Advances in anti-HER2 therapy in metastatic breast cancer
Background
Worldwide, breast cancer is the most common malignancy in women. The American Cancer Society estimated that there were 252,710 new breast cancer cases and 40,610 breast cancer deaths occurred in the United States, in 2017 (1). Although breast cancer is the most commonly diagnosed cancer, it is the second causes of death. With the invention of therapeutic drugs, especially targeted drugs, most patients with early stage breast cancer were cured, the patients with metastases also had a notable improvement of progression-free survival (PFS) and overall survival (OS).
Human epidermal growth factor receptor 2 (HER2) has been identified as oncogenes in about 20% of all breast cancer patients. Since the last 1980s, many studies had identified that breast cancer, with HER2-positive had a more aggressive biology and were associated with poorer outcomes (recurrence and survival) (2-4). Since 1998, the first anti-HER2 agent (trastuzumab) got into clinical, which significantly improved the prognosis of HER2-positive breast cancer patients in both the early stage and metastatic settings.
In the last 20 years, multiple anti-HER2 agents span a number of drug classes stepped into clinical practice, included the monoclonal antibodies (trastuzumab and pertuzumab), the small-molecule intracellular tyrosine kinase inhibitors (lapatinib and neratinib) and the antibody-drug conjugate (ADC) ado-trastuzumab emtansine (T-DM1).
In this article, we will review a number of trials, primarily in phase III, in HER2-positive metastatic breast cancer (MBC) which have indicated significant improvement outcomes with anti-HER2 therapy in the first-line setting and beyond (Table 1) and constantly change clinical practice, then, discuss some new targeted agents in the current management and anti-HER2 therapy in HER2 low-or non-expression breast cancer.
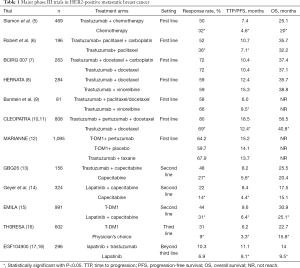
Full table
First-line therapy
Trastuzumab
Trastuzumab, a murine monoclonal antibody, directed against the extracellular domain of HER2, is the first anti-HER2 antibody approved by Food and Drug Administration (FDA) in 1998 for the treatment of patients with HER2-positive MBC (19).
The first phase III clinical trial result provided by Slamon et al. (5) in 2001, demonstrated trastuzumab increased the clinical benefit of first-line chemotherapy in MBC that overexpresses HER2. Patients with HER2-positive MBC were randomly divided into two group in the first-line therapy, one was chemotherapy with trastuzumab (n=235) and the other was chemotherapy alone (n=234). A significant improvement in time to progression (TTP) (7.4 vs. 4.6 months; P<0.001) and OS (25.1 vs. 20.3 months; P=0.01) had been obtained at a median follow up of 30 months. The most important adverse event was cardiac dysfunction. Higher rates of cardiac dysfunction were seen with the combination of anthracycline and trastuzumab than anthracycline alone (27% vs. 8%). Though, there was also higher in paclitaxel-trastuzumab than paclitaxel alone (8% vs. 1%), the overall rates were low.
Then, trastuzumab were tested in combination with several chemotherapy drugs. Robert et al. (6) randomized 196 patients to trastuzumab and paclitaxel with or without carboplatin. Though, the addition of carboplatin to paclitaxel and trastuzumab improved objective response rate (ORR) (52% vs. 36%) and PFS (10.7 vs. 7.1 months) in women with HER2-positive MBC, no significant difference in OS (35.7 vs. 32.2 months, P=0.76) and more hematologic toxicity was observed.
Similarly, The Breast Cancer International Research Group (BCIRG) 007 study researched trastuzumab and docetaxel with or without carboplatin, and found that adding carboplatin did not enhance antitumor activity (7).
Two phase III trials compared trastuzumab plus taxane or vinorelbine as first-line therapy (8,9). Both trastuzumab/taxane and trastuzumab/vinorelbine treatments were efficacy and tolerability for HER2-positive MBC. And, they both could be considered as an alternative first-line option.
