
Total skin electron beam therapy in mycosis fungoides—a shift towards lower dose?
Introduction
Cutaneous T-cell lymphoma (CTCL) is a heterogenous group of rare non-Hodgkin lymphoma (NHL) characterized by uncontrolled clonal proliferation of malignant T-lymphocytes in the skin (1,2). Mycosis fungoides (MF) is the most common CTCL subset, which combined with the more advanced Sézary syndrome (SS) accounts for >60% of all CTCL cases. A recent Surveillance, Epidemiology and End Results analysis demonstrated an annual incidence rate of MF of about 5.6 per million persons, which has stabilized since 1995 (3). The median age at diagnosis is 55–60 years with incidence increasing with advancing age. Males and African Americans are more commonly affected.
Patients with early-stage disease present with limited patches and plaques suspicious only to the experienced physician, while late stage MF is characterized by severe disease including tumors, ulceration, systemic involvement and death. Stage at diagnosis, including lymphatic and extracutaneous disease involvement, are important prognostic factors for disease progression (4), and have been incorporated in the staging of MF/SS (5). Most patients present with early-stage disease and thus have a good prognosis for long-term survival. Nonetheless, MF is considered to be an incurable disease requiring lifelong treatment.
Skin-directed therapies, such as topical steroids, chemotherapy, retinoids, imiquimod phototherapy, photochemotherapy (PUVA) and radiotherapy (RT) (6-10), are the recommended first-line options for stage IA–III MF and most patients can look forward to a normal life expectancy. RT, in particular total skin electron beam therapy (TSEBT), has a long history in the treatment of MF and is considered to be the most effective single modality treatment for MF (11). Lately, low-dose (ld) TSEBT regimens (in the range of 10–12 Gy) have been gaining traction due improved toxicity profile, shorter treatment length, and multiple applications in relapsed disease (12).
This review of TSEBT highlights techniques, clinical outcomes, evolution of dose (high to low), and toxicities, in the treatment of MF/SS. In addition, future directions are discussed highlighting the potential of ld-TSEBT as part of a promising therapeutic approach.
RT in CTCL
RT was first employed in the early 1900s (13) and TSEBT has been used to treat CTCL since 1951 (14,15). The neoplastic T-cells in MF are extremely radiosensitive with high levels of response seen even with low doses of RT (16,17). In addition, RT has the advantage of simultaneously treating large extents of disease while penetrating deeper skin layers. Both photons and electrons can be used in MF; however, electrons are particularly effective due to their short, well-defined range, thus optimizing dose delivery to the skin surface, as well as a more rapid dose falloff that limits RT exposure to deeper, healthy tissues.
When a significant amount of body surface is involved with disease such that the entire skin surface requires irradiation, TSEBT is employed. TSEBT is a technically challenging RT technique requiring significant physics and dosimetry support as well as special commissioning of a linear accelerator. Accordingly, TSEBT is typically offered only at large institutions or facilities with a large population of MF patients. The objective of TSEBT is to provide a relatively homogenous RT dose to the entire skin while limiting toxicities (18). Acute adverse effects are normally limited to the skin, hair, and nails with minimal serious long-term complications (19,20). To minimize toxicity, external and internal eye shields (21) are used with further consideration of lips, fingernail, scalp, and testes shielding.
TSEBT can be administered using large electron field techniques (i.e., the Stanford technique) (15), rotational techniques (22,23), or techniques where the patient is shifted during irradiation (24). Currently, the Stanford technique is the most commonly employed TSEBT method. Conventional-dose (cd) TSEBT consists of 30–36 Gy delivered over a period of 8–10 weeks. During irradiation, patients stand in an upright position on a static base. Electron beams with 6–9 MeV energy are usually used (depending on the depth of skin infiltration) to treat 3 anterior and posterior treatment positions each (Figure 1), with both superior and inferior beam angulations. Two treatment cycles are delivered per week, with one cycle consisting of irradiating anteroposterior and 2 posterior oblique fields on day 1 followed by posteroanterior and 2 anterior oblique fields on day 2 (18). A boost is often given to areas that may be “underdosed” including the perineum, plantar surfaces, medial thigh, inframammary fold, behind pannus, and/or scalp (25).
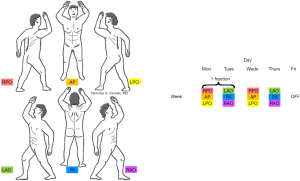
TSEBT dose determination
Both the European Organization for the Research and Treatment of Cancer (EORTC)/International Society for Cutaneous Lymphoma (ISCL) (26) and National Comprehensive Cancer Network (NCCN) guidelines (27) approve of TSEBT in refractory stage IA and stage IB–IV MF as well as in SS. Cd-TSEBT of 30–36 Gy achieves a high remission rate with acceptable toxicity. In selected patients with relapse after good initial response, TSEBT can be successfully repeated; however, RT toxicities are cumulative resulting in clinical reluctance to administer more than 2 lifetime cd-TSEBT courses to a patient. As such, more recent studies are investigating the impact of lowering the total TSEBT dose. Herein, we will examine the data for both cd- and ld-TSEBT (10–12 Gy) approaches.
