Intrahepatic cholangiocarcinoma surgery: the impact of lymphadenectomy
Introduction
Intrahepatic cholangiocarcinoma (ICC), second most common primary liver cancer after the hepatocellular carcinoma (HCC), originates from the intrahepatic bile duct (1). Although the annual incidence of ICC remains low, over the past two decades it has been a dramatic rise from 0.32 per 100,000 in 1975 to 1 per 100,000 in 2000 (2). Studies that examined the international time trends in mortality rates using the World Health Organization’s (WHO) database found that the age standardized mortality for ICC had increase in several countries at different rates (3). ICC is classified into three morphologic types: mass-forming which is the more common, periductal infiltrating and intraductal growth types. Some clinical cases with mixed tumor patterns have also been described (4). Radical surgical treatment is considered the only real effective therapy and many centers have recommended an aggressive surgical approach, including major liver resection with extended systematic lymph node (LN) dissection for improving outcomes (5). Unfortunately, the resectability rate of ICC remains low and varies worldwide between 19% and 74% (4). Prognosis of advanced ICC is still unsatisfactory, principally due to the high intrahepatic recurrence rate. Five year survival following resection ranges from 14% to 40% and several prognostic factors have been associated with outcome (6). The majority of studies have identified curative resection (R0), number of tumors (single or multiple), presence of vascular invasion and lymph node metastases (LNM) as the most important independent predictors of survival (7). Pathological LNM in patients with ICC are known to be an extremely poor prognostic risk factor, even when a curative resection is performed (8-10). Considering such circumstances, it is of critical value to determine the validity of surgical resection for LNM diagnosed preoperatively, or whether routine or prophylactic lymphadenectomy needs to be performed. No definitive evidence about the use of LN dissection for ICC was published up to date. In this review, we analyzed and summarized some anatomic considerations of the lymphatic anatomy of the liver and the current knowledge and potential advantages of performing a routine lymphadenectomy in patients with ICC, especially looking at pathological staging, prognosis, prevention of local recurrence and outcome. New areas like lymphadenectomy in cirrhotic patients and laparoscopic lymphadenectomy are also discussed.
Anatomic considerations of the hepatic lymphatic system
The liver produces a large amount of lymph, estimated to be 1–3 L/day in a normal adult liver, which means 25–50% of the lymph of the entire body. Hepatic lymph originates from the perisinusoidal space of Disse, located between hepatocytes and the sinusoids (11). This perisinusoidal space is the site of exchange of materials between blood and liver cells and contains interstitial fluid, mostly plasma, and migrating cells. Interstitial fluid passes through channels between hepatocytes and through the space along the initial segment of the hepatic sinusoids to enter the connective tissue following in small lymphatic capillaries along the branches of portal and hepatic vein or following the hepatic capsule as hepatic lymph. These lymphatic capillaries converge to thicker lymph vessels that drain into the first LN station or communicate directly with the general lymphatic system (11). The hepatic lymphatic system can be classified into a deep and a superficial lymphatic system (12). The deep system follows the portal triads and the hepatic veins, while the superficial system lies in the connective tissue of the convex and inferior hepatic surfaces.
Deep lymphatic system
Deep lymphatic system can be divided into two categories, the periportal system and the hepatic venous systems. In the periportal system, lymphatic vessels run in the Glisson’s sheath along with the portal vein, hepatic artery, or bile duct and converge in 12 to 15 separate lymphatic vessels at the hepatic hilum. The periportal lymphatic tract is responsible for 80% of the hepatic lymph drainage and flows in the same direction as bile. Outside the liver, the efferent lymphatic vessels communicate with hilar and peripancreatic LNs that are the first LN station. After this station, hilar LNs present connection with celiac, juxtaesophageal and gastro-cardiac LNs along the lesser omentum. Peripancreatic LNs connect with the superior mesenteric LNs. Subsequently, hilar and peripancreatic routes connect with the cisterna chyli through the paraaortic LNs while the juxtaesophageal route directly connects with the general lymphatic system of the posterior mediastinum. In reference to the hepatic venous system, which is the second deep system, 5–6 separate vessels follows the inferior vena cava and lymph directly flows into the general lymphatic system of the posterior mediastinum. Also, some lymph along the right hepatic vein flows into paraaortic LNs through the right hepatorenal ligament (13).
