
Neoadjuvant chemotherapy and interval debulking surgery for advanced ovarian cancer, an alternative with multiple advantages
Introduction
Ovarian cancer (OC) is one of the tumors among women with higher mortality rates; it has a 5-year survival below 45%. It is the seventh most common malignancy and the eighth cause of cancer death among women (1,2). It is also the second most common gynecologic cancer and the leading cause of gynecologic cancer death (3,4). Worldwide, Central and Eastern Europe followed by Northern America have the highest incidence rates where they exceed 8 per 100,000 (Table 1) (1,5). Non-Hispanic, white women also have the leadership in incidence among different ethnicities (1). Every year, OC accounts for an estimated of 239,000 new cases and 152,000 deaths worldwide (1,5). Lifetime risk for women of developing OC is 1 in 75 and chances of dying is 1 in 100 (6).
Age of incidence varies, but it usually affects women older than 45 years with a median age of diagnosis at 63. Women younger than 40 are usually affected by germinal ovary tumors, contrary to those in older groups where epithelial tumors are the most common. By stage, less than 15% of OC are diagnosed in stage 1 when the 5-year survival is 92% (5). The 5-year survival in advanced epithelial ovary cancer (EOC) is of 30%, approximately (7).
OC is classified according to the tissue where they originate: the epithelium, stromal endocrine cells and germ cells neoplasms of the ovary. EOC accounts for 90% of these malignancies (8). It is most common in women in the 6th and 7th decades. Family history is one of the most significant risk factors; up to 7% of women with OC have a positive family history (3). Hereditary syndromes with mutations in BRCA1 and BRCA2 genes increase the risk by 44% and 27%, respectively (3). Another genetic syndrome associated with OC is Lynch syndrome also known as non-polyposis colorectal cancer syndrome with mutations in MLH1, MSH2, PMS1, and MSH6. Factors associated with greater lifetime ovulation or estrogen exposure as nulliparity, early age of menarche or late age of menopause, and use of hormone replacement therapy have been established as risk factors for the development of this disease (3,8). On the other hand, some factors that include suppression of ovulation such as increased number of parities and use of oral contraceptives are considered as protective factors for EOC.
CA-125, a protein encoded by the MUC16 gene, is the most frequently used tumor marker for OC. It is an easy measurement that reflects the tumor growth and it is efficient to evaluate treatment response (9). Levels should be below 35 U/mL. It is a predictor factor during the treatment and useful marker for follow-up of EOC. It is important to mention that it should not be used as a diagnostic test because it may be elevated in other cancers situations such as endocervical carcinoma and pancreatic cancer; or physiologic conditions pancreatitis, endometriosis, peritonitis, cirrhosis or first trimester of pregnancy.
The current evaluation for identifying patients with advanced EOC is limited. The combination of CA-125, imaging and a physical examination is mandatory for staging the patient. Laparoscopy is a new model used for defining stage and type of surgery that should be done.
Treatment for EOC
Since the introduction of platinum-based therapy more than 30 years ago for treatment of OC, survival in these patients has increased in small proportions. Five-year survival increased less than 5% between 1980 and 2004 (10). PDS followed by adjuvant chemotherapy (ACT) with paclitaxel and carboplatin is the standard of treatment for EOC. This approach was introduced after a retrospective study in 1975 that demonstrated that postoperative largest residual tumor, decreased survival (11). The aim is to achieve optimal cytoreduction which is defined as having less than 1 cm of residual disease, ideally to achieve no visible disease (12). Relapses account for 75–80% within the 5 years after diagnosis (13). Although this is the standard of care, PDS has been associated with increased perioperative morbidity, mortality and diminished QOL. Because of these disadvantages, new randomized trials have shown an alternative with a three-cycle neoadjuvant chemotherapy (NAC) followed by IDS and fulfillment of the rest of chemotherapy after it, usually 3 more ACT cycles. This approach has demonstrated a higher rate of optimal cytoreduction surgery in advanced EOC.
As many authors agree, undergoing an extensive cytoreductive surgery is justified because residual disease, at the end of surgery, is the major prognostic factor for survival (14). Nowadays, the main goal of PDS and IDS is to avoid leaving any macroscopic residual tumor.