Conventional dose
The effectiveness of cd-TSEBT has been confirmed by multiple large single and multi-institutional series (Table 1) (28-35). Overall response (OR) rates range from 94.7–100% range using 30–36 Gy TSEBT. In terms of complete response (CR) rates, there is a well-established linear dose-dependent relationship. Jones et al. (28) found a significantly higher CR in stage IA–III patients treated with 35 vs. 30 Gy (85% vs. 64%). Additionally, lower rates of CR are seen in higher stage disease, despite RT doses >30 Gy. Quirós (29) et al. and Ysebaert et al. (32) both noted lower CR rates in T2 compared to T1 disease treated with 30 or 36 Gy (84.8% vs. 87.5% or 87% vs. 97%), respectively. Similarly, CR following 32 Gy TSEBT was only 60% in 45 patients with stage III–IV disease.

Full table
The largest cohort of patients treated with cd-TSEBT is at Stanford with the latest updated published in 2011 (33). A total of 180 patients (T2: 103; T3: 77) received 36 Gy TSEBT over 9 weeks. Clinical improvement was achieved in all patients, with 60% demonstrating a CR. Higher CR rates were seen in T2 vs. T3 disease (75% vs. 47%). The median duration of response for CR patients was longer for those with T2 than T3 disease, 29 vs. 9 months, respectively. The 5- and 10-year overall survival (OS) rate for the entire cohort was 63% and 44%, respectively. Both median OS (10.9 vs. 4.7 years, P<0.001) and progression-free survival (PFS) (8.5 vs. 2.9 years, P<0.001) were also significantly longer in T2 vs. T3 disease.
The United Kingdom (UK) Cutaneous Lymphoma Group (35) implemented a shortened TSEBT treatment regimen with comparable results to 9-week TSEBT. A total of 30 Gy in 20 fractions was administered over a course of 5 weeks (4 days/week) using the Stanford 6-dual-field approach. Of the 41 patients, 17 were stage IB, 19 stage IIB, 3 stage III, and 2 stage IV. The OR and CR was 95% and 51%, respectively. The median time to relapse, time to systemic therapy, and time to modified severity weighted assessment tool (mSWAT) progression above baseline was 12, 15, and 44 months, respectively. The median OS was 35 months overall: stage IA patients had increased OS rates and longer time to relapse than stage IIB.
Low dose
Despite the proven benefit of cd-TSEBT, there are associated toxicities that limit its use following disease recurrence. As a result, lower dose TSEBT has been explored. Extrapolating the very favorable clinical response of very lose-dose RT in indolent low-grade NHL (36-38), Kamstrup et al. (39) first explored very ld-TSEBT (4 Gy in 4 fractions over 4 days) as a second-line treatment in ten patients with stage IB–II MF who did not achieve CR or relapsed within 4 months following psoralen plus ultraviolet-A. Two patients had a CR, but relapsed after 3.5 months. Six patients had a partial response (PR), with a mean duration of 2.0 months. One patient had no clinical response and one patient died without receiving treatment. This report showed that even 4 Gy TSEBT can obtain clinical response in MF; however, the insufficiently low duration of response does not allow very ld-TSEBT to be recommended as standard management for early stage MF.
In 2011, Harrison et al. (40) published the Stanford University experience of ld-TSEBT and evaluated the impact of various radiation doses. A total of 102 patients (T2: 51; T3: 29; T4: 22) received between 5 to <30 Gy TSEBT from 1958-1993; those with visceral or peripheral blood disease were excluded. For purposes of comparison, the authors also compared results to a cohort of patients who received ≥30 Gy TSEBT from 1970 to 2007. OR & CR rates were 90% & 16%, 98% & 35%, and 97% & 34% for those receiving 5 to <10 Gy (n=19), 10 to <20 Gy (n=51), and 20 to <30 Gy (n=22), respectively. Furthermore, no significant differences in OR, PFS, RFS, or OS was seen among the low-dose groups (10 to <20 Gy and 20 to <30 Gy) and those who received ≥30 Gy. Based on these findings, the authors concluded that TSEBT within the lower dose range of 10 to <20 Gy merits further investigation, particularly in the context of multi-modality treatment.
