OS; interaction P=0.37 for DFS) but did vary by age for OS (younger than age 70 v 70+ interaction P=0.039). Oxaliplatin significantly improved OS in patients younger than age 70 (HR, 0.80; 95% CI, 0.68 to 0.95; P=0.013), but no positive effect was evident in older patients. (Yothers JCO 2011). The FLOX regimen is less used than FOLFOX or capecitabine plus oxaliplatin (XELOX).
Based on the results of the X-ACT trial, which showed noninferiority of XELOX to bolus 5-FU/LV in stage III colon cancer, and the likely desirability of oral therapy to infusional approaches, the XELOXA trial was initiated (32). Stage III patients were randomly assigned to XELOX (oxaliplatin 130 mg/m2 on day 1 plus capecitabine 1,000 mg/m2 twice daily on days 1 to 14 every 3 weeks for 24 weeks) or a standard bolus 5-FU/LV adjuvant regimen. The primary study end point was disease-free survival (DFS). The 3-year DFS rate was 70.9% with XELOX and 66.5% with FU/LV. The HR OS for XELOX compared to FU/LV was 0.87 (95% CI, 0.72 to 1.05; P=0.1486). The 5-year OS for XELOX and FU/FA were 77.6% and 74.2%, respectively. These results were comparable to the MOSAIC and FLOX trials at similar time point analyses.
Two large trials assessed the benefits of irinotecan in adjuvant therapy of stage II/III colon cancer. The first trial performed in the US utilized a bolus 5-FU/LV plus irinotecan regimen (IFL) compared to bolus 5-FU/LV alone (33). 1,264 patients were randomly assigned to treatment; the primary end points of the study were overall survival (OS) and disease-free survival (DFS). There were no differences in either DFS or OS between the two treatment arms, but toxicity, including lethal toxicity, was significantly higher on IFL. As IFL was found to be inferior to FOLFOX in advanced disease (34), it was postulated that the results of a trial (PETACC-3) comparing LV5FU2 plus irinotecan (FOLFIRI) to LV5FU2, mirroring the MOSAIC trial, might also show more benefit than IFL for combination adjuvant therapy (35). 2,094 treated patients with stage III disease were assessed for the primary endpoint, DFS in stage III disease. After a median follow-up of more than 5 years, the 5-year DFS rate was 56.7% with irinotecan/LV5FU2 and 54.3% with LV5FU2 alone (P=0.106). Overall survival was not improved compared with LV5FU2 alone (5-year OS 73.6% vs. 71.3%, respectively; log-rank P=0.094). Based on the results of these two trials, there is no role for irinotecan in the standard management of stage II/III colon cancer.
The increased efficacy of regimens containing either bevacizumab or cetuximab in advanced colorectal cancer supported the initiation of these agents in the adjuvant setting, using FOLFOX as the backbone and comparator. Two trials were performed with bevacizumab, NSABP C-08 and AVANT. In advanced disease, with combination therapy such as FOLFOX, the benefit of bevacizumab is primarily seen in prolonging progression-free survival rather than increasing response rate (36). Therefore, the effect of this drug in the adjuvant setting was uncertain. NSABP C-08 compared six months of FOLFOX to six months of FOLFOX with bevacizumab, followed by an additional six months of bevacizumab alone (37). In 2,673 analyzed patients, with a median follow-up of 5 years, the addition of bevacizumab to mFOLFOX6 did not result in an overall significant increase in DFS (HR, 0.93; 95% CI, 0.81 to 1.08; P=0.35). At 12 months, there was a significant reduction in the hazard ratio, but exploratory analyses found that the effect of bevacizumab on DFS was different before and after a 1.25-year landmark (time-by-treatment interaction P value<0.0001). OS was not different between the two study arms for all patients (HR, 0.95; 95% CI, 0.79 to 1.13; P=0.56) and for stage III disease (HR, 1.0; 95% CI, 0.83 to 1.21; P=0.99).