Chemotherapy with taxanes and platinum has a high sensibility of up to 80% response. NAC-IDS has been demonstrated as a valid strategy in patients with stage IV, unresectable tumors with large ascites and diffuse disseminations have a dramatic disappearance after it. For this reason, in the coming years, it is expected that NAC-IDS becomes the gold standard treatment.
First reports of NAC application were in 1990. A group at Yale University reported a group of 17 patients treated with NAC who were diagnosed with advanced EOC. The median survival was 15 months and survival curves were the same between patients at the International Federation of Gynecology and Obstetrics (FIGO) stage III and IV (15). After that, in 1991 a retrospective case-control study using NAC-IDS was done at MD Anderson Cancer Center. A total of 22 patients were registered with FIGO stage III or IV EOC who underwent to laparotomy and biopsy. Thereafter, they received 2 to 4 cycles of chemotherapy followed by IDS and ACT. Comparisons were done among two control groups, one with suboptimal debulking surgery with platinum chemotherapy and another group with laparotomy and PDS followed by ACT. Results showed no difference in median survival among the 3 groups concluding that patients with bulky disease have a poorer prognosis despite of the treatment given (16).
Several clinical trials have compared both approaches. In 1994, the first prospective randomized clinical trial was published. Seventy-nine patients underwent suboptimal PDS and under randomization either to platinum chemotherapy alone or to platinum-based NAC followed by IDS if chemotherapy response was observed. They didn’t find any difference in median survival of both groups. After that, the European Organization for Research and Treatment of Cancer (EORTC-55865) study was done in 1995. It included OC FIGO stages IIB, IIC, III, and IV who had residual lesions measuring more than 1 cm in diameter after PDS. After 3 cycles of cyclophosphamide and cisplatin, patients were randomly assigned to undergo either debulking surgery or no surgery, followed by ACT. A total of 319 patients were eligible for the study, 278 patients were evaluated: 140 patients treated with three cycles of cisplatin and cyclophosphamide followed by IDS and three additional ACT and 138 patients who received chemotherapy without IDS. The group who underwent IDS have a statistically significant advantage in median survival (6 months) and a difference of 10% in the survival at 2 years. Surgery reduced the risk of death by 33% (17).
After that, in 2004 another randomized phase III trial was done by Gynecologic Oncology Group (GOG), which enrolled 550 patients with stage III or IV of EOC and residual intraperitoneal tumor larger than 1 cm in diameter: all received 3 NAC cycles. After it, a total of 448 patients with no progression and limited extraperitoneal disease were randomly assigned either to a secondary PDS followed by 3 more cycles as ACT or to three more chemotherapy cycles alone. They didn’t find improvement in progression-free survival (PFS) or overall survival (OS): OS was 10.5 vs. 33.7 months (18). The study found no benefit of secondary cytoreduction in patients with maximal PDS but the group of patients with inadequate primary surgery could earn some advantage of it.
Lee et al., in 2006 evaluated the efficacy of NAC. Of the 40 patients included, 22 were treated with PDS and 18 with NAC-IDS. Optimal IDS was possible in the 77.8% of the NAC group compared to 45.5% in the PDS group; concluding higher rate of optimum cytoreduction with less invasive surgery and reduced morbidity (19).
In 2010, a new randomized trial was performed comparing PDS and NAC-IDS by the European Organization for Research and Treatment of Cancer Gynecological Cancer Group-National Cancer Institute of Canada Clinical Trials Group (EORTC-NCIC 55971) from the Gynecologic Cancer Intergroup. Vergote et al. recruit 670 patients FIGO stage IIIC and IV that were randomly assigned to these approaches. They found that after NAC-IDS, optimal cytoreduction with the largest residual tumor ≤1 cm was in the 80.6% of the patients compared to PDS with 41.6%. Hazard ratio (HR) for death was 0.98 and HR for progression of disease was 1.01 in the NAC compared to the PDS group. PFS (12 months in both approaches) and OS (20 in PDS and 30 months in NAC) were almost the same, but complications after PDS were higher (20).