Given their prior experience and encouraging findings from Stanford, Kamstrup et al. (41) conducted an open multi-institutional clinical study evaluating ld-TSEBT (10 Gy). A total of 21 patients with stage IB-IV MF/SS received 10 Gy (1 Gy per fraction; 4 fractions per week) TSEBT from 2009 to 2012. The median follow-up was 15.7 months (3.9–40.2 months). The OR rate was 95%. Moreover, 58% of patients achieved either a CR (29%) or very good PR (VGPR; 29%) (<1% involvement of body surface area). The median duration of cutaneous response was 5.8 months (2–22.2 months) overall, 9.1 months (2.7–22.2 months) for those with CR + VGPR, and 4.5 months (2–13.3 months) in patients with a PR. Only grade 1–2 side effects were seen, at a lower frequency than what is reported with cd-TSEBT. The 10 Gy regimen appears acceptable, and future studies should determine if the combination with other agents could increase the rate of CR and response duration.
Hoppe et al. subsequently started a phase II trial of 12 Gy TSEBT (ClinicalTrials.gov NCT00985140), and later published (42) a pooled analysis of three Phase-II clinical trials of ld-TSEBT from Stanford University and MD Anderson Cancer Center. The primary end point for all trials was the clinical response rate. In all, 33 enrolled patients with stage IB–IIIA MF were treated with 12 Gy TSEBT over a 3-week span. The OR, CR, and PR rate was 88%, 27%, and 61%, respectively. The median overall reduction in mSWAT score was 93.5%, with the largest reduction (median: 45%) occurring between 1–2 months post-TSEBT. The median time to response and median duration of clinical benefit (time from initial response until initiation of any total skin-equivalent treatment, systemic therapy, or progressive disease) was 1.7 months (0.7–2.9 months) and 16.3 months (95% CI, 9.6–30.8 months), respectively. The majority of recorded toxicities were mild and reversible with only two grade III adverse effects noted. These findings further confirm the clinical utility of ld-TSEBT.
Moreover, a direct comparison between conventional versus ld-TSEBT was provided by a retrospective German study (43). The median dose was 30 Gy (30–36 Gy) for the cd-cohort [n=24 (19 MF; 5 SS)] and 20 Gy (12–28 Gy) for the ld-cohort [n=12 (7 MF; 5 SS)]. Overall response & CR was 92% & 50% and 70% & 50% for the entire MF and SS cohorts, respectively. No significant outcomes differences were seen between MF/SS patients treated with cd- vs. ld-TSEBT: OR—92% vs. 75% (P=0.09); CR—63% vs. 25%; event-free survival (EFS)—15 vs. 6 months (P=0.053); median OS—77 vs. 16 months (P=0.237). ld-TSEBT patients had significantly lower median treatment length {32 [16–186] vs. 49 [25–323] days, P=0.009}. Of note, the cd-cohort experienced statistically significant higher grade II toxicities (82% vs. 50%, P=0.043).
Most recently, The UK Cutaneous Lymphoma Group published the largest prospective series of MF patients treated with ld-TSEBT (44). A total of 103 MF patients (52.4%, 32.0%, 11.7%, 3.9%—stages IB, IIB, III, and IV, respectively) were irradiated with 12 Gy TSEBT in 8 fractions over a period of 2 weeks. The median age was 68 years. The ORR was 87% in all patients: 18% achieved a CR, 69% achieved a PR, and stable disease was present in 8% of patients. In those with CR, the median time to relapse was 7.3 months. The median response duration was 11.8 months. The median PFS rate for all patients was 13.2 months, with higher rates seen in lower stages of disease. Treatment was well tolerated with <5% grade 3+ toxicity (lower extremity edema, skin blisters, radiation dermatitis) seen.
TSEBT toxicity
For patients receiving conventional 30–36 Gy TSEBT, the most frequently seen acute side effects are erythema and dry desquamation (76%), blisters (52%), hyperpigmentation (50%), skin pain (48%), and skin infections requiring antibiotics (32%) (20). Alopecia and temporary damage or loss of nails are common, and alopecia potentially irreversible with doses >25 Gy. Repeat TSEBT (45,46), while effective, can be associated with more significant long-term toxicities, such as hypo- or anhidrosis, chronic dry skin, and scattered telangiectasias. There is also a slightly increased risk of secondary non-melanoma skin malignancies (47). Toxicity is dose-dependent with reluctance to administer >2 cd-TSEBT courses in a patient’s lifetime.
A significant more favorable toxicity profile is associated with ld-TSEBT. Kroeger et al. (48) found significantly less grade 2 (33% vs. 79%) and grade 3 (6% vs. 15%) toxicities treated with low-dose (median surface dose: 12 Gy) vs. high-dose (median surface dose: 30 Gy) TSEBT. Multiple/salvage ld-TSEBT were not associated with increased risk of acute adverse events. In accordance with these findings, the UK Lymphoma Group found a significant reduction in grade 2 toxicities (7% vs. 38%—fatigue; 3% vs. 19%—skin blisters; 13% vs. 47%—radiation dermatitis; 5% vs. 31%—skin infection) with low- vs. higher-dose TSEBT (44).