Pertuzumab is a recombinant, humanized monoclonal antibody that bind to a different HER2 epitope than trastuzumab, is known as an inhibitor of heterodimerization of the HER receptors. Phase I/II clinical trial by Cortés et al. (20) showed pertuzumab as monotherapy or in combination with chemotherapy was not effective in patient that progressed during therapy with trastuzumab. But, in the phase III trial CLEOPATRA, pertuzumab added to the first-line treatment standard of docetaxel plus trastuzumab and challenged classic. Patients (n=808) with HER2-positive MBC randomized to receive trastuzumab plus docetaxel plus placebo (control group) or trastuzumab plus docetaxel plus pertuzumab (pertuzumab group) as first-line therapy. The median independently assessed PFS for addition of pertuzumab was prolonged by 6.1 months (12.4 vs. 18.5 months, P<0.001) (10). The final prespecified OS results with a median follow-up of 50 months reported 56.5 months in the pertuzumab group and significantly improved compared with 40.8 months in the control group (P<0.001) (11). Dang et al. (21,22) performed a phase II study of weekly paclitaxel along with trastuzumab and pertuzumab in 69 HER2-positive MBC patients (74% and 26% treated in first- and second-line), the result showed weekly paclitaxel added to trastuzumab and pertuzumab was associated with a favorable OS and PFS and offered an alternative to docetaxel-based therapy.
The MARIANNE trial (12) showed that T-DM1 arm and T-DM1 plus pertuzumab arm had noninferior PFS compared with trastuzumab plus taxane (T-DM1, 14.1 months; T-DM1 plus pertuzumab, 15.2 months; trastuzumab plus taxane, 13.7 months) and neither experimental arm showed PFS superiority to trastuzumab plus taxane for first-line treatment of HER2-positive MBC. The incidence of grade ≥3 adverse events was significant higher in the trastuzumab plus taxane arm (54.1%) versus the T-DM1 arm (45.4%) and T-DM1 plus pertuzumab arm (46.2%). In the T-DM1 arms, there were fewer patients discontinued treatment because of adverse events and health-related quality of life was maintained for longer.
Therefore, nowadays pertuzumab, trastuzumab plus docetaxel is the standard treatment in the first-line therapy for HER2-positive MBC, T-DM1 may be an alternative.
Second-line therapy
Patients in MBC with the first-line therapy always recurred or progressed. Therapeutic options with evidenced based include continuation of trastuzumab with a different chemotherapy parter. The phase III trial by German Breast Group 26 study (13) showed continuation of trastuzumab plus capecitabine had a significant improvement in overall response and TTP compared with capecitabine alone in patients with HER-2-positive breast cancer who experienced progression during trastuzumab treatment, but, the OS was not statistically significant (25.5 vs. 20.4 months, P=0.257).
Lapatinib is an orally available small molecule tyrosine kinase inhibitor of both epidermal growth factor receptor (EGFR) and HER2. The Canadian-led MA.31 study (23) by the NCIC clinical trials group, compared lapatinib with trastuzumab, both combined with a taxane in the first-line setting showed lapatinib with taxane therapy is associated with a shorter PFS compared to trastuzumab with taxane. But, lapatinib plus capecitabine is a choice for the patients progressed for trastuzumab. An phase III trial by Geyer et al. (14) comparing lapatinib plus capecitabine versus capecitabine alone in patients who have progressed on prior trastuzumab-based therapy, in 2006, interim analyzed that there was a significant improvement in PFS (8.4 vs. 4.4 months, P<0.001). And, the improvement was got without an increase in serious toxic effects or symptomatic cardiac event. Although premature enrollment termination and subsequent crossover resulted in insufficient power to detect differences in OS, the final exploratory analyses demonstrated a trend toward a survival advantage with lapatinib plus capecitabine (24).
T-DM1 is a HER2-positive directed antibody drug conjugate that incorporates the HER2-targeted anti-tumor properties of trastuzumab with the cytotoxic activity of the microtubule-inhibitory agent DM1. The EMILA trial (15), comparing T-DM1 and lapatinib-capecitabine for patients who had previously been treated with trastuzumab and taxane, reported a statistically significant OS advantage in favor of T-DM1 at the second interim analysis in 2012, and it led to registration and rapid clinical adoption of T-DM1. In 2017, the EMILA final analysis showed median OS was longer with T-DM1 than with lapatinib-capecitabine (29.9 vs. 25.9 months), though 136 (27%) of 496 patients crossed over from control to T-DM1 after the second interim overall survival analysis. Additional, the rates of grade 3 or 4 adverse events were numerically higher in lapatinib plus capecitabine versus T-DM1 (57% vs. 41%). But, thrombocytopenia and increased serum aminotransferase levels were favored for T-DM1.
