Management of fibrolamellar hepatocellular carcinoma
Introduction
Fibrolamellar hepatocellular carcinoma (FLHCC) is a primary liver tumor. It was first described by Edmondson in 1956 (1). It is pathologically distinct from hepatocellular carcinoma (HCC). The term ‘fibrolamellar’ is derived from the histological presence of thick fibrous collagen bands surrounding the tumor cells. It is a relatively rare tumor of unknown biology. Due to its low incidence, limited clinical information is available. It comprises approximately 1–9% of all HCCs with an age adjusted incidence rate estimated at 0.02 per 100,000 (2,3). Unlike “classic”/conventional HCC, it has a distinctive predilection for adolescents and young adults with no underlying liver disease or cirrhosis (4).
FLHCC was initially considered to have an indolent course, but it is now recognized to demonstrate a spectrum of aggressive behaviour. Surgery is the current mainstay of treatment and remains the only potentially curative option. As recurrences are common, alternative therapies are under investigation. FLHCCs have traditionally been considered less chemo-responsive than their conventional HCC counterparts, but in advanced cases multimodality treatments can be effective (4). The goal of this section is to review this primary liver neoplasm of “uncertain origin and unveiled outcome” in detail.
Epidemiology
The rarity of this tumor limits clear assessment of the difference in its incidence rates across countries. Less than 1% of primary liver tumors in the USA, and 5.8% of liver tumors in Mexico are FLHCC (5). However, incidence rates are relatively homogeneous among various racial and ethnic groups throughout the world (6,7).
Compared to HCC, FLHCC typically affects a younger population between the age groups of 14–33 years, with a median age of 21 years (3). A majority of cases (64%) are diagnosed before the age of 40 years (6). Bimodal age distribution has been reported with incidence peaks observed between the 10–30-year age group with a second peak noted in the 60–69-year age group (8). Most studies report equal incidence in both sexes while few have reported slight male preponderance (M:F—1.7:1) (6,7).
Clinical presentation
FLHCC is a rare tumor with unknown etiology. Patients tend to present young, asymptomatic and typically without any underlying liver disease. In a study by Mavros et al. only 3% of patients had underlying cirrhosis, 2% were positive for hepatitis B, and 1% were positive for hepatitis C (3).
Symptomatic patients present with pain, nausea, abdominal fullness, malaise, weight loss, palpable abdominal mass, hepatomegaly with or without right upper quadrant pain at a duration typically ranging from 1 to 6 months (9,10). Abdominal pain is the most common symptom (72%) followed by abdominal distention (44%), anorexia (32%), fever, and jaundice (20%) (11).
Other reported rare presentations include gynecomastia in males (due to aromatization of androgens), fulminant liver failure, recurrent deep vein thrombosis, encephalopathy, thrombophlebitis of the lower extremity, anaemia and hypoglycaemia (glucose utilization by growing tumor cells) (4).
Biochemical evaluation
At presentation, haematological studies and liver function tests such as aspartate aminotransferase, alanine aminotransferase, and alkaline phosphatase levels are normal or mildly elevated (9). Elevated alkaline phosphatase levels are likely due to growth of the tumor into the biliary tree with partial or complete biliary obstruction.
Tumor markers
Alpha-fetoprotein (AFP) levels can be normal or near normal in patients with FLHCC. Particularly in males, the diagnosis of testicular neoplasm along with FLHCC should be carefully considered in the context of marked elevation of AFP levels. On biopsy, a minority of these tumors are immunohistochemically positive for AFP (12). Elevated levels of serum transcobalamin I (haptocorrin) and vitamin B12 binding capacity were anecdotally reported in several case reports (13-17). However, additional evidence is needed to determine their diagnostic role. Serum neurotensin levels have also been found to be elevated, but this test is neither sensitive nor specific for FLHCC (17-19). Des-gamma carboxyprothrombin is elevated in FLHCC along with conventional HCC and hence may be less useful (19). Overall these tumor markers lack the predictive value required for non-invasive diagnosis of FLHCC.
Imaging
Ultrasound (US)
FLHCCs have nonspecific sonographic features. On ultrasonography (USG) they appear as well-defined masses of variable echogenicity (20). USG provides a diagnosis of liver mass which mandates further evaluation with a contrast enhanced CT (computed tomography) scan using a liver protocol or dynamic contrast-enhanced MRI (magnetic resonance imaging) for further characterization.