Discussion
TSEBT remains one of the most effective treatment modalities for MF. Historically, TSEBT doses increased from 8 to ≥30 Gy as higher response rates were seen with an acceptable increase in acute toxicity associated with incremental dose increases. Since then, the efficacy of 30–36 Gy TSEBT in inducing CR has been validated (Table 1), indicating the dose-response relationship between neoplastic MF cells and RT. Although CR is the preferred result for all therapeutic modalities in oncology, the chance of inducing and sustaining a CR in MF/SS is particularly challenging. Despite receiving cd-TSEBT, many patients experience relapse within 2 years. Although TSEBT repetition (45,46) following standard-dose regimens has been reported, the RT dose in second or third courses is often reduced due to the significant risk of severe and/or irreversible toxicities such as skin atrophy, xerosis, and alopecia.
ld-TSEBT is becoming increasingly popular over the past several years. Studies have shown very similar OR, OS, and EFS rates, albeit at the cost of decreased CR rates (Table 2). Nonetheless, ld-TSEBT is able to produce clinically significant benefits (i.e., reduction in mSWAT scores, very-good PR) resulting in reduced disease burden and associated symptomatic relief (Figure 2). Indeed, Hoppe et al. (42) described an outstanding 93.5% median reduction in disease burden and 16.3-month duration of clinical benefit. For patients at high risk of recurrence, the resultant quality of life improvement is often more beneficial than a CR. Of note, it is very difficult to predict the length of cutaneous. The UK Lymphoma Group have published modern results of both cd- (35) and ld- (44) TSEBT and have noted similar response duration (~12 months), despite higher CR with high-dose TSEBT. Similarly, Kamstrup et al. (41) noted an overlap in duration of cutaneous response among patients who experienced PR or CR + VGPR. Toxicity is also reduced using ld-TSEBT. MF is considered to be an incurable disease with patients often requiring multiple lifetime treatments. ld-TSEBT thus has the benefit of multiple applications without inducing severe radiation-associated toxicity.

Full table
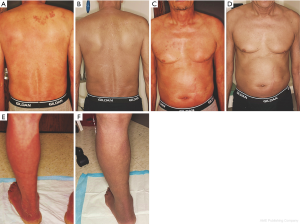
Finally, ld-TSEBT is able to produce a rapid and significant symptomatic relief in a shortened duration. Many patients find the length of cd-TSEBT treatment to be cumbersome (49) and are hesitant to repeat the treatment if necessary. Thus, the less intensive treatment course of ld-TSEBT provides the opportunity to improve patient compliance (50) and satisfaction, while also reducing costs (42). Due to these benefits, the updated NCCN guidelines (27) recommend a dose range for TSEBT to be between 12–36 Gy, generally 4–6 Gy per week.
Future directions
There has been a steady development of newer systemic, targeted, and immunotherapeutic agents in MF/SS. Histone deacetylase inhibitors, such as vorinostat (51) and romidepsin (52), modulate single- and double-stranded repair proteins and may have a radiosensitization effect when combined with RT (53,54). An imbalance between Th1/Th2 CD4+ T-lymphocyte exists in MF, with a disproportionate amount of Th2 cytokines present in advanced-stage disease (55). A phase II study (56) of the Th1 promoting cytokine, human recombinant interleukin 12, has shown a 43% PR, though brief (median: 3 months) antitumor response. Brentuximab, an anti-CD30+ monoclonal antibody (mAb), is now approved for CD30+ CTCL following the results of the phase III ALCANZA trial (57). Other promising mAb include zanolimumab (anti-CD4+ mAb) (58) and mogamulizumab (anti-CCR4 mAb) (59), which have shown OR rates reaching 56% and 37%, respectively. Finally, the anti-PD-1 antibody, nivolumab (60), has shown a modest OR of 15% in recurrent or refractory MF.
Multi-modality treatment with TSEBT remains controversial due to inconsistent findings, particular when different agents are utilized. For example, one study showed, for early stage patients, adjuvant PUVA was shown to improved 5-year disease-free survival (DFS) (29). Combining TSEBT with interferon (IFN) resulted in increased though acceptable toxicities; however, the addition of IFN did not appear to improve the CR rate, DFS or OS in MF (61). For patients with extracutaneous involvement, the combination of TSEBT with conventional chemotherapy has been shown to improve CR rates in several studies (62-64). The updated 2017 EORTC/ISCL recommendations for the treatment of MF/SS suggest following remission-inducing treatment, such as TSEBT, with maintenance topical (i.e., topical steroids, PUVA, ultraviolet B, mechlorethamine) or systemic therapies (i.e., low dose methotrexate, IFN, retinoids, extracorporeal photopheresis) in order to improve and/or sustain the clinical benefit of TSEBT (26).