Superficial lymphatic system
Superficial lymphatic system exists in the subserosal connective tissue of the hepatic surface and develops from the convex surface along the bilateral coronary ligament, bilateral triangular ligament and falciform ligament and from the inferior surface of the liver communicating with distant LNs or the general lymphatic system. In the hepatic convex surface system lymph enters directly the distant thoracic LNs including pericardial, superior phrenic, and juxta esophageal LNs flowing into the general lymphatic system of the anterior mediastinum or paraaortic LNs through the right or left phrenic artery. In the inferior hepatic surface of the superficial system, lymphatic vessels converge in the liver pedicle, and connect with the different regional LNs. In addition, lymphatic vessels from the gallbladder connect with the cystic LNs. Posteriorly to the caudate lobe and liver bare area, lymphatics follow the inferior vena cava and flow into the posterior mediastinum general system (12).
Imaging & staging
The accuracy of preoperative imaging assessment for LNM by CT scan has been unsatisfactory, and the current imaging methods had not provided yet an accurate LN status (9). Sensitivity and specificity of CT scan evaluation for detecting LNM had been reported in the range of 40–50% and 77–92% respectively (12). In a study by Park et al. a higher sensitivity (80%) and specificity (92%) of PET-CT over CT in detecting LNM in ICC patients was shown, but in a small population (14). Further studies are needed to define the exact role of PET-CT in the preoperative evaluation of patients with ICC and suspected LNM.
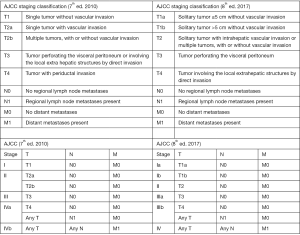
According to the 7th edition of the manual introduced by the American Joint Committee on Cancer (AJCC) in 2010 a new TNM staging system for ICC was introduced and the relevance of performing a nodal basin evaluation in this disease was established (15). More recently, the updated 8th edition of the AJCC classification presented several modifications for ICC staging (Figure 1) as the inclusion of tumor size, vascular invasion and the recommendation that ≥6 LNs needs to be evaluated to properly stage ICC patients (16). An expert panel sponsored by the Americas Hepato-Pancreato-Biliary Association (AHPBA) already recommended LN dissection as part of the standard surgical treatment for patients undergoing curative resection of ICC (7). A retrospective study based in the US National Cancer Database defined that the optimal extent or a lymphadenectomy for resected ICC should include ≥3 LNs (17). A recent multicenter study analyzed 1,154 patients undergoing hepatectomy for ICC in 14 major hepatobiliary centers (18). While pathological characteristics of the LNs were strongly associated with long term survival, only one fourth of the population treated by liver resection for ICC had an adequate LN staging according to the newly proposed AJCC 8th edition staging system (18). Moreover, it was demonstrated that radiological LN evaluation could be inaccurate in up to 40% of patients with ICC, so it shouldn’t be considered a valid alternative to LN dissection. The best results of lymphadenectomy to differentiate between patients with favorable and poor prognosis was reached when ≥6 LNs were harvested (18).