So, at the present time, T-DM1 is the standard treatment in the second-line therapy for HER2-positive MBC.
Third-line therapy and beyond
Phase III trial for anti-HER2 therapy in the third-line setting is seldom reported. The TH3RESA trial (16) is the only one we know. Eligible patients were men and women (n =602) previously treated with both trastuzumab and lapatinib (advanced setting) and a taxane (any setting) and with progression on two or more HER2-directed regimens in the advanced setting. Patients were randomized in a 2:1 fashion to T-DM1 and physician’s choice of therapy. At data cutoff, 47% patients in the physician’s choice group had crossed over to T-DM1. OS was significantly longer with T-DM1 versus treatment of physician’s choice (22.7 vs. 15.8 months, P=0.0007).
The EGF104900 trial (17) is a phase III for patients who received a median of three prior trastuzumab-containing treatment, compared the combination of lapatinib plus trastuzumab to lapatinib alone. It reported lapatinib plus trastuzumab showed superiority to lapatinib monotherapy in PFS (11.1 vs. 8.1 weeks, P=0.011) and OS (14 vs. 9.5 months, P=0.026) (17,18). Thus offer a chemotherapy-free option with an acceptable safety profile to patients with heavily pretreated HER2-positive MBC.
Hormone receptor(HR)-positive tumors
For patients with HR-positive and HER2-positive breast cancer who have light tumor burden or be old, the combination of anti-HER2 therapy and endocrine therapy may considered.
The combination of trastuzumab and endocrine therapy had been tested in postmenopausal HR-positive and HER2-positive MBC patients. The TAnDEM study (25) showed trastuzumab plus anastrozole having an improvement PFS than anastrozole alone(4.8 vs. 2.4 months, P<0.001), but non-significant increase in OS (28.5 vs. 23.9 months). The eLEcTRA trial (26) compared efficacy and safety of trastuzumab plus letrozole to letrozole alone in patients with HER2-positive and HR-positive MBC as first-line treatment and demonstrated that the combination of trastuzumab and letrozole was a safe and effective treatment option.
A letrozole and lapatinib combination was tested in the first-line setting for HR-positive HER2-positive MBC. Results in 219 patients, letrozole plus lapatinib significantly reduced the risk of disease progression versus letrozole plus placebo [hazard ratio (HR) =0.71; 95% CI, 0.53 to 0.96; P=0.019]; median PFS was 8.2 vs. 3.0 months, respectively (27).
The ALTERNATIVE study (28) evaluated the efficacy and safety of dual HER2 blockade (lapatinib plus trastuzumab) plus aromatase inhibitor (AI) in postmenopausal women with HR-positive and HER2-positive MBC who received prior endocrine therapy and prior neo(adjuvant)/first-line trastuzumab (TRAS) plus chemotherapy. LAP + TRAS + AI showed a more superior PFS than TRAS + AI (11 vs. 5.7 months, P=0.0064). And LAP + AI had a more superior PFS than TRAS + AI (8.3 vs. 5.7 months, P=0.0361). Overall response rate, clinical benefit rate, and OS also favored LAP + TRAS + AI.
Although improvements in PFS are seen with the combination of anti-HER2 therapy and endocrine therapy for this subset of patients with HR-positive and HER2-positive disease, only one study has demonstrated an improvement in OS. So, the use of such an approach has to be weighed and only offers a safe chemotherapy-sparing alternative treatment regimen for patient without aggressive disease and who are not candidates for chemotherapy.
New agents in anti-HER2
Neratinib is an orally active, irreversible inhibitor of the HER-2 tyrosine kinase that blocks signal transduction through a broad spectrum of 3 receptors, erbB-1, erbB-2 and erbB-4 (29,30). The first open-label multicenter phase II trial with neratinib monotherapy for patients with advanced HER2-positive breast cancer was reported in 2010. Patients were divided into two cohorts, the prior trastuzumab cohort (n=66) and no prior trastuzumab cohort (n=70). The results showed 16-week PFS rates were 59% for patients with prior trastuzumab treatment and 78% for patients with no prior trastuzumab treatment. Median PFS was 22.3 and 39.6 weeks. Diarrhea was the most frequent grades 3 to 4 adverse event, occurring in 30% of patients with prior trastuzumab treatment and in 13% of patients with no prior trastuzumab treatment (31). A phase I/II trial (32) had been conducted, evaluating the role of neratinib plus capecitabine for patients with trastuzumab-pretreated HER2-positive MBC. The objective response rate (ORR) was 64% (n=39 of 61) in patients with no prior lapatinib exposure and 57% (n=4 of 7) in patients previously treated with lapatinib. Median PFS was 40.3 and 35.9 weeks. The NEfERT-T Randomized Clinical Trial (33) showed in first-line HER2-positive MBC, neratinib-paclitaxel was not superior to trastuzumab-paclitaxel in terms of PFS. But, neratinib-paclitaxel may delay the onset and reduce the frequency of central nervous system progression. These results need to be confirmed by large-scale randomized trials.