Investigations are ongoing with newer agents may help augment the CR rates of ld-TSEBT. There are two clinical trials actively recruiting patients for combined ld-TSEBT and systemic/targeted therapy. The first is a phase I trial (ClinicalTrials.gov NCT02822586) evaluating treatment response and cutaneous toxicity of concurrent brentuximab and 12 Gy TSEBT. The other is a phase 2 multi-institutional trial (ClinicalTrials.gov NCT02542124) assessing the toxicity of concurrent recombinant interleukin 12 and 12 Gy TSEBT. Finally, another multi-institutional phase II study (ClinicalTrials.gov NCT01187446) of 12 Gy TSEBT combined with vorinostat has completed, with final results pending.
Despite these promising advancements, patients with advanced disease only achieve temporary remission. Autologous hematopoietic stem cell transplantation (HCT) is the only way to potentially produce a cure and is an area under ongoing investigation (65,66). Ideally, patients should be in CR prior to initiating the conditioning regimen for HCT. TSEBT has the ability to control cutaneous disease and achieve remission prior to transplant. Duvic et al. (67) reported on 19 patients (3 stage IIB; 6 stage IVA; 10 stage IVB) who received cd-TSEBT immediately before allogeneic HCT for refractory MF. Following TSEBT and HCT, 58% of patients were able to achieve a CR. At 2 years, OS was 79% and PFS was 53%. In addition, the authors suggested that the use of TSEBT to debulk the skin immediately before transplant may have led to a decrease in the severity of graft-versus-host disease, one of the most commonly seen toxicities with HCT (68). This combination may yet prove to be a promising approach for this difficult to treat population.
Conclusions
MF/SS is a rare and challenging disease to treat with minimal randomized data to support optimal initial treatment choice. RT, in particular TSEBT, is a well-established and efficacious treatment option for MF. The efficacy of cd-TSEBT (30–36 Gy) has been validated by numerous institutional studies and gives the highest chance of achieving a CR, albeit with the costs of greater toxicity and limited opportunities for retreatment. ld-TSEBT (10–12 G7) has been gaining traction due to its ability to provide rapid and clinically significant disease reduction, improved side effect profile, convenience, and repeatability following disease recurrence. Future studies identifying combinations of novel systemic agents with ld-TSEBT may help improve response durability. Nonetheless, it is clear that ld-TSEBT should be considered as a safe and effective skin-directed therapy in the treatment of MF/SS.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood 2005;105:3768-85. [Crossref] [PubMed]
- Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016;127:2375-90. [Crossref] [PubMed]
- Korgavkar K, Xiong M, Weinstock M. Changing Incidence Trends of Cutaneous T-cell Lymphoma. JAMA Dermatol 2013;149:1295-9. [Crossref] [PubMed]
- Scarisbrick JJ, Kim YH, Whittaker SJ, et al. Prognostic factors, prognostic indices and staging in mycosis fungoides and Sezary syndrome: where are we now? Br J Dermatol 2014;170:1226-36. [Crossref] [PubMed]
- Olsen E, Vonderheid E, Pimpinelli N, et al. Revisions to the staging and classification of mycosis fungoides and Sezary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the cutaneous lymphoma task force of the European Organization of Research and Treatment of Cancer (EORTC). Blood 2007;110:1713-22. [Crossref] [PubMed]
- Zackheim HS. Topical carmustine (BCNU) in the treatment of mycosis fungoides. Dermatol Ther 2003;16:299-302. [Crossref] [PubMed]
- Breneman D, Duvic M, Kuzel T, et al. Phase 1 and 2 trial of bexarotene gel for skin-directed treatment of patients with cutaneous T-cell lymphoma. Arch Dermatol 2002;138:325-32. [Crossref] [PubMed]
- Coors EA, Schuler G, Von Den Driesch P. Topical imiquimod as treatment for different kinds of cutaneous lymphoma. Eur J Dermatol 2006;16:391-3. [PubMed]
- Diederen PV, van Weelden H, Sanders CJ, et al. Narrowband UVB and psoralen-UVA in the treatment of early-stage mycosis fungoides: a retrospective study. J Am Acad Dermatol 2003;48:215-9. [Crossref] [PubMed]