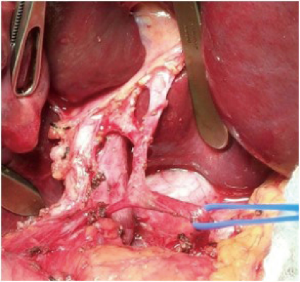
Lymphadenectomy in ICC
The value of lymphadenectomy at the time of radical surgical resection has already been established for several gastrointestinal malignancies such as those with gastric, pancreatic and colorectal origin (19). Lymphadenectomy is also the standard of care for some primary hepato-biliary tumors such as fibrolamellar HCC and gallbladder cancer (20). LNM have demonstrated a strong negative prognostic effect in patients with ICC but the exact role of performing a lymphadenectomy has not been standardized yet. Although some series from Asia have described that most centers do not regularly perform lymphadenectomy, other studies from the West suggested that the technique is becoming more routine (8,19,21,22). A recent study demonstrated a growing adoption of lymphadenectomy all over the world over the past 16 years. In a 15-year period, the percentage of patients undergoing lymphadenectomy for ICC doubled (year 2000: 44.4% versus year 2015: 81.5%) (23). In contrast with previous reports, comparison between Eastern and Western centers in the review by Zhang et al. didn’t show any differences between both regions after controlling tumor factors such as size, number, adjacent organ invasion and T status (23). Given the poor accuracy of preoperative imaging assessment and the absence of models that can identify patients at high risk of LNM, routine histological examination with lymphadenectomy remains to be the only accurate method to diagnose this entity (1). In the ideal lymphadenectomy all regional nodal stations must be included. As was stated previously, clinical and pathological data showed that the first stations to become involved in the metastatic process are LNs of the hepatoduodenal ligament (station 12) and of the hepatic artery (station 8) so this LN should be removed in all patients (7). Retropancreatic LNs (station 13) are still considered as first echelon nodes and their routine removal is recommended in ICC localized in the right hemi-liver. Another direct lymphatic pathway is recognized as running from the left hemi-liver through the lesser omentum. So, for left sided tumors, routine removal of left gastric artery nodes along the lesser curvature (station 7) and right esophageal crus nodes (station 1) around the cardiac portion of the stomach is also recommended (23). At present time the dissected area selected for lymphadenectomy depends on the Institution or surgeon’s policy and is not commonly defined in all studies. Hepatoduodenal ligament (Figure 2), sometimes including celiac axis or peripancreatic LNs are the most common dissected areas in most centers (1). A cutoff of six LNs is the recommendation to N stage patients adequately (18). As we see in Table 1, lymphadenectomy had been performed in a range of 41% to 98% of the patients treated by liver resection for ICC in 12 recent studies. It is remarkable that 2 systematic reviews with 2,358 and 4,756 patients demonstrated higher rates of lymphadenectomy, 67% and 78.5% respectively (16,28). In terms of complication of this procedure, a French multicenter study with 522 resected ICC patients demonstrated a 42% morbidity rate with 21.6% of major complications but there was no association of morbidity with the performance of lymphadenectomy (29).

Full table
Potential advantages of lymphadenectomy in ICC
The potential advantages of lymphadenectomy can be divided into three principal arguments: (I) improvement of pathological staging and prediction of prognosis after resection; (II) prevention of LN recurrence and local control; (III) increment of survival time in carefully selected cases (1).
Pathological staging and prognosis
Several studies have noted that the condition of the LNs is a relevant prognostic factor among ICC patients. In addition to tumor size, number of lesions and vascular invasion, LNM had been referred systematically as one of the most important negative prognostic factors associated with long term outcome (5,8,9,16,18,30,31). In recent studies, incidence of LNM ranges from 16% to 45% (see Table 1). As already stated, LN status cannot be evaluated unless a surgical procedure is performed and LNs are harvested along the different LN stations already described. Avoiding LNs staging can lead to a wrong prognosis for the patient, as well as preclude preoperative discussions about indications of adjuvant therapy. Patients with unstaged N disease (Nx) have been reported to have a worse survival compared with negative LN patients (N0) within the first 18 months following resection although a similar outcome was shown in those who survived longer than 18 months (18). Similarly, a recent multicenter study showed an intermediate prognosis of Nx patients with a median overall survival of 43 months (23). Heterogeneous outcome of Nx patients suggests that this group is a combination of N0 and under-staged N1 patients and emphasizes the impact that failure of performing a routine lymphadenectomy could have on ICC prognostic classification in this population. Some authors still sustains that lymphadenectomy can be omitted in selected patients as those with ICC less than 5 cm (10). However, according to a recent multicenter study that found 24% of LNM in T1a and 22% in T1b patients, routine lymphadenectomy should be considered independently of tumor size (23).