The Primary Chemotherapy Versus Primary Surgery for Newly Diagnosed Advanced Ovarian Cancer study (CHORUS), a phase 3 noninferiority trial done in 2013 that had the objective of demonstrating the noninferiority of NAC compared to PDS. This was a randomized, controlled trial done in 87 hospitals which enrolled women with suspected FIGO stage III or IV OC. Women were randomly assigned either to PDS followed by 6 cycles of ACT or to 3 cycles of NAC and then surgery. Like EORTC, median OS was 22.6 months in the PDS compared to 24.1 of the NAC group. The HR for death was 0.87 in favor of NAC and PFS of 12 vs. 10.7 months for PDS. This study concluded that NAC-IDS was non-inferior to PDS followed by ACT recommending that NAC-IDS is an acceptable standard of care for women with advanced OC (21). Disadvantages of this study were: not standardization of surgical procedures leading to suboptimal ways of doing cytoreduction, and skewed selection of patients due to the inclusion of cases with heavy tumor burden (22).
More recent studies have confirmed the advantages of NAC-IDS. Shimoji et al. reported a retrospective study of 51 cases with stage III and IV EOC treated in a hospital located in Japan between 2012 and 2016. A total of 29 cases were treated with NAC followed by IDS and 22 with PDS. The NAC-IDS group achieved complete cytoreductive surgery. Blood loss was greater in PDS group. PFS and OS were similar in both groups (23).
The SCORPION (Surgical complications related to Primary or Interval debulking in Ovarian Neoplasm) trial published in 2016 was aimed to establish whether NAC followed by IDS was superior to PDS in terms of clinical outcome and peri-operative morbidity in advanced EOC. This phase III randomized clinical trial included 110 eligible women, 55 assigned to PDS arm and 55 to the NAC-IDS arm. The rate of complete residual disease was superimposable: 45.5% in PDS vs. 57.7% in NAC-IDS. Complications grade III and IV were more common in NAC-IDS. Perioperative morbidity and QOL scores were better in the NAC-IDS arm (24).
A National Cancer Database from 1998–2011 was analyzed to compare the OS between PDS and NAC-IDS in women with stage III or IV OC. The representative cohort for NAC-IDS group was low and they had greater comorbidities. The OS in PDS group was higher among women with stage III but not stage IV. It concluded that patients with PDS are well selected rather than for NAC-IDS (25).
A retrospective study was conducted at our institution. Patients with advanced epithelial OC in stages IIIC and IV without parenchymal metastasis were included. Patients were classified according to the procedure. Group A with 42 patients had PDS followed by ACT ×6 cycles, and group B with 63 patients had NAC with 6 cycles followed by IDS. A R0 cytoreduction was performed in 35.5% of group A and 64.5% of group B. Median PFS between both groups was 14.7 and 17.5 months for groups A and B and OS 33.59 and 56.4, respectively. These results lead us to conclude that although the rate of complete debulking surgery was higher, long-term outcome is not improved (26).
Well-designed algorithms are needed for doing a NAC-IDS procedure instead of just indicating it to patients with the worst prognosis.
Several meta-analyses have been done. In 2009, 21 studies between 1989 and 2008 were included in a meta-analysis. This study concluded that those patients who received NAC had a lower risk of suboptimal cytoreduction helping oncologists to achieve an optimal surgery (22). Another study made in 2013, which included 2 randomized controlled trials with 1,200 women didn’t find a statistical difference in OS and PFS between patients who underwent either to NAC or PDS (27). In 2016, Zeng et al. included in their meta-analysis four randomized controlled trials and found that NAC contributed to the completeness of debulking removal, but no significant difference was found in OS compared to PDS group (28). A recent one, published in 2017 by Yang et al. compared four randomized controlled trials in which NAC or PDS was done. A total of 1,607 patients with advanced EOC were included and they found that NAC-IDS was associated with a higher rate of complete cytoreduction, lower peri-operative morbidity, and less post-surgical mortality, in addition to a best QOL compared to the group in which PDS was done (12). Table 2 summarize these results.

Full table
FIGO recommends NAC-IDS for selected patients with stage III and IV disease and bulky tumors. A 25-year experienced group recommends treatment with PDS to all patients stage IIIC who can achieve R0 resection with acceptable morbidity (29). Most patients in stage IV should be included in NAC treatment group. The Leuven criteria for considering NAC and IDS can be a useful parameter for guiding the decision. Different variables are considered in Leuven criteria. For NAC to be considered as an option, following aspects should be positive in the patients:
- At diagnosis, patients should have one of the followings:
- Biopsy with histologically proven epithelial ovarian or tubal or peritoneal, FIGO stage IIIC and IV;
- Fine needle aspiration that proves the presence of carcinoma cells in patients with a suspicious pelvic mass if CA-125/CEA ratio >25. If serum CA-125/CEA ratio is ≤25, imaging or endoscopy is obligatory to exclude a primary gastric, colon, or breast carcinoma.