- Almohideb M, Walsh S, Scott W, et al. Bath Psoralen-ultraviolet A and Narrowband Ultraviolet B Phototherapy as Initial Therapy for Early-stage Mycosis Fungoides: A Retrospective Cohort of 267 Cases at the University of Toronto. Clin Lymphoma Myeloma Leuk 2017;17:604-12. [Crossref] [PubMed]
- Hoppe RT. Mycosis fungoides: radiation therapy. Dermatol Ther 2003;16:347-54. [Crossref] [PubMed]
- Chowdhary M, Chhabra AM, Kharod S, et al. Total Skin Electron Beam Therapy in the Treatment of Mycosis Fungoides: A Review of Conventional and Low-Dose Regimens. Clin Lymphoma Myeloma Leuk 2016;16:662-71. [Crossref] [PubMed]
- Scholtz W. Ueber den Einfluss der Röntgenstrahlen auf die Haut in gesundem und krankem Zustande. Archiv für Dermatologie und Syphilis 1902;59:421-46. [Crossref]
- Trump JG, Wright KA, Evans WW, et al. High energy electrons for the treatment of extensive superficial malignant lesions. Am J Roentgenol Radium Ther Nucl Med 1953;69:623-9. [PubMed]
- Karzmark CJ, Loevinger R, Steele RE, et al. A technique for large-field, superficial electron therapy. Radiology 1960;74:633-44. [Crossref] [PubMed]
- Trowell OA. The sensitivity of lymphocytes to ionizing radiation. J Pathol Bacteriol 1952;64:687-704. [Crossref] [PubMed]
- Kim JH, Nisce LZ, D’Angle GJ. Doseetime fractionation study in patients with mycosis fungoides and lymphoma cutis. Radiology 1976;119:439-42. [Crossref] [PubMed]
- Specht L, Dabaja B, Illidge T, et al. Modern radiation therapy for primary cutaneous lymphomas: field and dose guidelines from the International Lymphoma Radiation Oncology Group. Int J Radiat Oncol Biol Phys 2015;92:32-9. [Crossref] [PubMed]
- Desai KR, Pezner RD, Lipsett JA, et al. Total skin electron irradiation for mycosis fungoides: relationship between acute toxicities and measured dose at different anatomic sites. Int J Radiat Oncol Biol Phys 1988;15:641-5. [Crossref] [PubMed]
- Lloyd S, Chen Z, Foss FM, et al. Acute toxicity and risk of infection during total skin electron beam therapy for mycosis fungoides. J Am Acad Dermatol 2013;69:537-43. [Crossref] [PubMed]
- Asbell SO, Siu J, Lightfoot DA, et al. Individualized eye shields for use in electron beam therapy as well as low-energy photon irradiation. Int J Radiat Oncol Biol Phys 1980;6:519-21. [Crossref] [PubMed]
- Podgorsak EB, Pla C, Pla M, et al. Physical aspects of a rotational total skin electron irradiation. Med Phys 1983;10:159-68. [Crossref] [PubMed]
- Piotrowski T, Malicki J. The rotary dual technique for total skin irradiation in the treatment of mycosis fungoides—a description of the applied method. Rep Pract Oncol Radiother 2006;11:29-37. [Crossref]
- Piotrowski T, Milecki P, Skórska M, et al. Total skin electron irradiation techniques: a review. Postepy Dermatol Alergol 2013;30:50-5. [Crossref] [PubMed]
- Weaver RD, Gerbi BJ, Dusenbery KE. Evaluation of dose variation during total skin electron irradiation using thermoluminescent dosimeters. Int J Radiat Oncol Biol Phys 1995;33:475-8. [Crossref] [PubMed]
- Trautinger F, Eder J, Assaf C, et al. European Organisation for Research and Treatment of Cancer consensus recommendations for the treatment of mycosis fungoides/Sezary syndrome - Update 2017. Eur J Cancer 2017;77:57-74. [Crossref] [PubMed]
- Network NCC. NCCN clinical practice guideline in oncology: T-cell lymphomas, version 3.2918. Accessed May 11 2018. Available online: https://www.nccn.org/professionals/physician_gls/pdf/t-cell.pdf
- Jones GW, Tadros A, Hodson D, et al. Prognosis with newly diagnosed mycosis fungoides after total skin electron radiation of 30 or 35 Gy. Int J Radiat Oncol Biol Phys 1994;28:839-45. [Crossref] [PubMed]
- Quirós PA, Jones GW, Kacinski BM, et al. Total Skin Electron Beam Therapy Followed By Adjuvant Psoralen/Ultraviolet-A Light In The Management of Patients with T1 and T2 Cutaneous T-Cell Lymphoma (Mycosis Fungoides). Int J Radiat Oncol Biol Phys 1997;38:1027-35. [Crossref] [PubMed]
- Jones GW, Rosenthal D, Wilson LD. Total Skin Electron Radiation for Patients with Erythrodermic Cutaneous T-Cell Lymphoma (Mycosis Fungoides and the Se’zary Syndrome). Cancer 1999;85:1985-95. [PubMed]
- Shouman T, Aguib N, El-Taher Z, et al. Total Skin Electron Beam Therapy (TSEBT) in the Management of Mycosis Fungoides: Single Institution Experience. J Egypt Natl Canc Inst 2003;15:275-83.