Afatinib is also an oral small molecule HER family tyrosine kinase inhibitor and has the ability to cross the blood-brain barrier, could play a role in patients with brain metastases from breast cancer (34). The Lux-Breast trials are evaluating afatinib in additional clinical settings. Compared with rastuzumab-based therapy, afatinib had no advantage for patients with HER2-positive MBC who had progressed on trastuzumab (35). The Lux-Breast III trial (36) is evaluating afatinib monotherapy or afatinib plus vinorelbine compared with the investigators’ choice of treatment for patients with HER2-positive MBC and progressive brain metastasis who have progressed on prior trastuzumab or lapatinib based therapy. Though patient benefit with afatinib-containing treatments was not different from that in patients given investigator's choice of treatments, adverse events were frequent and afatinib-containing treatments seemed to be less well tolerated. A phase II small exploratory study showed afatinib combined with letrozole was able to induce disease stabilization in 54% of hormone-refractory MBC patients previously progressing on letrozole (37).
So far, Neratinib and Afatinib didn’t show superiority for MBC, how to make them play an important role needs to be further practiced.
Margetuximab is a Fc-modified anti-HER2 antibody that binds with elevated affinity to both the lower and higher affinity forms of CD16A, important for antibody dependent cell-mediated cytotoxicity (ADCC) against tumor cells. A phase I study in patients with HER2-positive MBC showed margetuximab was well-tolerated and has promising single-agent activity. Further development efforts of single agent or in combination with other therapeutic agents are ongoing (38).
MM-302 is a HER2-targeted PEGylated liposome that encapsulates doxorubicin to facilitate its delivery to HER2-overexpressing tumor cells. The combination of MM-302 and trastuzumab induced synergistic antitumor activity in HER2-overexpressing xenograft models of breast (39). A randomized Phase 2 trial (HERMIONE) of MM-302 plus trastuzumab versus chemotherapy of physician’s choice plus trastuzumab in patients with previously treated, anthracycline-naïve, HER2-positive MBC is going (40).
SYD985 is a HER2-targeting ADC based on trastuzumab and vc-seco-DUBA. In vivo antitumor studies in breast cancer xenograft models showed that SYD985 is very active in HER2 3+, 2+, and 1+ models (41). Clinical trial (NCT03262935) of SYD985 vs. Physician’s Choice in participants with HER2-positive locally advanced or MBC in third line-setting and beyond is conducted.
New combinations (with mTOR inhibitors or PI3K inhibitors)
Mutations in the PI3K pathway are frequent in breast cancer, causing resistance to HER 2-targeted agents. PI3K is downstream from HER2 and mTOR is downstream from PI3K, target this pathway may be an important step in overcoming trastuzumab resistance in HER2-positive MBC (42,43). BOLERO-1 and BOLERO-3 trials had evaluated mTOR inhibition in HER2-positive MBC. The BOLERO-3 trials showed addition of everolimus to trastuzumab plus vinorelbine significantly prolongs PFS (7.0 vs. 5.78, P=0.0067) in patients with trastuzumab-resistant and taxane-pretreated, HER2-positive MBC. But, serious adverse events were reported in the everolimus group (44). The phase III BOLERO-1 trial compares paclitaxel and trastuzumab with or without everolimus for first-line treatment of women with HER2-positive MBC (45). Although PFS was not significantly different between groups in the full population, the 7.2 months prolongation was noted with the addition of everolimus in the HR-negative HER2-positive population.
Pilaralisib (SAR245408) is a pan-class I PI3K inhibitor. A phase I/II study of pilaralisib in combination with trastuzumab or paclitaxel plus trastuzumab was conducted on patients with HER2-positive MBC who had progressed on a prior trastuzumab-containing regimen and resulted that pilaralisib in combination with trastuzumab with or without paclitaxel had an acceptable safety profile and with clinical activity in the paclitaxel arm (46).