Contrast-enhanced CT (CECT) (Figure 1)
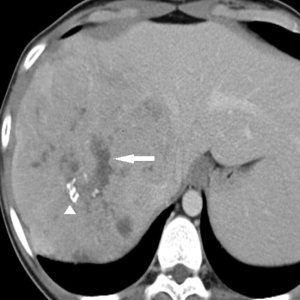
CECT with a liver protocol is commonly used for detection and characterization of liver lesions. An unenhanced phase followed by intravenous contrast-enhanced hepatic arterial phase (starting at 12 seconds after the trigger threshold of 120 HU is reached at the level of the abdominal aorta), portal venous phase (starting at 55 seconds after the trigger threshold), and delayed phase (starting at 180 seconds after the trigger threshold) are used for tumor characterization. On CT, FLHCCs usually present as large heterogeneous well-defined lesions (80–100%) with a lobulated outline. The tumors are hypoattenuating on the unenhanced images. Calcification and central stellate scar are seen in 65–70% along with tumor necrosis (21). A large scar (width >2 cm) and presence of radiating fibrotic bands or septa are common, and pathognomonic for FLHCC (20). Most FLHCCs (94%) show heterogeneous hyperattenuation on arterial phase images due to the presence of hypervascular tumor cells arranged around hypovascular fibrotic bands along with tumor necrosis (22). They have variable enhancement pattern in the portal venous and delayed phases. On the portal venous phase, approximately 50% are isoattenuating to liver (50%), 36% are hyperattenuating and 16% are hypoattenuating (22). On delayed phase images, FLHCCs may be hypoattenuating, isoattenuating, or hyperattenuating. Delayed enhancement of the central scar may be seen in 25–65% of FLHCC due to the presence of an increased number of vessels and cellular or myxomatous tissues within the scar. Biliary obstruction and portal vein thrombosis (5–10%) may also be diagnosed at scan in these patients (23).
MRI (Figure 2)
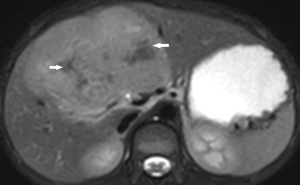
On MRI, FLHCC are hypointense on T1-weighted images and hyperintense on T2-weighted images. The fibrous central scar is hypointense on both T1- and T2-weighted images, distinguishing it from focal nodular hyperplasia (FNH). FLHCCs do not have intralesional fat (24). Calcification is rarely seen on MRI. On contrast injection with Gadolinium, the enhancement pattern is similar to CT scan with heterogeneous contrast enhancement on the arterial phase and becoming isointense or hypointense on the portal venous and delayed phase (25). FLHCC does not typically retain hepatobiliary-specific contrast agents, such as gadoxetate disodium and gadobenate dimeglumine. This finding is also a useful differentiator from FNH (26,27). FLHCC’s show restricted diffusion on diffusion-weighted imaging.
Radiological staging
Nodal metastases occur in up to 50–65% of cases and this is an important prognostic factor. Nodal metastases are seen commonly in the hepatic hilum, left gastric and hepatoduodenal ligament distributions and can extend to the retroperitoneum, pelvis, and mediastinum.
Distant metastases are seen in 20–30% of FLHCC with common sites being lung, peritoneum, and adrenal gland (28).
Nuclear medicine
Nuclear medicine imaging is occasionally useful in diagnosing FLHCC. These tumors show increased uptake of 99mTc-labeled RBCs during the arterial phase and wash out on delayed phase images. They appear photopenic on 99mTc-sulfur colloid scanning (22). The role of 18FDG PET/CT in FLHCC is not clear, however it may be useful in initial staging and in restaging in recurrent cases (29). Further studies are required before PET/CT can be recommended for routine use in clinical practice.
Role of biopsy
The diagnosis of FLHCC can often be made by characteristic CT and MRI imaging findings. For indeterminate cases, CT guided core needle biopsy or fine needle aspiration (FNA) can be useful in differentiating FLHCC from non FLHCC. A biopsy may be necessary for patients who have unresectable tumors or who have underlying medical conditions that preclude resection, in order to initiate palliative therapies.
Pathology
FLHCC is distinctive at the histological level and a further deeper level of understanding has emerged with the novel discovery of the DNAJB1-PRKACA fusion gene that drives the pathogenesis of this unique tumor in greater than 95% of cases (6).
Gross pathology
FLHCC’s appear as solitary tan masses on gross examination with the central scar seen in approximately one-third of cases (10). A green hue may be noted against the tan backdrop due to bile production by the neoplastic cells. They tend to infiltrate the porta hepatis with spread to regional nodes. The uninvolved liver tissue is usually grossly and histologically unremarkable with few patients having mild fatty changes. The presence of background fibrosis favours against the diagnosis of FLHCC.
Microscopic pathology (Figure 3)
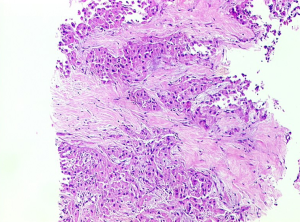
The microscopic characteristics of FLHCC are large eosinophilic cells, prominent nucleoli, and intra-tumoral fibrosis. The tumor cells are large and polygonal and almost three-times larger compared to surrounding cells (6). The presence of abundant mitochondria accounts for the eosinophilic cytoplasm. The nuclei often show vesiculated chromatin and essentially all cases show prominent macronuclei. The tumor cells are arranged in trabecular cords separated by intervening ribbons of fibrosis. The fibrous bands are usually arranged in parallel arrays. Various patterns of intra tumoral fibrosis also exist with tumors showing broad sheets or solid patterns.