The AVANT trial contained the same two comparison arms as C-08, with an additional arm of XELOX plus bevacizumab (38). 2,867 patients had stage III disease. After a median follow-up of 48 months the DFS hazard ratio for bevacizumab-FOLFOX4 versus FOLFOX4 was 1.17 (95% CI, 0.98-1.39; P=0.07), and for bevacizumab-XELOX versus FOLFOX4 was 1.07 (0.90-1.28; P=0.44). With a minimum follow-up of 60 months, the OS hazard ratio for bevacizumab-FOLFOX4 versus FOLFOX4 was 1.27 (1.03-1.57; P=0.02), and for bevacizumab-XELOX versus FOLFOX4 was 1.15 (0.93-1.42; P=0.21). These disappointing results have effectively eliminated bevacizumab from further development in the adjuvant setting, although some have discussed continuing the drug for 2-5 years to maintain the early benefit in DFS seen in both trials. Cost and uncertainty over toxicity related to long-term antiangiogenic therapy have been cited as reasons for not pursuing this possibility.
Cetuximab has activity as a single agent and increased response rates in trials with chemotherapy, raising hopes that this agent in adjuvant treatment might improve results with FOLFOX alone. The first trial to be reported in KRAS wild-type patients was N0147 (39). The trial was halted after a planned interim analysis of 48% of predicted DFS events (246/515) occurred in 1,863 (of 2,070 planned) patients. With a median follow-up of 28 months, 3-yr DFS for mFOLFOX6 alone was 74.6% vs. 71.5% with cetuximab (HR, 1.21; 95% CI, 0.98-1.49; P=0.08) even when restricted to patients with wild-type KRAS. Gr 3 or higher adverse events [72.5% vs. 52.3%; odds ratio (OR), 2.4; 95% CI, 2.1-2.8; P<0.001] and failure to complete 12 cycles (33% vs. 23%; OR, 1.6; 95% CI, 1.4-1.9; P<0.001) were significantly higher with cetuximab, with increased toxicity and worse outcomes in patients aged 70 years or older.
A similar trial was performed outside of the US, PETACC-8 (40). 1,602 KRAS wt patients were randomized to FOLFOX4 or FOLFOX4 plus cetuximab. At a median follow-up of 40 months, an interim analysis showed no difference between arms for DFS (HR 1.05, 95% CI, 0.85-1.29; P=0.66) or OS (HR 1.09, 95% CI, 0.81-1.47; P=0.55). As in N0147, worse outcomes were seen in patients >70 years. Whether the negative results in both trials were due to increased toxicity, reduced chemotherapy dosing, inactivity of cetuximab in micrometastases—or all three—is presently uncertain.
At the present time the thrust of adjuvant therapy is stage III colon cancer focuses on the duration of treatment, in part motivated by the dose-related neuropathy associated with oxaliplatin. The IDEA consortium will accumulate data from multiple trials worldwide to assess the non-inferiority of 3 months of FOLFOX compared to a standard 6 months. More than 12,000 patients will be accrued by the end of 2013, with results expected in 2014-15.
Age and adjuvant therapy
Many studies have suggested that adjuvant therapy may be less effective in the elderly, based on a number of factors including increased toxicity, reduced dosing, biologic variability in tumors and comorbidities. It is beyond the scope of this article to review completely this issue, but a recent report from the ACCENT collaboration (described elsewhere in this issue) summarized the largest database on adjuvant treatment. Individual studies and small pooled analysis have shown that patients with stage II/III colon cancer receive similar benefit from single-agent fluoropyrimidine adjuvant therapy regardless of age. The ACCENT group analyzed 11,953 patients age <70 and 2,575 age ≥70 years from seven adjuvant therapy trials comparing IV 5-FU with oral fluoropyrimidines or combinations of fluoropyrimidines with oxaliplatin or irinotecan (41). End points were DFS, OS and time to recurrence. In three studies comparing oxaliplatin-based chemotherapy with 5-FU, statistically significant interactions were not observed between treatment arm and age (P interaction=0.09 for DFS, 0.05 for OS, and 0.36 for TTR). Point estimates suggested limited benefit from the addition of oxaliplatin in elderly patients [DFS hazard ratio (HR), 0.94; 95% CI, 0.78 to 1.13; OS HR, 1.04; 95% CI, 0.85 to 1.27]. No significant interactions by age were detected with oral fluoropyrimidine therapy compared with IV FU; noninferiority was supported in both age populations.
These data suggest that decisions for adjuvant treatment should not take only age into consideration, but also comorbidities that may impact on overall survival. Many physicians consider these patients to represent the “fit” elderly, which would describe patients who may have been eligible for randomized trials. Particularly in low-risk stage III disease, consideration should be made for single-agent capecitabine, rather than observation or FOLFOX as the only treatment alternatives.
