
Gentle skin care guidelines for patients with mycosis fungoides
Gentle skin care is an umbrella term used to describe protective and non-interventional skin care regimens for patients to adhere to within the confines of their own home. Simple recommendations such as cleanser/moisturizer choices and shower habits are mainstays of gentle skin care regimens. These non-pharmacologic interventions are oftentimes the primary treatment modality for many dermatologic conditions and can improve outcome and symptoms before instituting an allopathic treatment. It has also been shown to be paramount treating individuals with a compromised cutaneous barrier. The layer of the skin targeted by interventions is the epidermis. The epidermis is the outermost layer of skin and the layer that interacts with the environment. The epidermis itself is composed of separate layers (from inferior to superior): the stratum basale, the stratum spinosum, the stratum granulosum, the stratum lucidum (in acral locations), and on top, the stratum corneum (SC) (1). Within the epidermis, the SC is the layer most essential to preserve and protect.
The SC is a dynamic epidermal layer where fully keratinized corneocytes (keratinocytes that underwent apoptosis as they migrated to the upper surface) function in conjunction with an enveloping lipid layer and natural moisturizing factor (NMF) (2,3). The lipid layer is composed of ceramides, cholesterol, fatty acids, and other lipids (4-6). Its function is to prevent desiccation and act as a physical barrier to the harsh environment outside the human body (2). The layer of hydrophobic molecules prevents transcutaneous water loss and entrance. NMF is a combination of multiple humectants (water-attracting elements) and comprises 15–20% of the SC (7-9). It is responsible for water retention and solvent supply for SC enzymatic activity (3). Although the SC does not have cellular life, it is of utmost importance for the health and maintenance of the entire epidermis.
Mycosis fungoides is the most common form of cutaneous T-cell lymphoma within the general population. Lesions are most commonly observed as erythematous patches and plaques with an overlying “cigarette-paper” scale (10). Scale is seen under microscopy as actively shedding SC. The inflammation and compromised SC leave an exposed and unprotected epidermis, rendering it more susceptible to infection. Infection has been shown to function as a catalyst for further disease progression in mycosis fungoides, due to further activation of the defective immune system (11). By nourishing and strengthening the SC via a prescribed gentle skin care regimen, an individual can maintain their primary barrier against external infection and prevent disease progression.
When a patient presents to the Jefferson Cutaneous Lymphoma Center, gentle skin care is an essential topic of conversation as it is a primary treatment modality for patients. The gentle skin care regimen is discussed with each patient on his or her primary visit and each patient is provided with a printed summary of the recommendations at the end of their visit. The discussion with patients often begins with a detailed question and answer session to provide information regarding their established skin care regimens at home and identify target areas that need to be modified. Table 1 provides questions that are commonly used to have a conversation with patients about their skin care.
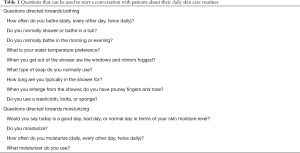
Full table
Bathing habits are most commonly the first topic discussed, as this is where many patients need adjustment to their daily routine. When discussing showering versus bathing in a tub, showering is always endorsed, as it is less drying to skin than bathing in a tub. This is because bathing increases cutaneous water exposure, thus increasing skin pH and decreasing cutaneous oil availability. These are discussed later in the article.
Before a patient enters the shower, he or she is instructed to massage his or her entire body (with the option of his or her scalp hair) with a thin layer of oil. Patients usually choose between coconut oil or baby oil. This thin layer of oil functions as a protective layer of exogenous oil that can rinse off in the shower while maintaining the patient’s natural oils. Inherent properties within the oil also prove to be invaluable when choosing which oil to apply. For example, coconut oil is most often recommended over all other oil options. Coconut oil contains lauric acid, which is approximately 50% of its overall fatty-acid content (12). This fatty acid has been shown in numerous studies to have antibacterial properties with functioning against both vegetative bacteria and those enveloped in pre-formed biofilm. Shilling et al. showed in their study that lipolyzed virgin coconut oil at a concentration of 1.2% inhibited 99.9% of C. difficile growth. When the concentration was reduced down to 0.15%, there was still an inhibition of growth by approx. 50%. Lauric acid, when isolated, was able to show inhibition of bacterial growth by approx. 100% (13). This data was then furthered by Yang et al. who were able to experimentally determine a minimum bactericidal concentration (MBC) of lauric acid, calculating it to be 0.3125 mg/mL. Using this number, they found that 0.25× MBC of lauric acid reduced biofilm production by 24.9 times and 47.2 times compared to controls for C. difficile strains R20291 and 630, respectively. They were also able to show that strains R20291 and 630’s total biofilm mass decreased when exposed to lauric acid at 2× MBC and 1× MBC, respectively. This experimentation then went further to propose reactive oxygen species formation with subsequent cell membrane damage as a mechanism of action (14). Coconut oil has also been shown to experimentally expedite the healing process of burns in rats. It was shown that coconut oil increased the rate of wound contraction 4 days prior to silver sulphadiazine (SSD) and SSD in combination with coconut oil. They theorized it to have anti-inflammatory properties due to its ability to hasten the healing process of burns, although how it does this was not elucidated (15). Palm kernel oil is another example of an oil high in lauric acid (16).