The presence of pale bodies and hyaline bodies are well-known features of FLHCC, seen in about one-half of the cases, but are not specific for diagnosis (10). Few cases also have pseudoglandular pattern mimicking cholangiocarcinoma. Rarely, cases of FLHCC may show a nodule within-a-nodule architecture which likely represents aggressive biology with tumor progression.
The tumors are relatively deficient in mitochondrial DNA.
Immunohistochemistry
Lysosomes in tumor cells can be detected by cluster of differentiation 68 (CD68) immunostaining (30). FLHCC also consistently express markers of hepatocellular differentiation, including HepPar1, arginase 1, and albumin mRNA as detected by in situ hybridization (FISH). FLHCC also stain positive for CAM5.2, cytokeratin7, and pan-keratin stains (31,32). Cytokeratin19 is positive in approximately 5% of FLHCC. cytokeratin7 and CD68 are sensitive markers and, when used together in cases with compatible morphology, confirm the diagnosis in 85–90% of cases (33,34). Anterior gradient-2 is another specific marker believed to be an oncogenic protein and is positive in 75% of cases of FLHCC. However, it is not widely available for clinical use (35).
Molecular genetics
Recently, Honeyman et al. identified a recurrent intrachromosomal deletion on chromosome 19 leading to fusion of DNAJB1-PRKACA genes in many patients with the diagnosis of FLHCC (36). The DNAJB1-PRKACA fusion includes the promoter of the constitutively active DNAJB1 and the kinase domain of PRKACA which encodes catalytic subunit of protein kinase A. The activation of protein kinase A is the key event in development of FLHCC. This fusion gene is formed by a 400 kb heterozygous deletion which brings DNAJB1 and PRKACA together.
The somatic recurrent intrachromosomal fusion gene—DNAJB1-PRKACA, is shown to be 100% specific for FLHCC in the context of HCC and has been independently validated with FISH probe (37). Detection of DNAJB1-PRKACA or PRKACA rearrangement is the most accurate ancillary test for the diagnosis of FLHCC (38).
Diagnosis
The diagnosis of FLHCC can be confirmed by demonstration of the key molecular genetic events (DNAJB1-PRKACA fusion or rarely complete loss of PRKAR1A) or by the findings of compatible histologic features with co-expression of keratin7 and CD68. The fusion gene can be detected by RT-PCR or fluorescence FISH. FISH is the preferred test as it requires only 50 tumor cells and can be easily performed in paraffin embedded tissues.
Differential diagnosis (Table 1)

Full table
Hypervascularity of FLHCC may mimic other hypervascular liver lesions. The differential diagnosis of FLHCC includes other benign and malignant lesions of liver like focal nodular hyperplasia, haemangioma, hepatic adenoma and HCC. These lesions show characteristic imaging features, however, biopsy and clinical correlation is required for the definitive diagnosis.
FNH
FNH arises from hepatocyte proliferation around a congenital arteriovenous malformation and contains scar-like tissue. It is most frequently incidentally found in women, though rarely has a causal relationship to oral contraceptives (39). As FNH and FLHCC occur in common age populations, and can have similar radiologic appearances, further studies are warranted. On MRI, the central scar in FNH is predominantly T2 hyperintense, whereas the scar in FLHCC is typically T2 hypointense. Calcification is rarely seen in FNH. Also, FNH retains liver-specific contrast agent during the hepatobiliary phase of liver MRI, whereas this is not commonly seen in FLHCC. Distinction is important as patients with FNH can be managed with observation only.
Hemangioma
Peripheral discontinuous nodular contrast enhancement on the arterial phase and gradual centripetal filling during the delayed phase are characteristics of hemangioma. This pattern is not commonly seen in FLHCC. Another characteristic contrasting feature of hemangioma is marked hyperintensity on T2-weighted MR images (40).
Hepatocellular adenoma
Hepatocellular adenomas are benign lesions which are typically seen in young women of child bearing age with a history of estrogen-based oral contraceptive use. Adenomas have the risk of rupture, bleed and malignant transformation (4.2%). These lesions appear hypervascular in the arterial phase in contrast to the heterogeneous arterial enhancement seen in FLHCC. They tend to become isoattenuating or isointense to the liver on the subsequent phase images (41). The diagnosis of adenoma is usually made based on the clinical and imaging findings and the findings of core biopsies obtained for diagnostic workup of a liver mass.
HCC
Compared to FLHCC, conventional HCC has well-defined risk factors such as chronic viral infection with hepatitis B and/or C and ingestion of alcohol or aflatoxin B1 contaminated food that leads to cirrhosis. Cirrhosis of the liver remains the strongest predisposing factor for HCC. Conventional HCC predominantly occurs in older males compared to FLHCC which typically affects adolescents and young adults (42). Conventional HCC’s show peripheral hypervascular enhancement on the arterial phase and prolonged and delayed enhancement in late phases, whereas FLHCC tends to be heterogeneously hyperattenuating on the arterial phase and may be hypoattenuating, isoattenuating, or even hyperattenuating on subsequent phases. Serum AFP levels are more often elevated in patients with conventional HCC. Patients with chronic hepatitis B may develop conventional HCC in the absence of fibrosis or cirrhosis, but most of these tumors tend to have the wash-in and wash-out enhancement pattern. Clinical and radiological features help to distinguish FLHCC from conventional HCC, however, biopsy may be required in some cases (2).