Anti-HER2 therapy in HER2 low-or non-expression breast cancer
In adjuvant, the National Surgical Adjuvant Breast and Bowel Project (NSABP) trial B-31 demonstrated the efficacy of adjuvant trastuzumab added to chemotherapy not only for HER2-positive breast cancer but also, surprisingly, for HER2-negative breast cancer (47-49) and which facilitated the NSABP B-47 trial for testing trastuzumab in HER2 low-expression patients (HER2 IHC score 1+, 2+ and FISH negative). Unfortunately, the results showed that adjuvant trastuzumab added to chemotherapy didn’t receive additional benefit, but increased severe toxicities (50).
Though some preclinical models showed pertuzumab inhibits tumor growth in the absence of HER2 overexpression, unlike trastuzumab, presumably by preventing ligand-stimulated HER2 heterodimer formation, a phase II multicenter, randomized study about pertuzumab in patients with HER2-negative MBC showed a limited efficacy (51).
More recently, research showed HER2 somatic mutations in breast cancer patients activating mutations that likely drive tumorigenesis, but the majority of HER2 somatic mutations had not been associated with concurrent HER2 gene amplification (52). Preclinical data suggest that functionally activating HER2 mutations may drive and maintain cancers and HER2 mutations may similarly confer sensitivity to HER2-directed drugs (53). Case report showed a triple-negative (ER/PR/HER2) inflammatory breast cancer but HER2 mutated was response to anti-HER2 therapy (lapatinib and capecitabine) (54) .
Trastuzumab deruxtecan (DS-8201) is an ADC, a novel enzyme-cleavable linker, and a topoisomerase I inhibitor payload. In a phase I study showed that in 23 evaluable patients (advanced breast and gastric or gastro-oesophageal tumours), including six low HER2-expressing patients, ten patients achieved an objective response (43%, 95% CI, 23.2–65.5) and disease control was achieved in 21 (91%) of 23 patients (55).
So, MBC with HER2 mutation-positive but HER2 negative may benefit from existing HER2-targeted drugs, further clinical studies are needed. Trastuzumab deruxtecan is worthy of further study for patients with low HER2-expressed.
Conclusions
The landscape of treatment for HER2-positive MBC continues to be refined, and evolves. Nowadays, the first line treatment is pertuzumab, trastuzumab plus docetaxel, and T-DM1 has great potential. However, drug resistance is inevitable in treatment. T-DM1 is the standard treatment in the second-line setting and beyond nowadays. For HR-positive and HER2-positive MBC patients who without aggressive disease and are not candidates for chemotherapy, the combination of dual anti-HER2 therapy and endocrine therapy may considered. The introduction of novel agents offers clinicians the ability to prolonged inhibition of the HER2 pathway across multiple lines of treatment. Active clinical trials of different agents combinations will hopefully result in continued improvements in outcomes for HER2-positive MBC patients. Then, patients for HER2-negative but HER2 mutation-positive may have benefit from anti-HER2 therapy.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7-30. [Crossref] [PubMed]
- Slamon DJ, Clark GM, Wong SG, et al. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 1987;235:177-82. [Crossref] [PubMed]
- Clark GM, McGuire WL. Follow-up study of HER-2/neu amplification in primary breast cancer. Cancer Res 1991;51:944-8. [PubMed]
- Seshadri R, Firgaira FA, Horsfall DJ, et al. Clinical significance of HER-2/neu oncogene amplification in primary breast cancer. The South Australian Breast Cancer Study Group. J Clin Oncol 1993;11:1936-42. [Crossref] [PubMed]
- Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001;344:783-92. [Crossref] [PubMed]
- Robert N, Leyland-Jones B, Asmar L, et al. Randomized phase III study of trastuzumab, paclitaxel, and carboplatin compared with trastuzumab and paclitaxel in women with HER-2-overexpressing metastatic breast cancer. J Clin Oncol 2006;24:2786-92. [Crossref] [PubMed]