Life style and diet after stage III colon cancer
Besides surveillance with physician visits, images, tests, and colonoscopy after curative resection, there are increased interest and some evidence in modifying behaviors and lifestyle as part of the efforts to improve overall outcome.
Multiple studies have shown that obesity is associated with an increased risk of developing colon cancer; however, the influence of obesity on the prognosis of colon cancer survivors and the recurrence of stage II and III colon cancer patients remains unclear. Further discussion of the role of diet and nutrition in presented in a separate article in this issue of CCO by Dignam et al. (42-45). The analysis from CALGB 89803 showed neither BMI nor weight change was significantly associated with an increased risk of cancer recurrence and death in patients with colon cancer. On the other hand, a recently published study which evaluated the association of body mass index with clinical outcome in colon carcinoma patients who participated in seven randomized trials of 5-fluorouracil–based adjuvant chemotherapy suggested the opposite results (46). There were 20% patients considered as obese (body mass index, ≥30 kg/m2) in the study. The study indicated that they were more likely to have distal tumors, MSS, and increased lymph node metastases. Obesity was shown as an independent and adverse prognostic variable in colon cancer survivors with distinct gender- related differences—although obesity showed reduced OS compared with normal weight in both men and women, overweight status was associated with improved OS in men (P=0.006), and underweight women had significantly worse OS (P=0.019). BMI was not predictive of therapeutic benefit. These data suggest that interventions to reduce rates of obesity may improve colon cancer outcomes. Although there are no definitive conclusions that could be made from these data, such information has the potential to influence patient management decisions and surveillance strategies. Further study is needed to determine the mechanism of the adverse effect of obesity on survivors of colon cancer, such as a prospective trial of diet and exercise in post-resection patients.
Although diet and lifestyle may influence colorectal adenoma/adenocarcinoma recurrence, the role of dietary supplement use in colorectal adenoma recurrence remains controversial. A prospective cohort study examined the association between dietary supplement use (vitamin C, vitamin B, calcium, or multivitamins), total colorectal adenoma recurrence and advanced adenoma recurrence showed that dietary supplement use was neither statistically significantly associated with total colorectal adenoma recurrence (HR=1.03; 95% CI, 0.79-1.34) nor with recurrent advanced adenomas (HR=1.59; 95% CI, 0.88-2.87) (47). However, at the same time, no harmful effects were noted. To make public health policies for prevention of recurrent adenomas and colorectal cancer, future studies with extensive information on regular dietary supplement use are needed.
Even there are no definitive answers from prospective intervention trials regarding diet, obesity, and exercise with surveillance of colon cancer, we should encourage such behavior change with increasing physical activity, healthy diet, and weight management since these may influence the risk of many adverse health conditions, such as coronary heart disease, diabetes, breast and colon cancers, all leading to shortened life expectancy (48).
Post-treatment surveillance
Surveillance after potential curative resection in stage II and III colon cancer patients is considered as standard in oncologic practice, since about 30-50% of patients will develop tumor relapse as locoregional recurrence, distant metastasis, or metachronous colorectal lesions after 5 years of follow-up (49). Early detection of asymptomatic recurrences by periodic images and monitoring of carcinoembryonic antigen (CEA) levels is likely associated with an increase in the potential for curative therapy and survival benefit, which has been shown from several meta-analyses, and randomized controlled studies (50-54). Long-term survival has been demonstrated for complete resection of local regional recurrences, and metastatic liver and lung recurrences. Detection of asymptomatic metachronous colorectal lesions including cancer and polyps via scheduled colonoscopy may also lead to cure (55). However, no uniform consensus has been reached as to the appropriateness of follow-up in colorectal cancer patients, and very little agreement on the modalities that should be employed or the frequency with which they should be used, and there have been no large prospective clinical trials to demonstrate effectiveness. There are several published guidelines from national and international professional socialites including the American Society of Clinical Oncology (ASCO), the European Society of Medical Oncology (ESMO) the National Comprehensive Cancer Network (US) National Health Service (UK), and the Program in Evidence-Based Care (Cancer Care, Ontario, Canada) (Table 2) (56-60). Each of these guidelines is based on literature review and opinions and consensus from regional experts. There are important differences among these guidelines despite being based on similar evidence. At a recent ASCO meeting, a British study of CEA and/or CT surveillance suggested that both regular CEA measurement and CT scanning results in significantly higher rates of diagnosis of operable recurrent colorectal cancer compared to minimal follow up. There appeared to be no differential benefit in monitoring with both CEA and CT, and no difference in the overall mortality was demonstrated. CEA monitoring combined with a single CT scan at 12-18 months was deemed likely to be cost-effective (61).