Staging
FLHCC patients are staged according to AJCC TNM staging for HCC (43). The median tumor size on presentation is 12 cm (range, 4–20 cm) (3). Only 16% of FLHCCs present with a diameter <5 cm (T2), compared to only 37% of conventional HCCs (7). The incidence of regional disease/node positive disease is 30–40% while 33% patients present with distant metastases (7,44). Most FLHCC cases present at an advanced stage at the time of initial diagnosis: AJCC stage III in 37.2% and AJCC stage IV in 42.3% (45).
In conventional HCC, lymph node involvement tends to correlate with tumor size and is a poor prognostic factor. HCC tumors less than 3 cm are rarely accompanied by nodal metastasis but more than 40% patients with HCC tumors larger than 10 cm have lymph node metastases. Also, patients with cirrhosis tend to have less nodal spread than non-cirrhotic patients. FLHCC patients have a higher incidence of regional lymph node involvement than conventional HCC patients, probably owing to larger median tumor size at presentation and non-cirrhotic livers. Regional nodal involvement constitutes stage IV disease (IVA) as per AJCC TNM staging. This stage grouping, though prognostically relevant, does not always define inoperability in FLHCC.
Treatment
Liver resection and liver transplantation are the two potentially curative surgical treatment options.
Surgical resection
Surgical resection is the treatment of choice for FLHCC. Young age at diagnosis and absence of cirrhosis frequently makes resection feasible, even with large size tumors.
The resection rate in FLHCC is as high as 60–70%, differing by age at presentation. Young patients (age less than 40 years) have higher resection rates when compared to older patients (6). Patients undergoing resection have a median tumor size of 10.5 cm (46).
More than 70% of patients require a major Hepatectomy (i.e., hemihepatectomy or extended hepatectomy). Around 24% of patients undergo partial or minor hepatectomy (46-48).
Unlike classic HCC, 30–60% of operated FLHCC patients have lymph node involvement. As lymph node positivity is an important prognostic factor, complete periportal lymphadenectomy must be routinely performed as part of radical resection in FLHCC patients.
Data from a fibrolamellar research consortium indicates that 42% patients have vascular invasion with a majority of them having major vascular invasion (major branch of hepatic or portal vein) (48).
Multiple series have demonstrated overall postoperative survivals of 26–76% at 5 years with a median survival of 32–174 months for resected patients (49-53). A SEER database analysis demonstrated an overall survival rate of 58.2% (44–70%) for patients undergoing resection (6).
R0 resection rates vary from 54% to 83% (46,48). R0 resection is associated with improved long-term overall survival. In a study by Darcy et al., which studied 21 patients who underwent resection for FLHCC, a complete (R0) resection was achieved in 17 (81.0%) patients. The overall 5-year survival in the entire cohort was 42.6%, while the 5-year overall survival of those who underwent complete resection was 51.6% (11).
Patients undergoing resection for FLHCC experience better survivals compared to conventional HCC. In the meta-analysis by Njei et al., which included 17 studies and 368 patients with FLHCC, a significant increase in mean OS was reported in patients with FLHCC vs. those with non-FLHCC (84.9±15.8 vs. 42.9±6.5 months) who underwent partial hepatectomy (54).
Even patients with advanced stage can benefit from resection. The reported 5-year survival rate for selected stage IV disease patients following resection is 66% with a median survival of 34 months (52,53).
Patients with recurrent disease also may benefit from surgical resection. In a series by Yamashita et al., recurrent disease was observed in 86% patients after resection of FLHCC. The most common sites of first recurrence were intra-abdominal and/or intrathoracic lymph nodes, liver, lungs and peritoneum. Surgical resection of recurrent disease was associated with improved median overall survival of 122 months, compared to 37 months without surgical resection (49).
Liver transplantation
Orthotopic liver transplantation (OLT) is considered only in select cases of unresectable FLHCC (51,55). Due to its rarity, the evidence regarding outcomes of patients undergoing liver transplantation for FLHCC is not robust, being based on small case reports and case series.
In a series of 41 patients by Pinna et al., 13 patients underwent liver transplantation for advanced FLHCC (50). The survival rates were superior for resection compared to transplantation and at 5 years the gap was 44%. The survival rates are superior for resection compared to transplantation likely due to differential selection of patients with advanced disease.
In a systematic review by Mavros et al., 14 studies reported on 109 patients undergoing liver transplantation for FLHCC. Six studies reported specific survival data on 79 patients (3). Survivals after 1, 3, and 5 years ranged from 63% to 100%, 43% to 75%, and 29% to 55%, respectively.