Olive oil use is discouraged. This is based on a study that found that daily application of olive oil caused erythema and SC discohesion in healthy skin (17). Olive oil contains many fatty acids, with the most prominent being oleic acid (OA). OA makes up between 67.9% and 82.2%, with variation based on year of harvest and olive type (18). OA is directly involved in the negative consequences that olive oil can produce. Schliemann-Willers et al. showed that palm oil containing the highest concentration of OA was found to induce the largest amount of transepidermal water loss (TEWL) (19). Jiang et al. discussed that water loss was most notable as destruction of the SC occurred, seconding the observation. They then demonstrated the relationship between OA and the induction of corneocyte separation and eventual sloughing. This was further seen upon electron microscopy, which revealed disruption of the corneocyte membrane lipid bilayer and the appearance of large gaps between cells in the SC (20). The mechanism of the SC disruption was investigated by Katsuta et al. They reported that when an NMDA receptor antagonist was applied in combination with OA, there was no increase in TEWL. Because of this study, it can be surmised that there is a direct relationship between epidermal NMDA receptors, OA, and overall skin health (21). The implications of this loss of primary barrier in an individual with a compromised cutaneous immune system can be immense. When counseling patients regarding their pre-shower oil application an appropriate amount should be emphasized. Oil should be massaged in well to avoid drips. Mats or other protective measures should be used to avoid falls caused from oils rinsing off the body.
After applying the protective layer of oil, the patient is then instructed to take showers no more than 5 minutes in duration. Additionally, the temperature of the water should be luke-warm. Hot water is extremely desiccating for the skin, so we advise patient avoidance of this temperature. Many patients report that they enjoy hot water during initial questioning, and that they fear it will be a difficult part of their routine to change. In this circumstance, we advise the patient to steam up the bathroom prior to showering, thus increasing ambient air temperature and minimizing perception of cooler water temperature. We suggest a duration of five minutes for showering because this reduces the amount of water a patient will be exposed to. While seemingly counterintuitive, the more water skin is exposed to, the more dehydrated it becomes. This is because as the lipid barrier is stripped, TEWL increases (22). As stated above, maintaining hydration in the epidermis is of utmost importance to skin protection.
During the allotted shower time, she or he is instructed to wash only particular areas of their body, as applying any type of soap will increase the likelihood of dehydration and irritation of the skin. We advise washing the axillae, genitals, perineum, perianal region, and feet using a pH balanced, moisturizing, hypoallergenic, and fragrance-free synthetic detergent (syndet). The permitted areas contain a high concentration of apocrine sweat glands; so, cleaning them allows the patient to minimize any malodor due to the new skin care regimen (23). Most patients insist on washing their face as well, which is permissible to increase compliance to the regimen. While washing, it is important to avoid scrubbing or exfoliating the skin as this can strip the natural oils and resident flora within the epidermis, leading to irritation and infection, as well as traumatizing and disrupting the skin barrier, allowing bacteria entry. Usage of loofas, sponges, exfoliants, and washcloths is not advised because of their rough surfaces and their potential to harbor pathogenic bacteria.
Using pH balanced cleansers and limiting the duration of daily showers is of utmost importance to patients diagnosed with mycosis fungoides. According to various studies, the natural pH of our skin is acidic and ranges between 4.0 to 5.6 (24-26). Over the counter cleansers are oftentimes alkaline, ranging in pH from 8 to 11. Use of these alkaline cleansers, as well as increased contact of the skin with water has the potential to alter the natural pH of our skin both short term and long term (25). Additionally, alkaline cleansers cause dissolution of the fat from the skin surface, effectively stripping the skin of its natural oils and leading to dehydration of the epidermal surface (27).