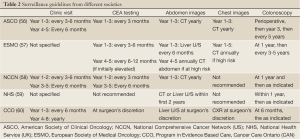
Full Table
Based on data from the ACCENT group showing later recurrence for stage II disease compared to stage III disease, some investigators believe—especially in clinical trials—that surveillance for recurrence should be extended as far as 6 years after surgery and adjuvant therapy (62).
All guidelines agree on a need for follow-up colonoscopy postoperatively to ensure the colon is clean of polyps. The joint updated guidelines by the American Cancer Society (ACS) and US Multi-Society Task Force on Colorectal Cancer recommends that: colonoscopy should be performed in one year to look for metachronous lesions. This recommendation is based on reports of a high incidence of apparently metachronous second cancers in the first 2 years after resection. If the examination at 1 year is normal, then the interval should be 3 years. If that colonoscopy is normal, then the next interval should be 5 years. Shorter intervals may be indicated by associated adenoma findings. Shorter intervals are also indicated if the patient’s age, family history, or tumor testing indicate definite or probable hereditary nonpolyposis colorectal cancer (63).
There has been increased interest in using 18FDG-PET as diagnostic imaging modality in evaluating recurrent colorectal cancer. It has been used to help select patients for hepatic resection and to evaluate patients with an elevated CEA and normal conventional imaging and colonoscopy (64,65). Although the results are interesting, it has not accepted as a routine surveillance method at this time.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Edge SB, Byrd DR, Compton CC, et al. AJCC cancer staging manual. 7th Ed. New York, NY: Springer, 2010.
- Available online: http://www.seer.cancer.gov. Accessed June 12, 2013
- Available online: http://www.nccn.org/professionals/physician_gls/f_guidelines. Accessed June 12, 2013
- McLeod HL, Murray GI. Tumour markers of prognosis in colorectal cancer. Br J Cancer 1999;79:191-203. [PubMed]
- Prolla TA. DNA mismatch repair and cancer. Curr Opin Cell Biol 1998;10:311-6. [PubMed]
- Jiricny J, Nystrom-Lahti M. Mismatch repair defects in cancer. Curr Opin Genet Dev 2000;10:157-61. [PubMed]
- Ribic CM, Sargent DJ, Moore MJ, et al. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N Engl J Med 2003;349:247-57. [PubMed]
- Popat S, Hubner R, Houlston RS. Systematic review of microsatellite instability and colorectal prognosis. J Clin Oncol 2005;23:609-18. [PubMed]
- Sargent DJ, Marsoni S, Thibodeau SN, et al. Confirmation of deficient mismatch repair (dMMR) as a predictive marker for lack of benefit from 5-FU based chemotherapy in stage II and III colon cancer (CC): a pooled molecular reanalysis of randomized chemotherapy trials. J Clin Oncol 2008;26:4008.
- Zaanan A, Cuilliere-Dartigues P, Guilloux A, et al. Impact of p53 expression and microsatellite instability on stage III colon cancer disease-free survival in patients treated by 5-fluorouracil and leucovorin with or without oxaliplatin. Ann Oncol 2010;21:772-80. [PubMed]
- Watanabe T, Wu TT, Catalano PJ, et al. Molecular predictors of survival after adjuvant chemotherapy for colon cancer. N Engl J Med 2001;344:1196-206. [PubMed]
- Ogino S, Nosho K, Irahara N, et al. Prognostic significance and molecular associations of 18q loss of heterozygosity: a cohort study of microsatellite stable colorectal cancers. J Clin Oncol 2009;27:4591-8. [PubMed]
- Bertagnolli MM, Redston M, Compton CC, et al. Microsatellite instability and loss of heterozygosity at chromosomal location 18q: prospective evaluation of biomarkers for stages II and III colon cancer–a study of CALGB 9581 and 89803. J Clin Oncol 2011;29:3153-62. [PubMed]
- Zhang B, Halder SK, Kashikar ND, et al. Antimetastatic role of Smad4 signaling in colorectal cancer. Gastroenterology 2010;138:969-80.e1-3.