Prevention of loco-regional recurrence
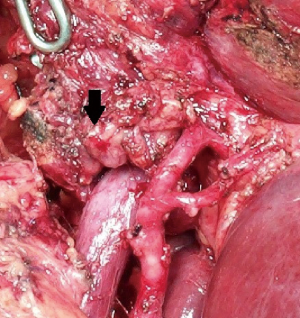
The value of LNs dissection in decreasing locoregional recurrence is still unclear (9,25). Some authors have suggested a therapeutic benefit in reducing jaundice due to biliary obstruction or relieving symptoms of LN swelling in patients with local recurrence (1) (Figure 3). No benefit in survival and similar recurrence patterns was demonstrated by Kim et al. in 215 ICC patients. Distant recurrence was more frequent (68.5%) than local recurrence with no significant differences between patients treated with or without lymphadenectomy (8).
Survival in patients with LNM
As depicted in Table 1, long term survival in patients with LN positive disease is rare but even possible (0–15%). Bagante et al. showed that ICC patients with N1 diseases had over a 3-fold increase risk of death and no patient with >3 LNM survived to 5 years (26). Jutric et al. demonstrated a poorest survival of LN positive patients in ICC >5 cm, older patient age and when no adjuvant chemotherapy was implemented (9). Staging systems like the AJCC system are helpful and applicable to population of patients but such staging schemas can be less useful in individual patients. The disease specific nomograms have been elaborated with the purpose of predicting long-term survival in daily practice for the individual patient (31). Two nomograms that consider nodal status as a variable had been published (31,32). The Wang nomogram includes three continuous variables as preoperative CA 19-9 and CEA levels plus tumor size. The dichotomous variables included in this nomogram are nodal involvement, vascular invasion and direct invasion or local metastases (32). The Hyder nomogram includes age and tumor size as continuous variables and number of tumors, nodal status, vascular invasion and underlying cirrhosis as categorical variables. In this nomogram nodal status includes not only pN0 and pN1 but also pNx (31). A study by Doussot et al. was the first to externally validate and compare both nomograms together with the Fudan risk score after resection of ICC (27). The utility of both nomograms was demonstrated by an accurate estimation of the patient prognosis after liver resection for ICC, prognosis that can help in decisions regarding the indication of adjuvant therapies (27). In a recent multicenter study adjuvant chemotherapy among patients with N1 disease tended to have a better 5-year OS (33). In fact, in patients with T3/T4 tumors and N1 disease, adjuvant chemotherapy demonstrated a strongest association with a better survival (33). Authors conclude that following surgical resection, although adjuvant chemotherapy did not influence the long-term prognosis of all ICC patients, a potential survival benefit in selected patients at increased risk for recurrence was established (33). In the multimodality treatment of ICC patients accurate pathologic staging with a formal LN evaluation by lymphadenectomy should be the rule.
New issues about lymphadenectomy in ICC
Lymphadenectomy in patients with cirrhosis
While among primary liver tumors, the association between HCC and cirrhosis is well established, more recently a significantly increase risk of ICC, with estimates ranging from 5% to 14% had been published (3). A 2018 study by Bagante et al. analyzed the impact of lymphadenectomy on peri-operative outcomes of 118 patients with cirrhosis who underwent hepatectomy for ICC (24). Compared with non-cirrhotic patients, only 20.4% of major liver resections and 19.5% of lymphadenectomies were performed for ICC in the presence of cirrhosis (24). In this population, an increased risk of complications such as superficial and surgical site infections, cardiovascular and respiratory complications had been recently reported (18). In conclusion, when operating on patients with ICC in a cirrhotic liver the recommendations of the 8th edition of the AJCC manual to perform extended LN harvest should be considered in light of these recent data that demonstrated a higher complication rate.