Atienza et al. analysed the United Network of Organ Sharing (UNOS) database between October 1988 and January 2013 to evaluate outcomes in patients with FLHCC undergoing liver transplantation in the United States (56). Mean Model for End-Stage Liver Disease (MELD) score at the time of transplantation was 11.3 and the mean cold ischemia time was 6 hours. Overall survival of FLHCC patients at 1, 3, and 5 years was 96%, 80%, and 48% as compared to HCC patients whose rates were 89%,77%, and 68%. There was no perioperative mortality for patients with FLHCC. The recurrence rate was 10%. MELD score and cold ischemic time were found to be the strongest predictors of overall survival in FLHCC patients. There was no effect of age or wait time for transplantation on prognosis. When compared with HCC and cholangiocarcinoma, FLHCC patients had similar graft and patient survivals.
In a meta-analysis of 17 studies involving 368 patients with FLHCC and 9,877 patients with conventional HCC, Njei et al. found a significant increase in mean overall survival in patients with FLHCC compared with the survival time of those with non-FLHCC undergoing partial hepatectomy, but there was no difference in patients undergoing liver transplant (54).
Patients with unresectable FLHCC at presentation have poor survival with median survival times of less than 12 months and so liver transplantation remains an important therapeutic option in these patients. Eggert et al. in their series reported better overall survival for patients with FLHCC who were younger than 40 years of age as compared to HCC (7). However, no such survival advantage could be demonstrated in the series by Atienza et al. (56).
Thus, the overall published data indicate acceptable outcome for patients undergoing liver transplantation in FLHCC despite advanced stage.
Systemic therapy
The role of chemotherapy in the management of FLHCC is not clear as these tumors tend not to be chemo-responsive. Some response has been observed with platinum based and interferon alpha 2b based regimens (57-59). Few reports have also suggested the use of gemcitabine-based regimens (60,61).
The role of chemotherapy in the neoadjuvant, adjuvant, and advanced tumor settings in FLHCC has not been studied adequately and studies show conflicting results for different regimens. In one study patients who had front-line surgery followed by adjuvant therapies had the longest overall survival of 110.5 months, and those who underwent neoadjuvant therapy followed by surgery (n=10) had a median OS of 60 months (47). In the same study, best response rates were seen with interferon and 5 Fluorouracil combination regimens.
In the paediatric population, a platinum-based chemotherapy regimen resulted in partial response in 31% with 3-year survival of only 22% (62).
In a series by Maniaci et al., patients who received multimodality therapy for recurrence had median overall survival of 9.3 years with two patients showing at least partial response to cisplatin and fluorouracil (57).
The role and utility of chemotherapy for FLHCC can be better defined with prospective trials and is an area of active research. For example, in a phase II trial, Patt et al. reported a complete or partial response in 5 out of 8 patients treated with a combination therapy of fluorouracil and recombinant interferon alpha-2b (59).
Prognostic factors
Despite presenting at an advanced stage, approximately 70% patients with FLHCC are amenable to surgical treatment and have a 5-year overall survival rate of 70% (3).
Initial reports suggested better survival for FLHCC compared to conventional HCC with an overall increase in the 5-year survival figures. Also, a significant increase in overall survival was reported in patients with FLHCC compared with Non FLHCC patients undergoing partial hepatectomy. However, survival is not significantly different when compared to non-cirrhotic conventional HCC patients (54). Matched for stage patients with FLHCC do not have a favourable prognosis and do not respond any differently to treatment than non-cirrhotic patients with HCC (49).
Various prognostic factors associated with FLHCC include age, stage of disease, multiplicity, tumor thrombosis, lymphovascular invasion, nodal and distant metastases and completeness of resection (4).
In a study by Kaseb et al., white race, female gender, early tumor stage, and tumor resection including metastasectomy, were positively associated with longer OS, while female gender was the only significant positive predictor of longer recurrence-free survival (47).
The data regarding sex as a prognostic factor is conflicting, as studies have variably reported female sex to be both a favourable and an adverse factor. In the data from the FLHCC consortium; on univariate analysis, female sex, macrovascular invasion, unresectable disease, and lymph node and extrahepatic metastases were significantly associated with worse overall survival. However, on multivariate analysis only female sex, lack of surgery, and extrahepatic metastases were found to independently predict poor overall survival (48).
Initial stage of disease is one of the most important determining factors associated with prognosis as patients with stages I–III fare better compared to stage IV patients (48,51). In SEER database analysis by Mayo et al., on multivariable analysis, the presence of regional nodal disease was independently associated with shorter overall survival (46).
The recurrence rate following surgical resection is 33–100%, likely due to large tumor size, node positivity and advanced stage at presentation (3,53). The median time to recurrence is between 10–33 months. The common sites of recurrence include intrahepatic, regional and non-regional nodes and peritoneal surfaces.
Presence of extrahepatic disease is independently associated with recurrence free survival. In a study by Yamashita et al., FLHCC patients had a recurrence rate of 86% following resection. Longer recurrence-free survival was associated with absence of vascular invasion and age >25 years. Three-year recurrence-free survival rates were 9% vs. 35%, with and without vascular invasion (49).
Patients remaining disease free for 4 years after initial resection rarely develop recurrent disease or die of cancer (53). Patients who recur beyond 4–5 years usually have a favourable long-term prognosis.