- Roth AD, Delorenzi M, Tejpar S, et al. Integrated analysis of molecular and clinical prognostic factors in stage II/III colon cancer. J Natl Cancer Inst 2012;104:1635-46. [PubMed]
- Sträter J, Herter I, Merkel G, et al. Expression and prognostic significance of APAF-1, caspase-8 and caspase-9 in stage II/III colon carcinoma: caspase-8 and caspase-9 is associated with poor prognosis. Int J Cancer 2010;127:873-80. [PubMed]
- Salazar R, Roepman P, Capella G, et al. Gene expression signature to improve prognosis prediction of stage II and III colorectal cancer. J Clin Oncol 2011;29:17-24. [PubMed]
- O’Connell MJ, Lavery I, Yothers G, et al. Relationship between tumor gene expression and recurrence in four independent studies of patients with stage II/III colon cancer treated with surgery alone or surgery plus adjuvant fluorouracil plus leucovorin. J Clin Oncol 2010;28:3937-44. [PubMed]
- Kennedy RD, Bylesjo M, Kerr P, et al. Development and independent validation of a prognostic assay for stage II colon cancer using formalin-fixed paraffin-embedded tissue. J Clin Oncol 2011;29:4620-6. [PubMed]
- Van Laar RK. An online gene expression assay for determining adjuvant therapy eligibility in patients with stage 2 or 3 colon cancer. Br J Cancer 2010;103:1852-7. [PubMed]
- Srivastrava G, Renfro LA, Behrens RJ, et al. Prospective evaluation of a 12-gene assay on treatment recommendations in stage II colon cancer patients. 2013 Gastrointestinal Cancers Symposium. J Clin Oncol 2012;30:abstr 453.
- O’Connell MJ, Lee M, Lopatin M, et al. Validation of the 12-gene colon cancer recurrence score (RS) in NSABP C07 as a predictor of recurrence in stage II and III colon cancer patients treated with 5FU/LV (FU) and 5FU/LV+oxaliplatin (FU+Ox). J Clin Oncol 2012;30:abstr 3512.
- Moertel CG, Fleming TR, Macdonald JS, et al. Fluorouracil plus levamisole as effective adjuvant therapy after resection of stage III colon carcinoma: a final report. Ann Intern Med 1995;122:321-6. [PubMed]
- Haller DG, Catalano PJ, Macdonald JS, et al. Phase III study of fluorouracil, leucovorin, and levamisole in high-risk stage II and III colon cancer: final report of Intergroup 0089. J Clin Oncol 2005;23:8671-8. [PubMed]
- O’Connell MJ, Laurie JA, Kahn M, et al. Prospectively randomized trial of postoperative adjuvant chemotherapy in patients with high-risk colon cancer. J Clin Oncol 1998;16:295-300. [PubMed]
- Wolmark N, Rockette H, Mamounas E, et al. Clinical trial to assess the relative efficacy of fluorouracil and leucovorin, fluorouracil and levamisole, and fluorouracil, leucovorin, and levamisole in patients with Dukes’ B and C carcinoma of the colon: results from National Surgical Adjuvant Breast and Bowel Project C-04. J Clin Oncol 1999;17:3553-9. [PubMed]
- Twelves C, Wong A, Nowacki MP, et al. Capecitabine as adjuvant treatment for stage III colon cancer. N Engl J Med 2005;352:2696-704. [PubMed]
- André T, Boni C, Mounedji-Boudiaf L, et al. Multicenter international study of oxaliplatin/5-fluorouracil/leucovorin in the adjuvant treatment of colon cancer (MOSAIC) investigators. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med 2004;350:2343-51. [PubMed]
- André T, Boni C, Navarro M, Tabernero J, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol 2009;27:3109-16. [PubMed]
- Tournigand C, André T, Bonnetain F, et al. Adjuvant therapy with fluorouracil and oxaliplatin in stage II and elderly patients (between ages 70 and 75 years) with colon cancer: subgroup analyses of the Multicenter International Study of Oxaliplatin, Fluorouracil, and Leucovorin in the Adjuvant Treatment of Colon Cancer trial. J Clin Oncol 2012;30:3353-60. [PubMed]