- Ysebaert L, Truc G, Dalac S, et al. Ultimate results of radiation therapy for T1-T2 mycosis fungoides (including reirradiation). Int J Radiat Oncol Biol Phys 2004;58:1128-34. [Crossref] [PubMed]
- Navi D, Riaz N, Levin YS, et al. The Stanford University Experience With Conventional-Dose, Total Skin Electron-Beam Therapy in the Treatment of Generalized Patch or Plaque (T2) and Tumor (T3) Mycosis Fungoides. Arch Dermatol 2011;147:561-7. [Crossref] [PubMed]
- Heumann TR, Esiashvili N, Parker S, et al. Total skin electron therapy for cutaneous T-cell lymphoma using a modern dual-field rotational technique. Int J Radiat Oncol Biol Phys 2015;92:183-91. [Crossref] [PubMed]
- Morris SL, McGovern M, Bayne S, et al. Results of a 5-week schedule of modern total skin electron beam radiation therapy. Int J Radiat Oncol Biol Phys 2013;86:936-41. [Crossref] [PubMed]
- Ganem G, Lambin P, Socié G, et al. Potential role for low dose limited-field radiation therapy (2 x 2 grays) in advanced low-grade non-Hodgkin's lymphomas. Hematol Oncol 1994;12:1-8. [Crossref] [PubMed]
- Sawyer EJ, Timothy AR. Low dose palliative radiotherapy in low grade non-Hodgkin's lymphoma. Radiother Oncol 1997;42:49-51. [Crossref] [PubMed]
- Jóhannsson J, Specht L, Mejer J, et al. Phase II study of palliative low-dose local radiotherapy in disseminated indolent non-Hodgkin's lymphoma and chronic lymphocytic leukemia. Int J Radiat Oncol Biol Phys 2002;54:1466-70. [Crossref] [PubMed]
- Kamstrup MR, Specht L, Skovgaard GL, et al. A prospective, open-label study of low-dose total skin electron beam therapy in mycosis fungoides. Int J Radiat Oncol Biol Phys 2008;71:1204-7. [Crossref] [PubMed]
- Harrison C, Young J, Navi D, et al. Revisiting low-dose total skin electron beam therapy in mycosis fungoides. Int J Radiat Oncol Biol Phys 2011;81:e651-7. [Crossref] [PubMed]
- Kamstrup MR, Gniadecki R, Iversen L, et al. Low-dose (10-Gy) total skin electron beam therapy for cutaneous T-cell lymphoma: an open clinical study and pooled data analysis. Int J Radiat Oncol Biol Phys 2015;92:138-43. [Crossref] [PubMed]
- Hoppe RT, Harrison C, Tavallaee M, et al. Low-dose total skin electron beam therapy as an effective modality to reduce disease burden in patients with mycosis fungoides: results of a pooled analysis from 3 phase-II clinical trials. J Am Acad Dermatol 2015;72:286-92. [Crossref] [PubMed]
- Elsayad K, Kriz J, Moustakis C, et al. Total Skin Electron Beam for Primary Cutaneous T-cell Lymphoma. Int J Radiat Oncol Biol Phys 2015;93:1077-86. [Crossref] [PubMed]
- Morris S, Scarisbrick J, Frew J, et al. The Results of Low-Dose Total Skin Electron Beam Radiation Therapy (TSEB) in Patients With Mycosis Fungoides From the UK Cutaneous Lymphoma Group. Int J Radiat Oncol Biol Phys 2017;99:627-33. [Crossref] [PubMed]
- Becker M, Hoppe RT, Knox SJ. Multiple courses of high-dose total skin electron beam thearpy in the management of mycosis fungoides. Int J Radiat Oncol Biol Phys 1995;32:1445-9. [Crossref] [PubMed]
- Wilson LD, Quiros PA, Kolenik SA, et al. Additional courses of total skin electron beam therapy in the treatment of patients with recurrent cutaneous T-cell lymphoma. J Am Acad Dermatol 1996;35:69-73. [Crossref] [PubMed]
- Abel EA, Sendagorta E, Hoppe RT. Cutaneous malignancies and metastatic squamous cell carcinoma following topical therapies for mycosis fungoides. J Am Acad Dermatol 1986;14:1029-38. [Crossref] [PubMed]
- Kroeger K, Elsayad K, Moustakis C, et al. Low-dose total skin electron beam therapy for cutaneous lymphoma: Minimal risk of acute toxicities. Strahlenther Onkol 2017;193:1024-30. [Crossref] [PubMed]
- Yu JB, Khan AM, Jones GW, et al. Patient perspectives regarding the value of total skin electron beam therapy for cutaneous T-cell lymphoma/mycosis fungoides: a pilot study. Am J Clin Oncol 2009;32:142-4. [Crossref] [PubMed]
- Chowdhary M, Kabbani AA, Rimtepathip P, et al. Rapidly progressive stage IVB mycosis fungoides treated with low-dose total skin electron beam therapy. Onco Targets Ther 2015;8:1597-601. [Crossref] [PubMed]