Conclusions
FLHCC has unique demographic, radiologic and pathological features. The somatic recurrent intrachromosomal fusion gene—DNAJB1-PRKACA is responsible for pathogenesis of this tumor. Surgical resection and liver transplantation are curative therapies. Despite R0 resection, most patients recur. However, surgical resection is feasible for many recurrences and is associated with good survival. The role of chemotherapy is evolving and further research is required to define its role in the management of this disease.
Acknowledgements
The authors would like to thank Professor Mukta Ramadwar, Department of Pathology for providing pathological slide image and Dr. Akshay Baheti, Assistant Professor, Department of Radiology for CT and MRI images.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Edmondson HA. Differential diagnosis of tumors and tumor-like lesions of liver in infancy and childhood. AMA J Dis Child 1956;91:168-86. [PubMed]
- National Cancer Institute D SRP, Cancer Statistics Branch. Surveillance, Epidemiology, and End Results (SEER) Program; 2015. Available online: http://www.seer.cancer.gov
- Mavros MN, Mayo SC, Hyder O, et al. A systematic review: Treatment and prognosis of patients with fibrolamellar hepatocellular carcinoma. J Am Coll Surg 2012;215:820-30. [Crossref] [PubMed]
- Lafaro KJ, Pawlik TM. Fibrolamellar hepatocellular carcinoma: current clinical perspectives. J Hepatocell Carcinoma 2015;2:151-7. [PubMed]
- Arista-Nasr J, Gutierrez-Villalobos L, Nuncio J, et al. Fibrolamellar hepatocellular carcinoma in mexican patients. Pathol Oncol Res 2002;8:133-7. [Crossref] [PubMed]
- El-Serag HB, Davila JA. Is fibrolamellar carcinoma different from hepatocellular carcinoma? A US population-based study. Hepatology 2004;39:798-803. [Crossref] [PubMed]
- Eggert T, McGlynn KA, Duffy A, et al. Fibrolamellar hepatocellular carcinoma in the USA, 2000-2010: a detailed report on frequency, treatment and outcome based on the surveillance, epidemiology, and end results database. United European Gastroenterol J 2013;1:351-7. [Crossref] [PubMed]
- Eggert T, McGlynn KA, Duffy A, et al. Epidemiology of fibrolamellar hepatocellular carcinoma in the USA, 2000-10. Gut 2013;62:1667-8. [Crossref] [PubMed]
- Moreno-Luna LE, Arrieta O, Leiva JG, et al. Clinical and pathologic factors associated with survival in young adult patients with fibrolamellar hepatocarcinoma. BMC Cancer 2005;5:142. [Crossref] [PubMed]
- Craig JR, Peters RL, Edmondson HA, et al. Fibrolamellar carcinoma of the liver: a tumor of adolescents and young adults with distinctive clinico-pathologic features. Cancer 1980;46:372-9. [Crossref] [PubMed]
- Darcy DG, Malek MM, Kobos R, et al. Prognostic factors in fibrolamellar hepatocellular carcinoma in young adults. J Pediatr Surg 2015;50:153-6. [Crossref] [PubMed]
- Berman MA, Burnham JA, Sheahan DG. Fibrolamellar carcinoma of the liver: an immunohistochemical study of nineteen cases and review of the literature. Hum Pathol 1988;19:784-94. [Crossref] [PubMed]
- Wheeler K, Pritchard J, Luck W, et al. Transcobalamin I as a "marker" for fibrolamellar hepatoma. Med Pediatr Oncol 1986;14:227-9. [Crossref] [PubMed]
- Lildballe DL, Nguyen KQ, Poulsen SS, et al. Haptocorrin as marker of disease progression in fibrolamellar hepatocellular carcinoma. Eur J Surg Oncol 2011;37:72-9. [Crossref] [PubMed]
- Paradinas FJ, Melia WM, Wilkinson ML, et al. High serum vitaminB12 binding capacity as a marker of the fibrolamellar variant of hepatocellular carcinoma. Br Med J (Clin Res Ed) 1982;285:840-2. [Crossref] [PubMed]
- Sheppard KJ, Bradbury DA, Davies JM, et al. HighserumvitaminB12binding capacity as a marker of the fibrolamellar variant of hepatocellular carcinoma. Br Med J (Clin Res Ed) 1983;286:57. [Crossref] [PubMed]
- Collier NA, Weinbren K, Bloom SR, et al. Neurotensin secretion by fibrolamellar carcinoma of the liver. Lancet 1984;1:538-40. [Crossref] [PubMed]
- Ehrenfried JA, Zhou Z, Thompson JC, et al. Expression of the neurotensin gene in fetal human liver and fibrolamellar carcinoma. Ann Surg 1994;220:484-9. [Crossref] [PubMed]
- Bertino G, Ardiri AM, Calvagno GS, et al. Prognostic and diagnostic value of des-gamma-carboxyprothrombin in liver cancer. Drug News Perspect 2010;23:498-508. [PubMed]