Resident skin microflora function at an optimum pH, providing what is commonly described as an “acid mantle” protecting the skin from infection and irritation. In a study by Grice et al, natural skin flora was analyzed by 16S ribosomal RNA and four main phyla were found to be the most common natural skin colonizers: Actinobacteria, Firmicutes, Bacteroidetes and Proteobacteria. Within the Actinobacteria phyla Corynebacterium species Propionibacterium species were the most common and within the Firmicutes phyla Staphylococcus species were the most common. Propionibacterium species dominate sebaceous regions while Staphylococcus and Corynebacterium species dominate moist areas such as the axilla (28). These microorganisms play a vital role within the skin, serving to help protect from the outside environment and even may aid in educating resident T-cells to help provide immunity against similar pathogenic organisms (29). For instance, Propionibacterium acnes helps contribute to the acidic pH of the skin by producing propionic acid (30). In vivo studies demonstrate that when the pH of the skin is disturbed and becomes more basic, the growth of pathogenic bacterial species such as Staphylococcus aureus and Streptococcus pyogenes flourish (31). Furthermore, mycosis fungoides patients are at an increased risk of Staphylococcus aureus infections due to their compromised immune systems, making this particular patient population increasingly sensitive to shifts in pH, and therefore at increased risk of infection (32). In summary, the use of non-balanced pH cleansers and the extended use of water for cleansing (pH 7) influences the natural pH of our skin barrier, and must be avoided during patient care in order to minimize disease progression and increase in symptom severity (pain, itch).
After exiting the shower, the patient will dry himself or herself off via light dabbing. This method is less harsh than rubbing the skin and allows the skin to maintain moisture from the shower immediately following drying. While the skin is still damp, patients are instructed to apply moisturizer onto their damp skin. Often, patients will report that they use a specific moisturizer at home, but a recommended list of ointments and creams are provided (Table 2) as many over the counter moisturizing lotions contain water as their first ingredient, evaporating quickly and dehydrating the skin.

Full table
Distinct classes of moisturizers contain differing concentrations of humectants, emollients and emulsifiers. Humectants allow for synthetic replacement of barrier losses of NMF, decreasing dehydration by increasing water holding capacity of the skin, while emollients replace the principle ceramides found in the SC (cholesterol, phospholipids, and glucosylceramides) (33,34). Emulsifiers stabilize the hydrophilic and lipophilic components of the moisturizer. Ointments are a class of moisturizers that act as an occlusive, containing the highest amount of emollients and humectants and the least amount of water (usually 80% oil and 20% water). Creams contain 50% water and 50% oil, while lotions contain a high percentage of water and low percentage of fats. Ointments are the most effective of the three with regard to replacing the natural lipid layer of the skin and preventing dehydration. Studies have demonstrated approximately 50% reduction in TEWL after application of petrolatum ointment (35). Despite ointments being the most effective, most patients refuse to use it on their entire body as it is heavy, greasy and takes a long time to dry. We advise spot-treating lesions with ointment and using oil-based cream moisturizers on the rest of the body. Whole body moisturizing is suggested twice daily, approximately spaced 12 hours apart.
Special considerations must be taken for concomitant moisturizing with specific treatment regimens. For example, while using topical steroids, we advise to place the topical steroids to lesions on damp skin immediately after showering, and subsequently placing a layer of moisturizer over the topical steroid while moisturizing the rest of the body as this increases the absorption of the therapy by sealing it in. For topical chemotherapy (mechlorethamine gel), the drug insert instructs moisturizing two hours before or after application as water can deactivate therapeutic compounds within the gel (36). Finally, the last major topical treatment class used for MF is bexarotene, a rexinoid. We normally suggest to patients that they moisturize their bodies about five to ten minutes after they spot treat themselves with bexarotene. The drug-insert instructs patients to wait 20 minutes following bathing before applying medication and to not bathe for at least three hours after application (37). To avoid increased absorption and subsequent irritation and side-effects, we advise patients to apply mechlorethamine or bexarotene at the opposite time as bathing (if they bathe in the morning, they put their medicine on in the evening prior to bed). This allows the product to dry completely and prevents the medication from spreading to non-lesional sites. It is important for patients to develop a routine for bathing and application their topical treatment and moisturizer. Adherence to moisturizing regimens allows for further protection of the skin improving symptoms of pain and pruritus in many patients.