- Valero V, Forbes J, Pegram MD, et al. Multicenter phase III randomized trial comparing docetaxel and trastuzumab with docetaxel, carboplatin, and trastuzumab as first-line chemotherapy for patients with HER2-gene-amplified metastatic breast cancer (BCIRG 007 study): two highly active therapeutic regimens. J Clin Oncol 2011;29:149-56. [Crossref] [PubMed]
- Andersson M, Lidbrink E, Bjerre K, et al. Phase III randomized study comparing docetaxel plus trastuzumab with vinorelbine plus trastuzumab as first-line therapy of metastatic or locally advanced human epidermal growth factor receptor 2-positive breast cancer: the HERNATA study. J Clin Oncol 2011;29:264-71. [Crossref] [PubMed]
- Burstein HJ, Keshaviah A, Baron AD, et al. Trastuzumab plus vinorelbine or taxane chemotherapy for HER2-overexpressing metastatic breast cancer: the trastuzumab and vinorelbine or taxane study. Cancer 2007;110:965-72. [Crossref] [PubMed]
- Baselga J, Cortes J, Kim SB, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med 2012;366:109-19. [Crossref] [PubMed]
- Swain SM, Baselga J, Kim SB, et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med 2015;372:724-34. [Crossref] [PubMed]
- Perez EA, Barrios C, Eiermann W, et al. Trastuzumab Emtansine With or Without Pertuzumab Versus Trastuzumab Plus Taxane for Human Epidermal Growth Factor Receptor 2-Positive, Advanced Breast Cancer: Primary Results From the Phase III MARIANNE Study. J Clin Oncol 2017;35:141-8. [Crossref] [PubMed]
- von Minckwitz G, du Bois A, Schmidt M, et al. Trastuzumab beyond progression in human epidermal growth factor receptor 2-positive advanced breast cancer: a german breast group 26/breast international group 03-05 study. J Clin Oncol 2009;27:1999-2006. [Crossref] [PubMed]
- Geyer CE, Forster J, Lindquist D, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med 2006;355:2733-43. [Crossref] [PubMed]
- Verma S, Miles D, Gianni L, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med 2012;367:1783-91. [Crossref] [PubMed]
- Krop IE, Kim SB, Martin AG, et al. Trastuzumab emtansine versus treatment of physician's choice in patients with previously treated HER2-positive metastatic breast cancer (TH3RESA): final overall survival results from a randomised open-label phase 3 trial. Lancet Oncol 2017;18:743-54. [Crossref] [PubMed]
- Blackwell KL, Burstein HJ, Storniolo AM, et al. Randomized study of Lapatinib alone or in combination with trastuzumab in women with ErbB2-positive, trastuzumab-refractory metastatic breast cancer. J Clin Oncol 2010;28:1124-30. [Crossref] [PubMed]
- Blackwell KL, Burstein HJ, Storniolo AM, et al. Overall survival benefit with lapatinib in combination with trastuzumab for patients with human epidermal growth factor receptor 2-positive metastatic breast cancer: final results from the EGF104900 Study. J Clin Oncol 2012;30:2585-92. [Crossref] [PubMed]
- Albanell J, Baselga J. Trastuzumab, a humanized anti-HER2 monoclonal antibody, for the treatment of breast cancer. Drugs Today (Barc) 1999;35:931-46. [PubMed]
- Cortés J, Fumoleau P, Bianchi GV, et al. Pertuzumab monotherapy after trastuzumab-based treatment and subsequent reintroduction of trastuzumab: activity and tolerability in patients with advanced human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol 2012;30:1594-600. [Crossref] [PubMed]
- Dang C, Iyengar N, Datko F, et al. Phase II study of paclitaxel given once per week along with trastuzumab and pertuzumab in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer. J Clin Oncol 2015;33:442-7. [Crossref] [PubMed]
- Smyth LM, Iyengar NM, Chen MF, et al. Weekly paclitaxel with trastuzumab and pertuzumab in patients with HER2-overexpressing metastatic breast cancer: overall survival and updated progression-free survival results from a phase II study. Breast Cancer Res Treat 2016;158:91-7. [Crossref] [PubMed]