- In the case of positive abdominal metastases at diagnosis, these should include either of these ones:
- Involvement of the superior mesenteric artery;
- Diffuse deep infiltration of the root of the small bowel mesentery;
- Diffuse and confluent carcinomatosis of the stomach and/or small bowel that involves such large parts that resection would lead to a short bowel syndrome or a total gastrectomy;
- Intrahepatic metastases;
- Infiltration of the duodenum and/or pancreas and/or the large vessels of the ligamentum hepatoduodenale, truncus coeliacus, or behind the porta hepatis.
- In the case of extra-abdominal metastases all of them should be considered for NAC-IDS, except in the cases of:
- Resectable inguinal lymph nodes;
- Solitary resectable retrocrural o paracardial nodes;
- Pleural fluid that contains cytologically malignant cells without proof of the presence of pleural tumors.
- Patients with impaired performance status and comorbidity that will not allow to achieve a complete resection or will not accept to use potential supportive measures, such as blood transfusions or temporary stoma NAC should be offered.
The last criteria of Leuven Group include the indications for IDS. This surgery should be done in patients that don’t have progression of disease and in the case of extra-abdominal disease at diagnosis, the extra abdominal disease should be in complete response or resectable. The performance status and comorbidities should allow a maximal surgical effort to no residual disease.
The feasibility of doing NAC-IDS can be assessed with a diagnostic laparoscopy. A validated laparoscopic scoring system was developed in 2013 in the multicentric trial Olympia-MITO 13 (30). Among the most used parameters omental cake, peritoneal and diaphragmatic carcinomatosis, stomach infiltration and superficial liver metastasis had the higher accuracy (Table 3).
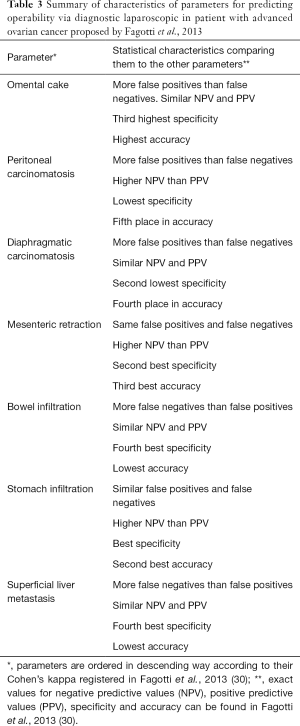
Full table
New approaches include hyperthermic intraperitoneal chemotherapy (HIPEC). A study published in 2014 included stage IIIC and IV OC patients treated during 2008 and 2013 (31). These patients were treated with NAC cycles of paclitaxel and carboplatin and thereafter cytoreduction HIPEC with paclitaxel 60 mg/m2 and cisplatin 75 mg/m2. A total of 66 patients were recruited, 9 patients were ≥75 years in which morbidity was higher compared to younger patients. This group had a median PFS of 6 months compared to 24 months of patients <75 years. Median OS in ≥75 years group was 13 months. This study concluded that patients ≥75 years do not benefit from IDS with HIPEC. In 2016, a phase II trial was done to assess the feasibility of PDS and HIPEC (cisplatin 50 mg/m2) followed by 6 cycles of ACT compared to 3–4 cycles of NAC and IDS with HIPEC followed by 3 cycles of ACT (32). HIPEC was done in patients with complete cytoreduction. A total of 19 patients were studied and NAC was administered to them, in 16 patients HIPEC was done in which outcome was good. For the 19 patients, median PFS was 33.2 months, with a 24-month PFS rate of 61.9%. OS rate at 24 months was 85.2%. In the HIPEC group, median PFS was 33.2 with a 24-month PFS rate of 69.2% and OS rate at 24-month of 92.3%.
Prognostic factors for success of NAC-IDS
The most important prognostic factor in the treatment of advanced stage ovarian continues to be the performance of an optimal surgery. Several factors that may affect the success of cytoreduction have been studied and analyzed. It is true that after NAC, the tissue is modified and measurements of residual tumor after surgery are not accurate. This may be a factor affecting the success of cytoreduction.