Lastly, measures must be taken for patients to identify and eliminate potential irritants. Patients are instructed to avoid fabric softeners and harsh perfumed detergents as many contain chemicals such as sodium lauryl sulfate, sodium dodecyl sulfate, and fragrance. All of these have been implicated as skin irritants (38). Minimally irritating brands that we suggest to our patients are all® Sensitive and Tide® Free and Gentle. Additionally, patients are told to keep rooms cooler at temperatures between 64 to 68 °F (17.8 to 20 °C). Cool mist humidifiers have also demonstrated importance as they prevent the drying of skin by increasing ambient air humidity. Lastly, harsh fabrics such as wool are to be avoided. Lanolin, the sebaceous secretions of the sheep that permeate wool, is a known allergen that can cause contact dermatitis. In a meta-analysis performed Lee et al., the percentage of patch test results positive for lanolin allergy in the general Dermatology population ranged from 0.2–7.4%. The percentage of patch test results positive for lanolin allergy was also determined for high-risk dermatology patients, with the results reaching as high as 30.6% of patients with leg ulcers (39). Zallmann et al. further discuss how the diameter of the fibers with a force of 75 mG cause activation of C-neuronal fibers, leading towards to initiation of pruritus (40). The itching to alleviate this sensation can lead to further inflammation and an increased risk of infection. 100% cotton clothing and sheets for his or her bed are recommended.
Many patients ask about their risk of progression and how they can best prevent it. Gentle skin care is often the first and most important topic discussed. It provides a way for patients to feel empowered to better their skin’s health. The techniques and suggestions for gentle skin care presented to patients requires dedication and motivation, but functions as a non-medicinal treatment modality that offers patients an opportunity to improve their own care outside of a physician’s office.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Yousef H, Sharma S. Anatomy, Skin (Integument), Epidermis. StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing, 2018.
- Haftek M. ‘Memory’ of the stratum corneum: exploration of the epidermis’ past. Br J Dermatol 2014;171:6-9. [Crossref] [PubMed]
- Choe C, Schleusener J, Lademann J, et al. Age related depth profiles of human Stratum Corneum barrier-related molecular parameters by confocal Raman microscopy in vivo. Mech Ageing Dev 2018;172:6-12. [Crossref] [PubMed]
- Gray GM, White RJ. Glycosphingolipids and ceramides in human and pig epidermis. J Invest Dermatol 1978;70:336-41. [Crossref] [PubMed]
- Gray GM, Yardley HJ. Lipid compositions of cells isolated from pig, human, and rat epidermis. J Lipid Res 1975;16:434-40. [PubMed]
- Elias PM, Brown BE, Fritsch P, et al. Localization and composition of lipids in neonatal mouse stratum granulosum and stratum corneum. J Invest Dermatol 1979;73:339-48. [Crossref] [PubMed]
- O'Regan GM, Kemperman PM, Sandilands A, et al. Raman profiles of the stratum corneum define 3 filaggrin genotype-determined atopic dermatitis endophenotypes. J Allergy Clin Immunol 2010;126:574-80.e1. [Crossref] [PubMed]
- Verdier‐Sévrain S, Bonté F. Skin hydration: a review on its molecular mechanisms. J Cosmet Dermatol 2007;6:75-82. [Crossref] [PubMed]
- Laden K. Moisturizing agent in skin. J Soc Cosmet Chem 1967;18:360.
- Pulitzer M. Cutaneous T-cell Lymphoma. Clin Lab Med 2017;37:527-46. [Crossref] [PubMed]
- Lebas E, Arrese JE, Nikkels AF. Risk factors for skin infections in mycosis fungoides. Dermatology 2016;232:731-7. [Crossref] [PubMed]
- McCarty MF, DiNicolantonio JJ. Lauric acid-rich medium-chain triglycerides can substitute for other oils in cooking applications and may have limited pathogenicity. Open Heart 2016;3:e000467. [Crossref] [PubMed]
- Shilling M, Matt L, Rubin E, et al. Antimicrobial effects of virgin coconut oil and its medium-chain fatty acids on Clostridium difficile. J Med Food 2013;16:1079-85. [Crossref] [PubMed]
- Yang HT, Chen JW, Rathod J, et al. Lauric Acid Is an Inhibitor of Clostridium difficile Growth in Vitro and Reduces Inflammation in a Mouse Infection Model. Front Microbiol 2018;8:2635. [Crossref] [PubMed]
- Srivastava P, Durgaprasad S. Burn wound healing property of Cocos nucifera: An appraisal. Indian J Pharmacol 2008;40:144. [Crossref] [PubMed]
- Ubgogu OC, Onyeagba RA, Chigbu OA. Lauric acid content and inhibitory effect of palm kernel oil on two bacterial isolates and Candida albicans. Afr J Biotechnol 2006;5:1045-7.