Laparoscopic lymphadenectomy
ICC is still a relatively uncommon indication for laparoscopic surgery due to the frequent indications in this type of tumor of major liver resections plus the requirement of regional lymphadenectomy. Previous reports have demonstrated the applicability of laparoscopy in gallbladder cancer that also requires a lymphadenectomy as a component of the radical surgical treatment of this entity (34,35). A recent study from a single Institution analyzed the safety and feasibility of laparoscopic liver resection with associated lymphadenectomy for ICC by a matching comparative study with open procedures (36). The laparoscopic approach resulted in less blood loss despite extensive use of the Pringle maneuver. The oncologic competence of the procedure was evidenced by the R0 resection rate, margin depth, number of LNs removed and overall and recurrence free survival, all parameters that resulted comparable with the open procedures (36). More time was required for the laparoscopic lymphadenectomy (260 versus 190 minutes in the open group) (36). Recently, Kobayashi et al. reinforced the value of laparoscopic LN sampling in patients with biliary tract cancers but selecting patients and indications for this procedure (37).
Conclusions
- In addition to other tumor characteristics LNM has constantly been identified as one of the most relevant prognostic factors associated with long-term outcomes of patients with ICC.
- Given the poor accuracy of preoperative clinical staging, routine histological assessment with lymphadenectomy appears to be the only accurate method to diagnose LNM at the present time.
- The rate of LNM (16–45%) occurs independently of T stage with one-fifth of patients with T1 disease having nodal metastases.
- Appropriate staging, prognosis and selection for adjuvant chemotherapy are the strongest arguments in performing routine lymphadenectomy.
- Avoiding LN staging can lead to heterogenous and incorrect prognostic classifications in some patients with ICC.
- The trend in increased use of lymphadenectomy all over the world suggests a growing adoption of the AJCC recommendation in the surgical therapy of ICC.
- The role of lymphadenectomy in decreasing locoregional recurrence and improving survival remains unclear.
- Accurate patient prognosis estimation after liver resection for ICC was demonstrated by two nomograms that may be useful in the decision process regarding adjuvant therapy indications after resection.
- In the cirrhotic patients lymphadenectomy had been associated with a higher risk of complications.
- Laparoscopic lymphadenectomy in ICC could be technically feasible and safe but more studies are required to validate the procedure.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Adachi T, Eguchi S. Lymph node dissection for intrahepatic cholangiocarcinoma: A critical review of the literature to date. J Hepatobiliary Pancreat Sci 2014;21:162-8. [Crossref] [PubMed]
- Wang K, Zhang H, Xia Y, et al. Surgical options for intrahepatic cholangiocarcinoma. Hepatobiliary Surg Nutr 2017;6:79-90. [Crossref] [PubMed]