Macroscopic residual disease after debulking surgery is an independent prognostic factor between NAC-IDS and PDS. Residual tumor less than 1 cm in IDS is associated with higher survival benefit compared to leaving more than 1 cm of residual tumor (33). Minimal gross residual tumor improves survival in IDS but not in PDS.
Macroscopic tumor on the omentum analyzed by pathologists is a poor prognosis factor. The OS after a macroscopic tumor is of 32.0 months compared to those with microscopic tumors in which it is 67.0 months (34). This is thought to be due to chemotherapy resistance.
Size of the tumor does not correlate with the number of NAC cycles that have been given and is another factor that needs to be taken into consideration. Administration of more than 4 NAC cycles correlates with poorer prognosis and decrease in the median survival time (35). It is still unclear whether if there is any advantage in administering more than 4 cycles of chemotherapy.
Completion of debulking surgery with lymphadenectomy increases the OS but currently, there’s lack of strong evidence for recommending lymphadenectomy after NAC (34). A multicentric descriptive study was done in 2015 comparing patients with initially inoperable advanced OC who underwent NAC-IDS with lymphadenectomy or without it. It concluded no significant difference between groups with HR of 1.88 and PFS with HR of 1.43 (36).
The number of chemotherapy cycles of NAC should be less than 5. Patients receiving ≥5 NAC cycles have shorter PFS contrary to patients with <5 cycles. In patients with less than 5 cycles of ACT after IDS, PFS and OS were shorter compared to those with 5 or more cycles of ACT (35).
Post-surgical levels of CA 125 correlates well with the success of NAC and of an optimal IDS. Its levels are significantly lower when compared to a group of patients in which conservative management with PDS was done. Patients with low preoperative CA 125, less than 35 U/mL, have better results in the cytoreduction surgery. Furthermore, longer PFS is observed compared to patients with CA 125 levels greater than 100 U/mL (37).
Ascites is another factor that predicts the outcome following NAC and IDS. The disappearance of ascites considered as a decrease in volume for less than 500 mL correlates with a longer PFS and OS compared to patients with increased residual ascites (38).
During follow up, imaging can be a predictor of progression after NAC. Those patients with progressive disease is an independent preoperative factor for 1-year PFS and OS (39).
Conclusions
Several studies conclude that chemotherapy increases the percentage of patients suitable for IDS. Optimal resection in IDS ranges from 77% to 94% (38). In addition, lower morbidity, requirement of intensive care unit, hospital stay and higher quality of life are some of the advantages for NAC-IDS (40). The patient’s personal desires, clinical features, and disease burden should be the determinants for choosing between PDS and NAC. The lack of consensus should be overcome in the next few years.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012. Estimated cancer incidence, mortality and prevalence in 2012. Fact Sheets by Population. 2012 [cited 2017 Sep 21]. Available online: http://globocan.iarc.fr/Pages/fact_sheets_population.aspx
- Webb PM, Jordan SJ. Epidemiology of epithelial ovarian cancer. Best Pract Res Clin Obstet Gynaecol 2017;41:3-14. [Crossref] [PubMed]
- Permuth-wey J, Sellers TA. Cancer Epidemiology. In: Methods of Molecular Biology [Internet]. Springer Protocols, 2009. Available online: http://link.springer.com/10.1007/978-1-60327-492-0
- Inciura A, Simavicius A, Juozaityte E, et al. Comparison of adjuvant and neoadjuvant chemotherapy in the management of advanced ovarian cancer: a retrospective study of 574 patients. BMC Cancer 2006;6:153. [Crossref] [PubMed]
