Palbociclib for treatment of metastatic melanoma with copy number variations of CDK4 pathway: case report
Introduction
The mortality associated with unresectable or metastatic melanoma remains high despite of the emergence of new anti-tumor agents such as small molecular tyrosine inhibitors (anti-BRAF and anti-MEK agents) and immune checkpoint inhibitors (anti-PD-1/PD-L1 and anti-CTLA4 antibodies). It is still challenging to explore new agents for the patients with advanced melanoma. We herein report two cases of metastatic melanoma with CNVs of CDK4 pathway-related genes, including CDK4, CCND1 and P16INK4a that was oncologically controlled with palbociclib, a highly specific inhibitor of cyclin dependent kinase 4 (CDK4) and CDK6, which has been approved for metastatic breast cancer by the US FDA in 2016.
Case presentation
Case 1
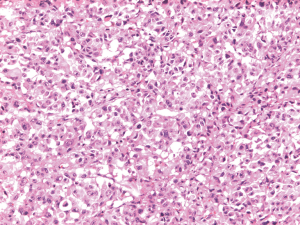
A 55-year-old woman was transferred to Beijing Cancer hospital because of progressive dysphagia. Upper endoscopy revealed a pigmented mass at the esophagogastric junction. No distant metastases were detected on computed tomography except a Para cardiac lymph node. Therefore, partial esophagectomy and gastrectomy were performed on August 25, 2014. Pathological examination revealed melanoma at the esophagogastric junction with a thickness of at least 12 mm to submucosa and lymph node metastases (1/18) (Figure 1). Temozolomide plus cisplatin and Endostar were given as adjuvant chemotherapy. But she declined more cycles of therapy because of grade 3 gastrointestinal reactions during the first cycle.
On November 11, 2015, positron-emission tomographic/computed tomographic scan (PET-CT) revealed an unresectable metastatic mass of 2 cm between the pancreas and the remnant stomach. The patient received the first-line treatment of nivolumab (3 mg/kg, every two weeks) combined with ipilimumab (1 mg/kg, every three weeks for four doses). After 6 months, her disease progressed, showing that the abdominal masses increased in numbers and in size (~9.3 cm) with massive ascites.
For her tumor sample, Sanger sequencing were used to detect somatic mutations of NRAS, BRAF and CKIT and Q-PCR was used to detect copy number variation of CDK4, CCND1 and P16INK4a. No somatic mutations were detected within exons 1 and 2 of the NRAS gene, exons 11 and 15 of the BRAF gene, and exons 11, 13, 15, 17, 18 of the CKIT gene. The copy number of CDK4, CCND1 and P16INK4a was 3.6, 3, 2 copies, respectively.
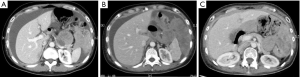
Therefore, CDK4/6 inhibitor palbociclib was recommended. It was initiated at a dose of 75 mg orally days 1–21 of each 28-day cycle from August 24, 2016. During the palbociclib therapy, the patient had done well with significant improvement in her overall function. CT showed stabilization of overall tumor size for approximately 6 months following initiation (Figure 2). Till Feb 28, 2017, new lesions appeared in liver, subcutaneous tissue and inguinal region. The progression-free survival (PFS) for palbociclib treatment lasted for 6 months. Grade 1 neutropenia as the only adverse events attributed to the medication.
Case 2
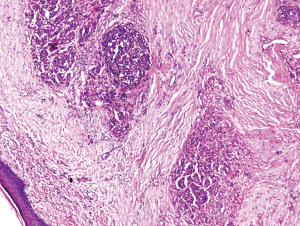
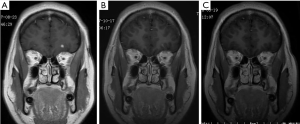
A 33-year-old female was diagnosed with scalp melanoma and got complete resection in 2000 (Figure 3). The tumor relapsed and was resected again in 2008. In 2016, computed tomographic (CT) scan revealed some pulmonary nodules, one of which was excised and founded to be melanoma. PET-CT confirmed melanoma metastases to the lung, and lymph nodes of the mediastinal and left hilus. From Aug, 2016 to Jan, 2017, she received dacarbazine plus cisplatin and Endostar as first-line therapy for 6 cycles with best response as partial response. By July, 2017, new metastatic nodules had developed in left frontal lobe of her brain. The patient underwent stereotactic radiosurgery for the metastatic loci in the brain and then took temozolomide as second line for one cycle. New metastatic lesions appeared in the left parotid gland and skin in bilateral frontal and bilateral parietal and occipital junction area. The workup revealed that the copy number of CDK4, CCND1 and P16INK4a of her tumor sample was 3, 2, 1 copies, respectively. While somatic mutations within exons 1 and 2 of the NRAS gene, exons 11 and 15 of the BRAF gene, and exons 11, 13, 15, 17, 18 of the CKIT gene were all wild types. So she took palbociclib as third-line therapy in Aug, 2017 and was evaluated every two to three months. At last follow-up (March, 2018), the metastatic lesions in the left parotid gland, lung, lymph nodes and skin in bilateral frontal and bilateral parietal and occipital junction area were stable. The metastatic lesion in her brain got shrunken gradually and disappeared in March 2018 (Figure 4). More than 7 months after starting treatment, she continued on palbociclib therapy, with no notable adverse effects.