- Gelmon KA, Boyle F, Kaufman B, et al. Open-label phase III randomized controlled trial comparing taxane-based chemotherapy (Tax) with lapatinib (L) or trastuzumab (T) as first-line therapy for women with HER2+ metastatic breast cancer: Interim analysis (IA) of NCIC CTG MA.31/GSK EGF 108919. J Clin Oncol 2012. [Epub ahead of print]. [Crossref]
- Cameron D, Casey M, Oliva C, et al. Lapatinib plus capecitabine in women with HER-2-positive advanced breast cancer: final survival analysis of a phase III randomized trial. Oncologist 2010;15:924-34. [Crossref] [PubMed]
- Kaufman B, Mackey JR, Clemens MR, et al. Trastuzumab plus anastrozole versus anastrozole alone for the treatment of postmenopausal women with human epidermal growth factor receptor 2-positive, hormone receptor-positive metastatic breast cancer: results from the randomized phase III TAnDEM study. J Clin Oncol 2009;27:5529-37. [Crossref] [PubMed]
- Huober J, Fasching PA, Barsoum M, et al. Higher efficacy of letrozole in combination with trastuzumab compared to letrozole monotherapy as first-line treatment in patients with HER2-positive, hormone-receptor-positive metastatic breast cancer - results of the eLEcTRA trial. Breast 2012;21:27-33. [Crossref] [PubMed]
- Johnston S, Pippen J Jr, Pivot X, et al. Lapatinib combined with letrozole versus letrozole and placebo as first-line therapy for postmenopausal hormone receptor-positive metastatic breast cancer. J Clin Oncol 2009;27:5538-46. [Crossref] [PubMed]
- Johnston SRD, Hegg R, Im SA, et al. Phase III, Randomized Study of Dual Human Epidermal Growth Factor Receptor 2 (HER2) Blockade With Lapatinib Plus Trastuzumab in Combination With an Aromatase Inhibitor in Postmenopausal Women With HER2-Positive, Hormone Receptor-Positive Metastatic Breast Cancer: ALTERNATIVE. J Clin Oncol 2018;36:741-8. [Crossref] [PubMed]
- Rabindran SK, Discafani CM, Rosfjord EC, et al. Antitumor activity of HKI-272, an orally active, irreversible inhibitor of the HER-2 tyrosine kinase. Cancer Res 2004;64:3958-65. [Crossref] [PubMed]
- Wong KK, Fracasso PM, Bukowski RM, et al. A phase I study with neratinib (HKI-272), an irreversible pan ErbB receptor tyrosine kinase inhibitor, in patients with solid tumors. Clin Cancer Res 2009;15:2552-8. [Crossref] [PubMed]
- Burstein HJ, Sun Y, Dirix LY, et al. Neratinib, an irreversible ErbB receptor tyrosine kinase inhibitor, in patients with advanced ErbB2-positive breast cancer. J Clin Oncol 2010;28:1301-7. [Crossref] [PubMed]
- Saura C, Garcia-Saenz JA, Xu B, et al. Safety and efficacy of neratinib in combination with capecitabine in patients with metastatic human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol 2014;32:3626-33. [Crossref] [PubMed]
- Awada A, Colomer R, Inoue K, et al. Neratinib Plus Paclitaxel vs Trastuzumab Plus Paclitaxel in Previously Untreated Metastatic ERBB2-Positive Breast Cancer: The NEfERT-T Randomized Clinical Trial. JAMA Oncol 2016;2:1557-64. [Crossref] [PubMed]
- Geuna E, Montemurro F, Aglietta M, et al. Potential of afatinib in the treatment of patients with HER2-positive breast cancer. Breast Cancer (Dove Med Press) 2012;4:131-7. [PubMed]
- Harbeck N, Huang CS, Hurvitz S, et al. Afatinib plus vinorelbine versus trastuzumab plus vinorelbine in patients with HER2-overexpressing metastatic breast cancer who had progressed on one previous trastuzumab treatment (LUX-Breast 1): an open-label, randomised, phase 3 trial. Lancet Oncol 2016;17:357-66. [Crossref] [PubMed]
- Cortés J, Dieras V, Ro J, et al. Afatinib alone or afatinib plus vinorelbine versus investigator's choice of treatment for HER2-positive breast cancer with progressive brain metastases after trastuzumab, lapatinib, or both (LUX-Breast 3): a randomised, open-label, multicentre, phase 2 trial. Lancet Oncol 2015;16:1700-10. [Crossref] [PubMed]
- Gunzer K, Joly F, Ferrero JM, et al. A phase II study of afatinib, an irreversible ErbB family blocker, added to letrozole in patients with estrogen receptor-positive hormone-refractory metastatic breast cancer progressing on letrozole. Springerplus 2016;5:45. [Crossref] [PubMed]