- Kuebler JP, Wieand HS, O’Connell MJ, et al. Oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: results from NSABP C-07. J Clin Oncol 2007;25:2198-204. [PubMed]
- Haller DG, Tabernero J, Maroun J, et al. Capecitabine plus oxaliplatin compared with fluorouracil and folinic acid as adjuvant therapy for stage III colon cancer. J Clin Oncol 2011;29:1465-71. [PubMed]
- Saltz LB, Niedzwiecki D, Hollis D, et al. Irinotecan fluorouracil plus leucovorin is not superior to fluorouracil plus leucovorin alone as adjuvant treatment for stage III colon cancer: results of CALGB 89803. J Clin Oncol 2007;25:3456-61. [PubMed]
- Goldberg RM, Sargent DJ, Morton RF, et al. Randomized controlled trial of reduced-dose bolus fluorouracil plus leucovorin and irinotecan or infused fluorouracil plus leucovorin and oxaliplatin in patients with previously untreated metastatic colorectal cancer: a North American Intergroup Trial. J Clin Oncol 2006;24:3347-53. [PubMed]
- Van Cutsem E, Labianca R, Bodoky G, et al. Randomized phase III trial comparing biweekly infusional fluorouracil/leucovorin alone or with irinotecan in the adjuvant treatment of stage III colon cancer: PETACC-3. J Clin Oncol 2009;27:3117-25. [PubMed]
- Saltz LB, Clarke S, Díaz-Rubio E, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol 2008;26:2013-9. [PubMed]
- Allegra CJ, Yothers G, O’Connell MJ, et al. Bevacizumab in stage II-III colon cancer: 5-year update of the National Surgical Adjuvant Breast and Bowel Project C-08 trial. J Clin Oncol 2013;31:359-64. [PubMed]
- de Gramont A, Van Cutsem E, Schmoll HJ, et al. Bevacizumab plus oxaliplatin-based chemotherapy as adjuvant treatment for colon cancer (AVANT): a phase 3 randomised controlled trial. Lancet Oncol 2012;13:1225-33. [PubMed]
- Alberts SR, Sargent DJ, Nair S, et al. Effect of oxaliplatin, fluorouracil, and leucovorin with or without cetuximab on survival among patients with resected stage III colon cancer: a randomized trial. JAMA 2012;307:1383-93. [PubMed]
- Taïeb J, Tabernero J, Mini E, et al. Subgroup analyses results of the PETACC8 phase III trial comparing adjuvant FOLFOX4 with or without cetuximab (CTX) in resected stage III colon cancer (CC). J Clin Oncol 2013;31:abstr 3525.
- McCleary NJ, Meyerhardt J, Green E, et al. Impact of Age on the Efficacy of Newer Adjuvant Therapies in Patients With Stage II/III Colon Cancer: Findings From the ACCENT Database. J Clin Oncol 2013; [PubMed]
- Pischon T, Lahmann PH, Boeing H, et al. Body size and risk of colon and rectal cancer in the European Prospective Investigation Into Cancer and Nutrition (EPIC). J Natl Cancer Inst 2006;98:920-31. [PubMed]
- Calle EE, Rodriguez C, Walker-Thurmond K, et al. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U S. adults. N Engl J Med 2003;348:1625-38. [PubMed]
- Dignam JJ, Polite BN, Yothers G, et al. Body mass index and outcomes in patients who receive adjuvant chemotherapy for colon cancer. J Natl Cancer Inst 2006;98:1647-54. [PubMed]
- Meyerhardt JA, Niedzwiecki D, Hollis D, et al. Impact of body mass index and weight change after treatment on cancer recurrence and survival in patients with stage III colon cancer: findings from Cancer and Leukemia Group B 89803. J Clin Oncol 2008;26:4109-15. [PubMed]
- Sinicrope FA, Foster NR, Sargent DJ, et al. Obesity is an independent prognostic variable in colon cancer survivors. Clin Cancer Res 2010;16:1884-93. [PubMed]
- Heine-Bröring RC, Winkels RM, Botma A, et al. Dietary supplement use is not associated with recurrence of colorectal adenomas: a prospective cohort study. Int J Cancer 2013;132:666-75. [PubMed]