- Reid BM, Permuth JB, Sellers TA. Epidemiology of ovarian cancer: a review. Cancer Biol Med 2017;14:9-32. [Crossref] [PubMed]
- Howlader N, Noone A, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2008, National Cancer Institute, 2011. Available online: http://seer.cancer.gov/csr/1975_2008/
- Seidman JD, Yemelyanova A, Cosin JA, et al. Survival rates for international federation of gynecology and obstetrics stage III ovarian carcinoma by cell type: a study of 262 unselected patients with uniform pathologic review. Int J Gynecol Cancer 2012;22:367-71. [Crossref] [PubMed]
- Jelovac D, Armstrong DK. Recent progress in the diagnosis and treatment of ovarian cancer. CA Cancer J Clin 2011;61:183-203. [Crossref] [PubMed]
- Rocconi RP, Matthews KS, Kemper MK, et al. The timing of normalization of CA-125 levels during primary chemotherapy is predictive of survival in patients with epithelial ovarian cancer. Gynecol Oncol 2009;114:242-5. [Crossref] [PubMed]
- Vaughan S, Coward JI, Bast RC Jr, et al. Rethinking ovarian cancer: recommendations for improving outcomes. Nat Rev Cancer 2011;11:719-25. [Crossref] [PubMed]
- Griffiths CT. Surgical resection of tumor bulk in the primary treatment of ovarian carcinoma. Natl Cancer Inst Monogr 1975;42:101-4. [PubMed]
- Yang L, Zhang B, Xing G, et al. Neoadjuvant chemotherapy versus primary debulking surgery in advanced epithelial ovarian cancer: A meta-analysis of peri-operative outcome. PLoS One 2017;12:e0186725. [Crossref] [PubMed]
- Sato S, Itamochi H. Neoadjuvant chemotherapy in advanced ovarian cancer: latest results and place in therapy. Ther Adv Med Oncol 2014;6:293-304. [Crossref] [PubMed]
- Winter WE 3rd, Maxwell GL, Tian C, et al. Prognostic factors for stage III epithelial ovarian cancer: a Gynecologic Oncology Group Study. J Clin Oncol 2007;25:3621-7. [Crossref] [PubMed]
- Chambers JT, Chambers SK, Voynick IM, et al. Neoadjuvant chemotherapy in stage X ovarian carcinoma. Gynecol Oncol 1990;37:327-31. [Crossref] [PubMed]
- Jacob JH, Gershenson DM, Morris M, et al. Neoadjuvant chemotherapy and interval debulking for advanced epithelial ovarian cancer. Gynecol Oncol 1991;42:146-50. [Crossref] [PubMed]
- van der Burg ME, van Lent M, Buyse M, et al. The effect of debulking surgery after induction chemotherapy on the prognosis in advanced epithelial ovarian cancer. Gynecological Cancer Cooperative Group of the European Organization for Research and Treatment of Cancer. N Engl J Med 1995;332:629-34. [Crossref] [PubMed]
- Rose PG, Nerenstone S, Brady MF, et al. Secondary surgical cytoreduction for advanced ovarian carcinoma. N Engl J Med 2004;351:2489-97. [Crossref] [PubMed]
- Lee SJ, Kim BG, Lee JW, et al. Preliminary results of neoadjuvant chemotherapy with paclitaxel and cisplatin in patients with advanced epithelial ovarian cancer who are inadequate for optimum primary surgery. J Obstet Gynaecol Res 2006;32:99-106. [Crossref] [PubMed]
- Vergote I, Tropé CG, Amant F, et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med 2010;363:943-53. [Crossref] [PubMed]
- Kehoe S, Hook J, Nankivell M, et al. Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): an open-label, randomised, controlled, non-inferiority trial. Lancet 2015;386:249-57. [Crossref] [PubMed]
- Kang S, Nam BH. Does neoadjuvant chemotherapy increase optimal cytoreduction rate in advanced ovarian cancer? Meta-analysis of 21 studies. Ann Surg Oncol 2009;16:2315-20. [Crossref] [PubMed]
- Shimoji K, Ito K, Tashima L, et al. Comparison between Primary Debulking Surgery and Neo-Adjuvant Chemotherapy Followed by Interval Debulking Surgery for Patients with Stage III-IV Ovarian Cancer. Gan To Kagaku Ryoho 2017;44:675-9. [PubMed]