- Bridgewater J, Galle PR, Khan SA, et al. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J Hepatol 2014;60:1268-89. [Crossref] [PubMed]
- Bektas H, Yeyrek C, Kleine M, et al. Surgical treatment for intrahepatic cholangiocarcinoma in Europe: a single center experience. J Hepatobiliary Pancreat Sci 2015;22:131-7. [Crossref] [PubMed]
- Luo X, Yuan L, Wang Y, et al. Survival Outcomes and Prognostic Factors of Surgical Therapy for All Potentially Resectable Intrahepatic Cholangiocarcinoma: A Large Single-Center Cohort Study. J Gastrointest Surg 2014;18:562-72. [Crossref] [PubMed]
- Lafaro KJ, Cosgrove D, Geschwind JFH, et al. Multidisciplinary care of patients with intrahepatic cholangiocarcinoma: Updates in management. Gastroenterol Res Pract 2015;2015. [Crossref] [PubMed]
- Weber SM, Ribero D, O’Reilly EM, et al. Intrahepatic Cholangiocarcinoma: Expert consensus statement. HPB (Oxford) 2015;17:669-80. [Crossref] [PubMed]
- Kim DH, Choi DW, Choi SH, et al. Is there a role for systematic hepatic pedicle lymphadenectomy in intrahepatic cholangiocarcinoma? A review of 17 years of experience in a tertiary institution. Surgery 2015;157:666-75. [Crossref] [PubMed]
- Jutric Z, Johnston WC, Hoen HM, et al. Impact of lymph node status in patients with intrahepatic cholangiocarcinoma treated by major hepatectomy: a review of the National Cancer Database. HPB (Oxford) 2016;18:79-87. [Crossref] [PubMed]
- Chang ME, Lei HJ, Chen MH, et al. Evaluation of prognostic factors and implication of lymph node dissection in intrahepatic cholangiocarcinoma: 10-year experience at a tertiary referral center. J Chin Med Assoc 2017;80:140-6. [Crossref] [PubMed]
- Tanaka M, Iwakiri Y. Lymphatics in the liver. Curr Opin Immunol 2018;53:137-42. [Crossref] [PubMed]
- Morine Y, Shimada M. The value of systematic lymph node dissection for intrahepatic cholangiocarcinoma from the viewpoint of liver lymphatics. J Gastroenterol 2015;50:913-27. [Crossref] [PubMed]
- Pupulim LF, Vilgrain V, Ronot M, et al. Hepatic lymphatics: anatomy and related diseases. Abdom Imaging 2015;40:1997-2011. [Crossref] [PubMed]
- Park TG, Yu Y, Park ÞBJ, et al. Implication of Lymph Node Metastasis Detected on 18 F-FDG PET/CT for Surgical Planning in Patients With Peripheral Intrahepatic Cholangiocarcinoma. Clin Nucl Med 2014;39:1-7. [Crossref] [PubMed]
- Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 2010;17:1471-4.
- Amini N, Ejaz A, Spolverato G, et al. Management of Lymph Nodes During Resection of Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma: A Systematic Review. J Gastrointest Surg 2014;18:2136-48. [Crossref] [PubMed]
- Brauer DG, Fields RC, Tan BR Jr, et al. Optimal extent of surgical and pathologic lymph node evaluation for resected intrahepatic cholangiocarcinoma. HPB (Oxford) 2018;20:470-6. [Crossref] [PubMed]
- Bagante F, Spolverato G, Weiss M, et al. Assessment of the Lymph Node Status in Patients Undergoing Liver Resection for Intrahepatic Cholangiocarcinoma: the New Eighth Edition AJCC Staging System. J Gastrointest Surg 2018;22:52-9.