- Bang YJ, Giaccone G, Im SA, et al. First-in-human phase 1 study of margetuximab (MGAH22), an Fc-modified chimeric monoclonal antibody, in patients with HER2-positive advanced solid tumors. Ann Oncol 2017;28:855-61. [PubMed]
- Espelin CW, Leonard SC, Geretti E, et al. Dual HER2 Targeting with Trastuzumab and Liposomal-Encapsulated Doxorubicin (MM-302) Demonstrates Synergistic Antitumor Activity in Breast and Gastric Cancer. Cancer Res 2016;76:1517-27. [Crossref] [PubMed]
- Miller K, Cortes J, Hurvitz SA, et al. HERMIONE: a randomized Phase 2 trial of MM-302 plus trastuzumab versus chemotherapy of physician's choice plus trastuzumab in patients with previously treated, anthracycline-naive, HER2-positive, locally advanced/metastatic breast cancer. BMC Cancer 2016;16:352. [Crossref] [PubMed]
- van der Lee MM, Groothuis PG, Ubink R, et al. The Preclinical Profile of the Duocarmycin-Based HER2-Targeting ADC SYD985 Predicts for Clinical Benefit in Low HER2-Expressing Breast Cancers. Mol Cancer Ther 2015;14:692-703. [Crossref] [PubMed]
- Baselga J. Targeting the phosphoinositide-3 (PI3) kinase pathway in breast cancer. Oncologist 2011;16 Suppl 1:12-9. [Crossref] [PubMed]
- Lauring J, Park BH, Wolff AC. The phosphoinositide-3-kinase-Akt-mTOR pathway as a therapeutic target in breast cancer. J Natl Compr Canc Netw 2013;11:670-8. [Crossref] [PubMed]
- André F, O'Regan R, Ozguroglu M, et al. Everolimus for women with trastuzumab-resistant, HER2-positive, advanced breast cancer (BOLERO-3): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet Oncol 2014;15:580-91. [Crossref] [PubMed]
- Hurvitz SA, Andre F, Jiang Z, et al. Combination of everolimus with trastuzumab plus paclitaxel as first-line treatment for patients with HER2-positive advanced breast cancer (BOLERO-1): a phase 3, randomised, double-blind, multicentre trial. Lancet Oncol 2015;16:816-29. [Crossref] [PubMed]
- Tolaney S, Burris H, Gartner E, et al. Phase I/II study of pilaralisib (SAR245408) in combination with trastuzumab or trastuzumab plus paclitaxel in trastuzumab-refractory HER2-positive metastatic breast cancer. Breast Cancer Res Treat 2015;149:151-61. [Crossref] [PubMed]
- Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med 2005;353:1673-84. [Crossref] [PubMed]
- Paik S, Kim C, Wolmark N. HER2 status and benefit from adjuvant trastuzumab in breast cancer. N Engl J Med 2008;358:1409-11. [Crossref] [PubMed]
- Pogue-Geile KL, Kim C, Jeong JH, et al. Predicting degree of benefit from adjuvant trastuzumab in NSABP trial B-31. J Natl Cancer Inst 2013;105:1782-8. [Crossref] [PubMed]
- Fehrenbacher L CR, Geyer CE, et al. NSABP B-47(NRG Oncology) phase III RCT Comparing adjuvant chemotherapy with trastuzumab for 1 year in high-risk, invasive breast cancer negative for HER2 by ISH and with IHC 1+ or 2+(HER2-Low IBC). San Antonio Breast Cancer Symposium 2017.
- Gianni L, Llado A, Bianchi G, et al. Open-label, phase II, multicenter, randomized study of the efficacy and safety of two dose levels of Pertuzumab, a human epidermal growth factor receptor 2 dimerization inhibitor, in patients with human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol 2010;28:1131-7. [Crossref] [PubMed]
- Bose R, Kavuri SM, Searleman AC, et al. Activating HER2 mutations in HER2 gene amplification negative breast cancer. Cancer Discov 2013;3:224-37. [Crossref] [PubMed]
- Connell CM, Doherty GJ. Activating HER2 mutations as emerging targets in multiple solid cancers. ESMO Open 2017;2. [Crossref] [PubMed]
- Ali SM, Alpaugh RK, Downing SR, et al. Response of an ERBB2-mutated inflammatory breast carcinoma to human epidermal growth factor receptor 2-targeted therapy. J Clin Oncol 2014;32:e88-91. [Crossref] [PubMed]
- Doi T, Shitara K, Naito Y, et al. Safety, pharmacokinetics, and antitumour activity of trastuzumab deruxtecan (DS-8201), a HER2-targeting antibody-drug conjugate, in patients with advanced breast and gastric or gastro-oesophageal tumours: a phase 1 dose-escalation study. Lancet Oncol 2017;18:1512-22. [Crossref] [PubMed]