- Friedman AC, Lichtenstein JE, Goodman Z, et al. Fibrolamellar hepatocellular carcinoma. Radiology 1985;157:583-7. [Crossref] [PubMed]
- Ichikawa T, Federle MP, Grazioli L, et al. Fibrolamellar hepatocellular carcinoma: imaging and pathologic findings in 31 recent cases. Radiology 1999;213:352-61. [Crossref] [PubMed]
- Blachar A, Federle MP, Ferris JV, et al. Radiologists’ performance in the diagnosis of liver tumors with central scars by using specific CT criteria. Radiology 2002;223:532-9. [Crossref] [PubMed]
- Ganeshan D, Szklaruk J, Kundra V, et al. Imaging features of fibrolamellar hepatocellular carcinoma. AJR Am J Roentgenol 2014;202:544-52. [Crossref] [PubMed]
- Brandt DJ, Johnson CD, Stephens DH, et al. Imaging of fibrolamellar hepatocellular carcinoma. AJR Am J Roentgenol 1988;151:295-9. [Crossref] [PubMed]
- Kadoya M, Matsui O, Takashima T, et al. Hepatocellular carcinoma: correlation of MR imaging and histopathologic findings. Radiology 1992;183:819-25. [Crossref] [PubMed]
- Ringe KI, Husarik DB, Sirlin CB, et al. Gadoxetate disodium-enhanced MRI of the liver. Part 1, Protocol optimization and lesion appearance in the noncirrhotic liver. AJR Am J Roentgenol 2010;195:13-28. [Crossref] [PubMed]
- Palm V, Sheng R, Mayer P, et al. Imaging features of fibrolamellar hepatocellular carcinoma in gadoxetic acid-enhanced MRI. Cancer Imaging 2018;18:9. [Crossref] [PubMed]
- Epstein BE, Pajak TF, Haulk TL, et al. Metastatic nonresectable fibrolamellar hepatoma: prognostic features and natural history. Am J Clin Oncol 1999;22:22-8. [Crossref] [PubMed]
- Liu S, Wah Chan K, Tong J, et al. PET-CT scan is a valuable modality in the diagnosis of fibrolamellar hepatocellular carcinoma: a case report and a summary of recent literature. QJM 2011;104:477-83. [Crossref] [PubMed]
- Vivekanandan P, Daniel H, Yeh MM, et al. Mitochondrial mutations in hepatocellular carcinomas and fibrolamellar carcinomas. Mod Pathol 2010;23:790-8. [Crossref] [PubMed]
- Ross HM, Daniel HD, Vivekanandan P, et al. Fibrolamellar carcinomas are positive for CD68. Mod Pathol 2011;24:390-5. [Crossref] [PubMed]
- Abdul-Al HM, Wang G, Makhlouf HR, et al. Fibrolamellar hepatocellular carcinoma: an immunohistochemical comparison with conventional hepatocellular carcinoma. Int J Surg Pathol 2010;18:313-8. [Crossref] [PubMed]
- Van Eyken P, Sciot R, Brock P, et al. Abundant expression of cytokeratin7 in fibrolamellar carcinoma of the liver. Histopathology 1990;17:101-7. [Crossref] [PubMed]
- Graham RP, Yeh MM, Lam-Himlin D, et al. Molecular testing for the clinical diagnosis of fibrolamellar carcinoma. Mod Pathol 2018;31:141-9. [Crossref] [PubMed]
- Vivekanandan P, Micchelli ST, Torbenson M. Anterior gradient-2 is over-expressed by fibrolamellar carcinomas. Hum Pathol 2009;40:293-9. [Crossref] [PubMed]
- Honeyman JN, Simon EP, Robine N, et al. Detection of a recurrent DNAJB1-PRKACA chimeric transcript in fibrolamellar hepatocellular carcinoma. Science 2014;343:1010-4. [Crossref] [PubMed]
- Graham RP, Jin L, Knutson DL, et al. DNAJB1-PRKACA is specific for fibrolamellar carcinoma. Mod Pathol 2015;28:822-9. [Crossref] [PubMed]
- Dinh TA, Vitucci EC, Wauthier E, et al. Comprehensive analysis of the cancer genome atlas reveals a unique gene and non-coding RNA signature of fibrolamellar carcinoma. Sci Rep 2017;7:44653. [Crossref] [PubMed]
- Mortelé KJ, Praet M, Van Vlierberghe H, et al. Focal nodular hyperplasia of the liver: detection and characterization with plain and dynamic-enhanced MRI. Abdom Imaging 2002;27:700-7. [Crossref] [PubMed]
- McFarland EG, Mayo-Smith WW, Saini S, et al. Hepatic hemangiomas and malignant tumors: improved differentiation with heavily T2-weighted conventional spin-echo MR imaging. Radiology 1994;193:43-7. [Crossref] [PubMed]
- Paulson EK, McClellan JS, Washington K, et al. Hepatic adenoma: MR characteristics and correlation with pathologic findings. AJR Am J Roentgenol 1994;163:113-6. [Crossref] [PubMed]
- Tsukuma H, Hiyama T, Tanaka S, et al. Risk factors for hepatocellular carcinoma among patients with chronic liver disease. N Engl J Med 1993;328:1797-801. [Crossref] [PubMed]
- American Joint Committee on Cancer. Amin MB, Edge S, Greene F, et al. editors. AJCC Cancer Staging Manual. 8th edition. New York: Springer, 2016.