Discussion
Cyclin-dependent kinases (CDK) are serine/threonine kinases that drive cell-cycle progression and regulate cell proliferation (1). Dysregulation of CDKs plays a central role in tumorigenesis and tumor progression. The P16INK4a (encoded by Cdkn2a)-cyclin D (popularly CCND1, encoded by Ccnd1)-CDK4/6-retinoblastoma protein (Rb1) pathway, well known as CDK4 pathway, promotes G1 to S cell-cycle transition, and is commonly dysregulated in most cancers (2). Gain or overexpression of CCND1, gain or active mutation of CDK4, and loss of P16INK4a are all common events in cutaneous melanoma development and progression (3-5).
Young et al. found that 37 of 47 melanoma cell lines were sensitive to palbociclib, and put forward that P16INK4a loss indicated the sensitivity to palbociclib while loss of Rb1 indicated palbociclib resistance (4). Kong et al. also reported that CDK4 pathway aberration is rather frequent in Asian melanoma patients. The melanoma cells and patient-derived xenograft (PDX) containing specific CDK4 pathway aberrations are sensitive to CDK4/6 inhibitors (6). In fact, in our data palbociclib could almost eliminate acral melanoma of PDX model with CDK4 gain plus Ccnd1 gain, indicating the CDK4 pathway as a potential therapeutic target (6).
Our first case indicates that CDK4 may be a potential target for mucosal melanoma treatment. Mucosal melanoma has significantly worse outcomes than cutaneous subtype. The 5-year disease-free survival rates range from 0% to 20% (7). Currently, few clinical results could be applied to mucosal melanoma treatments. And its molecular profile has not been extensively investigated. It is expected that more detailed molecular characterisations need to be identified for searching the potential targets. In our report, this patient’s disease got controlled for about 6 months after taking CDK4/6 inhibitor palbociclib.
The second case is more intriguing. Brain metastasis is hardly controlled clinically. The patient’s brain lesion was radiated In July, 2017. Then she took palbociclib one month later. How the lesion in her brain kept shrinking and finally disappeared? This phenomenon is seldom seen in foci treated by radiation alone. Is it the clinical effect of CDK4 inhibitor or the combination of CDK4 inhibitor and radiation? It is worth of researching further.
Conclusions
To our knowledge, this is the first report about CDK4/6 inhibitor for metastatic melanoma with copy number variations of CDK4 pathway-related genes. Although these two patients did not get complete recession or partial recession clinically, their diseases have been controlled for over 6 months after failed in chemotherapy or immunotherapy, indicating the CDK4 pathway is a potential therapeutic target.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: The study protocol and informed consent forms were reviewed and approved by the institutional independent ethics committee. Written informed consent was obtained from the patient for publication of this manuscript and any accompanying image.
References
- Nurse PM. Nobel lecture. Cyclin dependent kinases and cell cycle control. Biosci Rep 2002;22:487-99. [Crossref] [PubMed]
- Ortega S, Malumbres M, Barbacid M. Cyclin D-dependent kinases, INK4 inhibitors and cancer. Biochim Biophys Acta 2002;1602:73-87. [PubMed]
- Curtin JA, Fridlyand J, Kageshita T, et al. Distinct sets of genetic alterations in melanoma. N Engl J Med 2005;353:2135-47. [Crossref] [PubMed]
- Young RJ, Waldeck K, Martin C, et al. Loss of CDKN2A expression is a frequent event in primary invasive melanoma and correlates with sensitivity to the CDK4/6 inhibitor PD0332991 in melanoma cell lines. Pigment Cell Melanoma Res 2014;27:590-600. [Crossref] [PubMed]
- Sauter ER, Yeo UC. Cyclin D1 is a candidate oncogene in cutaneous melanoma. Cancer Res 2002;62:3200-6. [PubMed]
- Kong Y, Sheng X, Wu X, et al. Frequent Genetic Aberrations in the CDK4 Pathway in Acral Melanoma Indicate the Potential for CDK4/6 Inhibitors in Targeted Therapy. Clin Cancer Res 2017;23:6946-57. [Crossref] [PubMed]
- Lian B, Guo J. Checkpoint inhibitors in treatment of metastatic mucosal melanoma. Chin Clin Oncol 2014;3:37. [PubMed]