- Danby SG, AlEnezi T, Sultan A, et al. Effect of olive and sunflower seed oil on the adult skin barrier: implications for neonatal skin care. Pediatr Dermatol 2013;30:42-50. [Crossref] [PubMed]
- Fuentes E, Paucar F, Tapia F, et al. Effect of the composition of extra virgin olive oils on the differentiation and antioxidant capacities of twelve monovarietals. Food Chem 2018;243:285-94. [Crossref] [PubMed]
- Schliemann-Willers S, Wigger-Alberti W, Kleesz P, et al. Natural vegetable fats in the prevention of irritant contact dermatitis. Contact Dermatitis 2002;46:6-12. [Crossref] [PubMed]
- Jiang SJ, Zhou XJ. Examination of the mechanism of oleic acid-induced percutaneous penetration enhancement: an ultrastructural study. Biol Pharm Bull 2003;26:66-8. [Crossref] [PubMed]
- Katsuta Y, Iida T, Hasegawa K, et al. Function of oleic acid on epidermal barrier and calcium influx into keratinocytes is associated with N‐methyl D‐aspartate‐type glutamate receptors. Br J Dermatol 2009;160:69-74. [Crossref] [PubMed]
- Nolan K, Marmur E. Moisturizers: reality and the skin benefits. Dermatol Ther 2012;25:229-33. [Crossref] [PubMed]
- De Fontaine S, Van Geertruyden J, Vandeweyer E. Apocrine hidrocystoma of the finger. J Hand Surg Br 1998;23:281-2. [Crossref] [PubMed]
- Blank IH. Measurement of pH of the Skin Surface: III. Measurements on the Arms of Children with No Apparent Skin Lesions. J Invest Dermatol 1939;2:231-4. [Crossref]
- Korting HC, Kober M, Mueller M. Influence of Repeated Washings with Soap and Synthetic Detergents on pH and Resident. Acta Derm Venereol 1987;67:41-7. [PubMed]
- Zlotogorski A. Distribution of skin surface pH on the forehead and cheek of adults. Arch Dermatol Res 1987;279:398-401. [Crossref] [PubMed]
- Gfatter R, Hackl P, Braun F. Effects of soap and detergents on skin surface pH, stratum corneum hydration and fat content in infants. Dermatology 1997;195:258-62. [Crossref] [PubMed]
- Grice EA, Segre JA. The skin microbiome. Nat Rev Microbiol 2011;9:244. [Crossref] [PubMed]
- Chen YE, Tsao H. The skin microbiome: current perspectives and future challenges. J Am Acad Dermatol 2013;69:143-55. [Crossref] [PubMed]
- Roth RR, James WD. Microbial ecology of the skin. Annu Rev Microbiol 1988;42:441-64. [Crossref] [PubMed]
- Schmid MH, Korting HC. The concept of the acid mantle of the skin: its relevance for the choice of skin cleansers. Dermatology 1995;191:276-80. [Crossref] [PubMed]
- Talpur R, Bassett R, Duvic M. Prevalence and treatment of Staphylococcus aureus colonization in patients with mycosis fungoides and Sézary syndrome. Br J Dermatol 2008;159:105-12. [Crossref] [PubMed]
- Lodén M. Role of topical emollients and moisturizers in the treatment of dry skin barrier disorders. Am J Clin Dermatol 2003;4:771-88. [Crossref] [PubMed]
- Sethi A, Kaur T, Malhotra SK, et al. Moisturizers: The Slippery Road. Indian J Dermatol 2016;61:279-87. [Crossref] [PubMed]
- Lodén M. The increase in skin hydration after application of emollients with different amounts of lipids. Acta Derm Venereol 1992;72:327-30. [PubMed]
- Valchlor [package insert]. Malvern, PA: Ceptaris Therapeutics, 2013. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/202317lbl.pdf
- Targretin [package insert]. San Diego, CA: Ligand Pharmaceuticals Incorporated. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2000/21056lbl.pdf
- Austoria AJ, Lakshmi C, Srinivas CR, et al. Irritancy potential of 17 detergents used commonly by the Indian household. Indian J Dermatol Venereol Leprol 2010;76:249-53. [Crossref] [PubMed]
- Lee B, Warshaw E. Lanolin allergy: history, epidemiology, responsible allergens, and management. Dermatitis 2008;19:63-72. [PubMed]
- Zallmann M, Smith PK, Tang MLK, et al. Debunking the Myth of Wool Allergy: Reviewing the Evidence for Immune and Non-immune Cutaneous Reactions. Acta Derm Venereol 2017;97:906-15. [Crossref] [PubMed]