- Zhang XF, Chen Q, Kimbrough CW, et al. Lymphadenectomy for Intrahepatic Cholangiocarcinoma: Has Nodal Evaluation Been Increasingly Adopted by Surgeons over Time?A National Database Analysis. J Gastrointest Surg 2018;22:668-75. [Crossref] [PubMed]
- Amini N, Kim Y, Wilson A, et al. Prognostic Implications of Lymph Node Status for Patients With Gallbladder Cancer: A Multi-Institutional Study. Ann Surg Oncol 2016;23:3016-23. [Crossref] [PubMed]
- Vitale A, Moustafa M, Spolverato G, et al. Defining the possible therapeutic benefit of lymphadenectomy among patients undergoing hepatic resection for intrahepatic cholangiocarcinoma. J Surg Oncol 2016;113:685-91. [Crossref] [PubMed]
- Miyata T, Yamashita Y-I, Yamao T, et al. Clinical benefits of lymph node dissection in intrahepatic cholangiocarcinoma: A retrospective single-institution study. Anticancer Res 2017;37:2673-7. [Crossref] [PubMed]
- Zhang X-F, Chakedis J, Bagante F, et al. Trends in use of lymphadenectomy in surgery with curative intent for intrahepatic cholangiocarcinoma. Br J Surg 2018;105:857-66. [Crossref] [PubMed]
- Bagante F, Spolverato G, Weiss M, et al. Surgical Management of Intrahepatic Cholangiocarcinoma in Patients with Cirrhosis: Impact of Lymphadenectomy on Peri-Operative Outcomes. World J Surg 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Gil E, Joh JW, Park HC, et al. Predictors and patterns of recurrence after curative liver resection in intrahepatic cholangiocarcinoma, for application of postoperative radiotherapy: A retrospective study. World J Surg Oncol 2015;13:227. [Crossref] [PubMed]
- Bagante F, Gani F, Spolverato G, et al. Intrahepatic Cholangiocarcinoma: Prognosis of Patients Who Did Not Undergo Lymphadenectomy. J Am Coll Surg 2015;221:1031-40.e1. [Crossref] [PubMed]
- Doussot A, Groot-Koerkamp B, Wiggers JK, et al. Outcomes after Resection of Intrahepatic Cholangiocarcinoma: External Validation and Comparison of Prognostic Models. J Am Coll Surg 2015;221:452-61. [Crossref] [PubMed]
- Mavros MN, Economopoulos KP, Alexiou VG, et al. Treatment and prognosis for patients with intrahepatic cholangiocarcinoma: Systematic review and meta-analysis. JAMA Surg 2014;149:565-74. [Crossref] [PubMed]
- Doussot A, Lim C, Gómez Gavara C, et al. Multicentre study of the impact of morbidity on long-term survival following hepatectomy for intrahepatic cholangiocarcinoma. Br J Surg 2016;103:1887-94. [Crossref] [PubMed]
- Spolverato G, Ejaz A, Kim Y, et al. Tumor Size Predicts Vascular Invasion and Histologic Grade Among Patients Undergoing Resection of Intrahepatic Cholangiocarcinoma. J Gastrointest Surg 2014;18:1284-91. [Crossref] [PubMed]
- Hyder O, Marques H, Pulitano C, et al. A nomogram to predict long-term survival after resection for intrahepatic cholangiocarcinoma : An Eastern and Western experience. JAMA Surg 2014;149:432-8. [Crossref] [PubMed]
- Wang Y, Li J, Xia Y, et al. Prognostic nomogram for intrahepatic cholangiocarcinoma after partial hepatectomy. J Clin Oncol 2013;31:1188-95. [Crossref] [PubMed]
- Reames BN, Bagante F, Ejaz A, et al. Impact of adjuvant chemotherapy on survival in patients with intrahepatic cholangiocarcinoma: a multi-institutional analysis. HPB (Oxford) 2017;19:901-9. [Crossref] [PubMed]
- de Aretxabala X, Leon J, Hepp J, et al. Gallbladder cancer: role of laparoscopy in the management of potentially resectable tumors. Surg Endosc 2010;24:2192-6. [Crossref] [PubMed]
- Agarwal AK, Javed A, Kalayarasan R, et al. Minimally invasive versus the conventional open surgical approach of a radical cholecystectomy for gallbladder cancer: A retrospective comparative study. HPB (Oxford) 2015;17:536-41. [Crossref] [PubMed]
- Ratti F, Cipriani F, Ariotti R, et al. Safety and feasibility of laparoscopic liver resection with associated lymphadenectomy for intrahepatic cholangiocarcinoma: a propensity score-based case-matched analysis from a single institution. Surg Endosc 2016;30:1999-2010. [Crossref] [PubMed]
- Kobayashi S, Tomokuni A, Takahashi H, et al. Laparoscopic hilar lymph node sampling in patients with biliary tract cancers that are rarely associated with nodal metastasis. Surg Laparosc Endosc Percutan Tech 2018;28:90-5. [PubMed]