- Fagotti A, Ferrandina G, Vizzielli G, et al. Phase III randomised clinical trial comparing primary surgery versus neoadjuvant chemotherapy in advanced epithelial ovarian cancer with high tumour load (SCORPION trial): Final analysis of peri-operative outcome. Eur J Cancer 2016;59:22-33. [Crossref] [PubMed]
- Seagle BL, Graves S, Strohl AE, et al. Survival After Primary Debulking Surgery Compared With Neoadjuvant Chemotherapy in Advanced Ovarian Cancer: A National Cancer Database Study. Int J Gynecol Cancer 2017;27:1610-8. [Crossref] [PubMed]
- Medina-Franco H, Cortés-González R, Lambreton-Hinojosa F, et al. Neoadjuvant Chemotherapy Increases R0 Cytoreduction Rate But Does Not Improve Final Outcome in Advanced Epithelial Ovarian Cancer. Ann Surg Oncol 2017;24:1330-5. [Crossref] [PubMed]
- Dai-yuan M, Bang-xian T, Xian-fu L, et al. A meta-analysis: neoadjuvant chemotherapy versus primary surgery in ovarian carcinoma FIGO stage III and IV. World J Surg Oncol 2013;11:267. [Crossref] [PubMed]
- Zeng LJ, Xiang CL, Gong YZ, et al. Neoadjuvant chemotherapy for Patients with advanced epithelial ovarian cancer: A Meta-Analysis. Sci Rep 2016;6:35914. [Crossref] [PubMed]
- Vergote IB, Van Nieuwenhuysen E, Vanderstichele A, et al. How to Select Neoadjuvant Chemotherapy or Primary Debulking Surgery in Patients With Stage IIIC or IV Ovarian Carcinoma. J Clin Oncol 2016;34:3827-8. [Crossref] [PubMed]
- Fagotti A, Vizzielli G, De Iaco P, et al. A multicentric trial (Olympia-MITO 13) on the accuracy of laparoscopy to assess peritoneal spread in ovarian cancer. Am J Obstet Gynecol 2013;209:462.e1-462.e11. [Crossref] [PubMed]
- Cascales-Campos P, Gil J, Gil E, et al. Cytoreduction and HIPEC after neoadjuvant chemotherapy in stage IIIC-IV ovarian cancer. Critical analysis in elderly patients. Eur J Obstet Gynecol Reprod Biol 2014;179:88-93. [Crossref] [PubMed]
- D'Hondt V, Goffin F, Roca L, et al. Interval Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy in First-Line Treatment for Advanced Ovarian Carcinoma: A Feasibility Study. Int J Gynecol Cancer 2016;26:912-7. [Crossref] [PubMed]
- Rutten MJ, Sonke GS, Westermann AM, et al. Prognostic Value of Residual Disease after Interval Debulking Surgery for FIGO Stage IIIC and IV Epithelial Ovarian Cancer. Obstet Gynecol Int 2015;2015:464123. [Crossref] [PubMed]
- Kaban A, Topuz S, Saip P, et al. Poor Prognostic Factors in Patients Undergoing Surgery After Neoadjuvant Chemotherapy for Ovarian, Tubal, or Peritoneal Cancer. J Obstet Gynaecol Can 2017;39:1163-70. [Crossref] [PubMed]
- Xu X, Deng F, Lv M, et al. The number of cycles of neoadjuvant chemotherapy is associated with prognosis of stage IIIc-IV high-grade serous ovarian cancer. Arch Gynecol Obstet 2017;295:451-8. [Crossref] [PubMed]
- Schwartz L, Schrot-Sanyan S, Brigand C, et al. Impact of Pelvic and Para-aortic Lymphadenectomy in Advanced Ovarian Cancer After Neoadjuvant Chemotherapy. Anticancer Res 2015;35:5503-9. [PubMed]
- Matsuhashi T, Takeshita T, Yamamoto A, et al. Serum CA 125 Level after Neoadjuvant Chemotherapy is Predictive of Prognosis and Debulking Surgery Outcomes in Advanced Epithelial Ovarian Cancer. J Nippon Med Sch 2017;84:170-6. [Crossref] [PubMed]
- Xu X, Deng F, Lv M, et al. Ascites regression following neoadjuvant chemotherapy in prediction of treatment outcome among stage IIIc to IV high-grade serous ovarian cancer. J Ovarian Res 2016;9:85. [Crossref] [PubMed]
- Baek MH, Lee SW, Park JY, et al. Preoperative Predictive Factors for Complete Cytoreduction and Survival Outcome in Epithelial Ovarian, Tubal, and Peritoneal Cancer After Neoadjuvant Chemotherapy. Int J Gynecol Cancer 2017;27:420-9. [Crossref] [PubMed]
- Hall TR, Dizon DS. Neoadjuvant Chemotherapy for Advanced Epithelial Ovarian Cancer – Hematology & Oncology. Clin Adv Hematol Oncol 2016;14. [PubMed]