- Lee CW, Chan KM, Lee CF, et al. Hepatic resection for hepatocellular carcinoma with lymph node metastasis: clinicopathological analysis and survival outcome. Asian J Surg 2011;34:53-62. [Crossref] [PubMed]
- Watanabe J, Nakashima O, Kojiro M. Clinicopathologic study on lymph node metastasis of hepatocellular carcinoma: a retrospective study of 660 consecutive autopsy cases. Jpn J Clin Oncol 1994;24:37-41. [PubMed]
- Mayo SC, Mavros MN, Nathan H, et al. Treatment and prognosis of patients with fibrolamellar hepatocellular carcinoma: a national perspective. J Am Coll Surg 2014;218:196-205. [Crossref] [PubMed]
- Kaseb AO, Shama M, Sahin IH, et al. Prognostic indicators and treatment outcome in 94 cases of fibrolamellar hepatocellular carcinoma. Oncology 2013;85:197-203. [Crossref] [PubMed]
- Ang CS, Kelley RK, Choti MA, et al. Clinicopathologic characteristics and survival outcomes of patients with fibrolamellar carcinoma: data from the fibrolamellar carcinoma consortium. Gastrointest Cancer Res 2013;6:3-9. [PubMed]
- Yamashita S, Vauthey JN, Kaseb AO, et al. Prognosis of Fibrolamellar Carcinoma Compared to Non-cirrhotic Conventional Hepatocellular Carcinoma. J Gastrointest Surg 2016;20:1725-31. [Crossref] [PubMed]
- Pinna AD, Iwatsuki S, Lee RG, et al. Treatment of fibrolamellar hepatoma with subtotal hepatectomy of transplantation. Hepatology 1997;26:877-83. [Crossref] [PubMed]
- El-Gazzaz G, Wong W, El-Hadary MK, et al. Outcome of liver resection and transplantation for fibrolamellar hepatocellular carcinoma. Transpl Int 2000;13:S406-9. [Crossref] [PubMed]
- Stipa F, Yoon SS, Liau KH, et al. Outcome of patients with fibrolamellar hepatocellular carcinoma. Cancer 2006;106:1331-8. [Crossref] [PubMed]
- Groeschl RT, Miura JT, Wong RK, et al. Multi-institutional analysis of recurrence and survival after hepatectomy for fibrolamellar carcinoma. J Surg Oncol 2014;110:412-5. [Crossref] [PubMed]
- Njei B, Konjeti VR, Ditah I. Prognosis of Patients With Fibrolamellar Hepatocellular Carcinoma Versus Conventional Hepatocellular Carcinoma: A Systematic Review and Meta-analysis. Gastrointest Cancer Res 2014;7:49-54. [PubMed]
- Fouzas I, Sotiropoulos GC, Molmenti EP, et al. “Preemptive” live donor liver transplantation for fibrolamellar hepatocellular carcinoma: A case report. Transplant Proc 2008;40:3806-7. [Crossref] [PubMed]
- Atienza LG, Berger J, Mei X, et al. Liver transplantation for fibrolamellar hepatocellular carcinoma: A national perspective. J Surg Oncol 2017;115:319-23. [Crossref] [PubMed]
- Maniaci V, Davidson BR, Rolles K, et al. Fibrolamellar hepatocellular carcinoma: prolonged survival with multimodality therapy. Eur J Surg Oncol 2009;35:617-21. [Crossref] [PubMed]
- Bower M, Newlands ES, Habib N. Fibrolamellar hepatocellular carcinoma responsive to platinum-based combination chemotherapy. Clin Oncol (R Coll Radiol) 1996;8:331-3. [Crossref] [PubMed]
- Patt YZ, Hassan MM, Lozano RD, et al. Phase II trial of systemic continuous fluorouracil and subcutaneous recombinant interferon Alfa-2b for treatment of hepatocellular carcinoma. J Clin Oncol 2003;21:421-7. [Crossref] [PubMed]
- Fonseca GM, Varella AD, Coelho FF, et al. Downstaging and resection after neoadjuvant therapy for fibrolamellar hepatocellular carcinoma. World J Gastrointest Surg 2014;6:107-11. [Crossref] [PubMed]
- Gras P, Truant S, Boige V, et al. Prolonged Complete Response after GEMOX Chemotherapy in a Patient with Advanced Fibrolamellar Hepatocellular Carcinoma. Case Rep Oncol 2012;5:169-72. [Crossref] [PubMed]
- Weeda VB, Murawski M, McCabe AJ, et al. Fibrolamellar variant of hepatocellular carcinoma does not have a better survival than conventional hepatocellular carcinoma—results and treatment recommendations from the Childhood Liver Tumour Strategy Group (SIOPEL) experience. Eur J Cancer 2013;49:2698-704. [Crossref] [PubMed]