- Olsen EA, Kim YH, Kuzel TM, et al. Phase IIb multicenter trial of vorinostat in patients with persistent, progressive, or treatment refractory cutaneous T-cell lymphoma. J Clin Oncol 2007;25:3109-15. [Crossref] [PubMed]
- Whittaker SJ, Demierre MF, Kim EJ, et al. Final results from a multicenter, international, pivotal study of romidepsin in refractory cutaneous T-cell lymphoma. J Clin Oncol 2010;28:4485-91. [Crossref] [PubMed]
- Baschnagel A, Russo A, Burgan WE, et al. Vorinostat enhances the radiosensitivity of a breast cancer brain metastatic cell line grown in vitro and as intracranial xenografts. Mol Cancer Ther 2009;8:1589-95. [Crossref] [PubMed]
- Chinnaiyan P, Vallabhaneni G, Armstrong E, et al. Modulation of radiation response by histone deacetylase inhibition. Int J Radiat Oncol Biol Phys 2005;62:223-9. [Crossref] [PubMed]
- Vowels BR, Lessin SR, Cassin M, et al. Th2 cytokine mRNA expression in skin in cutaneous T-cell lymphoma. J Invest Dermatol 1994;103:669-73. [Crossref] [PubMed]
- Duvic M, Sherman ML, Wood GS, et al. A phase II open-label study of recombinant human interleukin-12 in patients with stage IA, IB, or IIA mycosis fungoides. J Am Acad Dermatol 2006;55:807-13. [Crossref] [PubMed]
- Prince HM, Kim YH, Horwitz SM, et al. Brentuximab vedotin or physician's choice in CD30-positive cutaneous T-cell lymphoma (ALCANZA): an international, open-label, randomised, phase 3, multicentre trial. Lancet 2017;390:555-66. [Crossref] [PubMed]
- Kim YH, Duvic M, Obitz E, et al. Clinical efficacy of zanolimumab (HuMax-CD4): two phase 2 studies in refractory cutaneous T-cell lymphoma. Blood 2007;109:4655-62. [Crossref] [PubMed]
- Duvic M, Pinter-Brown LC, Foss FM, et al. Phase 1/2 study of mogamulizumab, a defucosylated anti-CCR4 antibody, in previously treated patients with cutaneous T-cell lymphoma. Blood 2015;125:1883-9. [Crossref] [PubMed]
- Lesokhin AM, Ansell SM, Armand P, et al. Nivolumab in Patients With Relapsed or Refractory Hematologic Malignancy: Preliminary Results of a Phase Ib Study. J Clin Oncol 2016;34:2698-704. [Crossref] [PubMed]
- Roberge D, Muanza T, Blake G, et al. Does adjuvant alpha-interferon improve outcome when combined with total skin irradiation for mycosis fungoides? Br J Dermatol 2007;156:57-61. [Crossref] [PubMed]
- Braverman IM, Yager NB, Chen M, et al. Combined total body electron beam irradiation and chemotherapy for mycosis fungoides. J Am Acad Dermatol 1987;16:45-60. [Crossref] [PubMed]
- Wilson LD, Licata AL, Braverman IM, et al. Systemic chemotherapy and extracorporeal photochemotherapy for T3 and T4 cutaneous T-cell lymphoma patients who have achieved a complete response to total skin electron beam therapy. Int J Radiat Oncol Biol Phys 1995;32:987-95. [Crossref] [PubMed]
- Duvic M, Lemak NA, Redman JR, et al. Combined modality therapy for cutaneous T-cell lymphoma. J Am Acad Dermatol 1996;34:1022-9. [Crossref] [PubMed]
- Duarte RF, Canals C, Onida F, et al. Allogeneic hematopoietic cell transplantation for patients with mycosis fungoides and Sezary syndrome: a retrospective analysis of the Lymphoma Working Party of the European Group for Blood and Marrow Transplantation. J Clin Oncol 2010;28:4492-9. [Crossref] [PubMed]
- Hosing C, Bassett R, Dabaja B, et al. Allogeneic stem-cell transplantation in patients with cutaneous lymphoma: updated results from a single institution. Ann Oncol 2015;26:2490-5. [PubMed]
- Duvic M, Donato M, Dabaja B, et al. Total skin electron beam and non-myeloablative allogeneic hematopoietic stem-cell transplantation in advanced mycosis fungoides and Sezary syndrome. J Clin Oncol 2010;28:2365-72. [Crossref] [PubMed]
- Wu PA, Kim YH, Lavori PW, et al. A meta-analysis of patients receiving allogeneic or autologous hematopoietic stem cell transplant in mycosis fungoides and Sezary syndrome. Biol Blood Marrow Transplant 2009;15:982-90. [Crossref] [PubMed]