- Ho JW, Lee AM, Macfarlane DJ, et al. Study protocol for “Moving Bright, Eating Smart”-- A phase 2 clinical trial on the acceptability and feasibility of a diet and physical activity intervention to prevent recurrence in colorectal cancer survivors. BMC Public Health 2013;13:487. [PubMed]
- Safi F, Beyer HG. The value of follow-up after curative surgery of colorectal carcinoma. Cancer Detect Prev 1993;17:417-24. [PubMed]
- Jeffery GM, Hickey BE, Hider P. Follow-up strategies for patients treated for non-metastatic colorectal cancer. Cochrane Database Syst Rev 2002;(1):CD002200. [PubMed]
- Renehan AG, Egger M, Saunders MP, et al. Impact on survival of intensive follow up after curative resection for colorectal cancer: systematic review and meta-analysis of randomised trials. BMJ 2002;324:813. [PubMed]
- Figueredo A, Rumble RB, Maroun J, et al. Follow-up of patients with curatively resected colorectal cancer: a practice guideline. BMC Cancer 2003;3:26. [PubMed]
- Grossmann EM, Johnson FE, Virgo KS, et al. Follow-up of colorectal cancer patients after resection with curative intent-the GILDA trial. Surg Oncol 2004;13:119-24. [PubMed]
- Rodríguez-Moranta F, Saló J, Arcusa A, et al. Postoperative surveillance in patients with colorectal cancer who have undergone curative resection: a prospective, multicenter, randomized, controlled trial. J Clin Oncol 2006;24:386-93. [PubMed]
- Winawer SJ, Zauber AG, O’Brien MJ, et al. The National Polyp Study Workgroup. Randomized comparison of surveillance intervals after colonoscopic removal of newly diagnosed adenomatous polyps. N Engl J Med 1993;328:901-6. [PubMed]
- Desch CE, Benson AB III, Somerfield MR, et al. American Society of Clinical Oncology. Colorectal cancer surveillance: 2005 update of an American Society of Clinical Oncology practice guideline. J Clin Oncol 2005;23:8512-9. [PubMed]
- Van Cutsem EJ, Oliveira J, ESMO Guidelines Working Group. Colon cancer: ESMO clinical recommendations for diagnosis, adjuvant treatment and follow-up. Ann Oncol 2008;19 Suppl 2:ii29-30. [PubMed]
- Available online: http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#colon. Accessed June 12, 2013.
- Scholefield JH, Steele RJ, British Society For Gastroenterology, et al. Guidelines for follow up after resection of colorectal cancer. Gut 2002;51:V3-5. [PubMed]
- Figueredo A, Rumble RB, Maroun J, et al. Gastrointestinal Cancer Disease Site Group of Cancer Care Ontario’s Program in Evidence-based Care. Follow-up of patients with curatively resected colorectal cancer: a practice guideline. BMC Cancer 2003;3:26. [PubMed]
- David Mant, Rafael Perera, Alastair Gray, et al. Effect of 3-5 years of scheduled CEA and CT follow-up to detect recurrence of colorectal cancer: FACS randomized controlled trial. J Clin Oncol 2013;31:abstr 3500.
- Sargent D, Shi Q, Yothers G, et al. Two or three year disease-free survival (DFS) as a primary end-point in stage III adjuvant colon cancer trials with fluoropyrimidines with or without oxaliplatin or irinotecan: data from 12,676 patients from MOSAIC, X-ACT, PETACC-3, C-06, C-07 and C89803. Eur J Cancer 2011;47:990-6. [PubMed]
- Rex DK, Kahi CJ, Levin B, et al. Guidelines for Colonoscopy Surveillance after Cancer Resection: A Consensus Update by the American Cancer Society and US Multi-Society Task Force on Colorectal Cancer. CA Cancer J Clin 2006;56:160-7. [PubMed]
- Fong Y, Saldinger PF, Akhurst T, et al. Utility of 18F-FDG positron emission tomography scanning on selection of patients for resection of hepatic colorectal metastases. Am J Surg 1999;178:282-7. [PubMed]
- Shen YY, Liang JA, Chen YK, et al. Clinical impact of 18F-FDG-PET in the suspicion of recurrent colorectal cancer based on asymptomatically elevated serum level of carcinoembryonic antigen (CEA) in Taiwan. Hepatogastroenterology 2006;53:348-50. [PubMed]



