Precision medicine in breast cancer
Introduction
Breast cancer is one of the most prevalent cancers in the world (1,2). Historically, breast cancer has been treated according to biomarkers such as the expression of estrogen receptor (ER), progesterone receptor (PgR), and the status of HER2 as assessed by immunohistochemistry (IHC) or in-situ hybridization (ISH). More recently, breast cancer has been subclassified to intrinsic subtypes on the basis of molecular analyses (3,4), subsequently approximated by IHC (5). This subtyping has been accepted in daily clinical practice, and adjuvant endocrine therapy, chemotherapy, and anti-HER2 therapy are recommended based on the subtypes (Table 1) (6).

Full table
Precision medicine is a strategy for disease treatment and prevention that considers individual variability in genes, environment, and lifestyle (7). The concept of an individualized approach is not new in the field of breast cancer; however, recent advances in omics technologies have focused on and allowed more precise approaches. This review describes the recent advances in precision medicine, especially, genome medicine, in breast cancer.
Multigene assays for resected breast cancer
Classical “subtyping” is essential for early breast cancer to determine which modality and which antitumor agents are best recommended to the patient. However, the adverse effects of cytotoxic chemotherapy are substantial, and there is an urgent need for predicting the risk of relapse and estimating the benefit of adjuvant chemotherapy so as to omit chemotherapy without disadvantage. Several multigene assays are available to estimate the risk for relapse after definitive surgery (Table 2). They are used mainly in ER-positive breast cancers. MammaPrint (Agendia) and Oncotype DX (Genomic Health, Inc., Redwood City, CA, USA) are the most widely used multigene assays worldwide.

Full table
MammaPrint uses fresh/frozen tissues or formalin-fixed paraffin-embedded (FFPE) samples and examines 1,391 genes by microarray assay, and the results of 70 genes are used to classify patients into high- to low-risk for relapse (8,9). In a retrospective study, 295 breast cancers were analyzed. The hazard ratio for 10-year distant disease-free survival (DFS) was 5.1 for high-risk compared to low-risk patients (P<0.001). In comparison with Adjuvant! Online, an internet tool to estimate the risk of relapse and benefit of adjuvant endocrine therapy and chemotherapy, MammaPrint was more useful to distinguish high risk from low risk (10). Currently, a prospective study of MINDACT, a randomized phase III trial investigating the utility of MammaPrint in addition to the clinicopathological factors in selecting operable breast cancer patients for adjuvant chemotherapy, is underway (11,12).
Oncotype DX, which uses FFPE samples, is another widely used multigene assay. Oncotype DX examines 21 genes (including five reference genes) and estimates the risk of relapse at 10 years and the benefit of adjuvant endocrine therapy and chemotherapy. Oncotype DX results are expressed as recurrence score (RS) from 0 to 100. Retrospective analysis of the National Surgical Adjuvant Breast and Bowel Project (NSABP) B14, a randomized study which compared 5 years of tamoxifen with the same period of placebo in early breast cancer patients after surgery and showed a significant improvement of DFS with tamoxifen, showed that the rate of 10-year distant recurrence in the low RS (RS <18), intermediate RS (RS 18–30), and high RS (RS ≥31) cohort were 6.8%, 14.3%, and 30.5%, respectively (13). Another retrospective analysis with the data of Trans-Arimidex, Tamoxifen, Alone or in Combination (TransATAC) study showed that RS is an independent risk factor, regardless of the presence of lymph node metastases (14). Oncotype DX also predicts the benefit of adjuvant chemotherapy. Retrospective analysis of the NSABP B20 study, in which ER-positive patients without lymph-node metastasis were enrolled, showed that in patients with high RS (RS ≥31), the risk of relapse was improved by adjuvant chemotherapy and endocrine therapy compared with endocrine therapy alone (relative risk, 0.26; 95% CI, 0.13–0.53, P<0.001); however, in patients with low RS, benefit of adjuvant chemotherapy was not demonstrated (relative risk, 1.31; 95% CI, 0.46–3.78, P=0.61) (15). Similarly, the benefit of adjuvant chemotherapy in patients with lymph node metastasis was reported (SWOG 8814 study) (16). Currently, prospective studies (TAILORx, RxPONDER) are underway (17-19).
PAM50-based Prosigna (NanoString Technologies) examines a 50-gene signature and estimates the risk of recurrence (ROR) (20). In a retrospective study of the TransATAC study, the predictive value of the ROR was demonstrated (21). A retrospective analysis of Austrian Breast & Colorectal Cancer Study Group-8 (ABCSG-8), investigating the efficacy of switching from tamoxifen to anastrozole compared with continuing to receive tamoxifen in patients with postmenopausal hormone receptor-positive early breast cancer, showed that PAM50 was useful, regardless of lymph node metastases (22). The prognostic utility of the breast-cancer index (BCI) assay was examined in TransATAC study (23). BCI showed a significant predictor for risk of both early and late distant recurrence.
EndoPredict (Myriad Genetics) is a clinically validated multianalyte gene expression test which could predict the ROR in patients with breast cancer at 10 years (24).
Curebest 95GC breast (Sysmex) is a 95-gene classifier that uses fresh samples to predict the prognosis for ER-positive and lymph node metastasis-negative resected breast cancer treated with adjuvant endocrine therapy alone (25). The capability of Curebest 95GC to predict prognosis seems to be comparable to that of Oncotype DX (26). These assays are currently employed in practice guidelines and have been used intensively in clinic.
Mutational landscape and molecular targeted agents for breast cancer
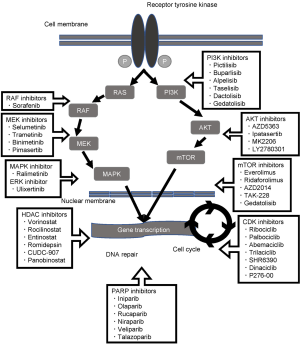
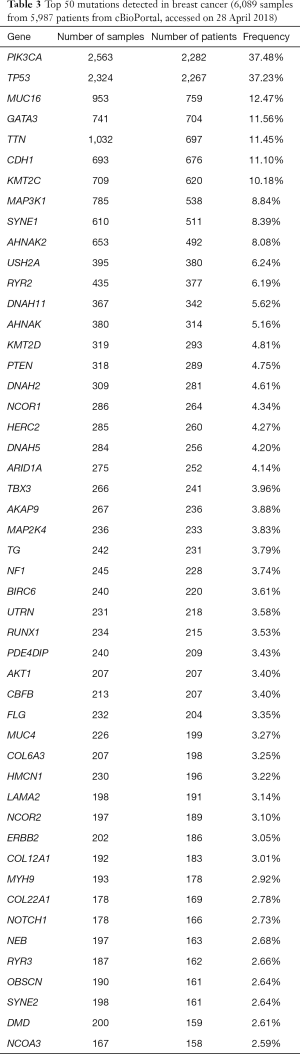
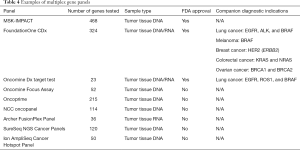
Recent advances in molecular medicine have been key to precision medicine. For example, epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors showed great benefit in patients with non-small cell lung cancer and activating EGFR mutations (27-29). In contrast, the evaluation of RAS mutation is useful to avoid anti-EGFR antibody for colorectal cancer (30,31). Druggable or actionable gene alterations which could affect treatment choice have been recently discovered across cancer types. In breast cancer, several possible druggable mutations have been identified (Table 3) (32). Several multiplex gene panels are available (Table 4). However, there is relatively less evidence to support the use of matched molecular targeted agents in breast cancer.
Full table
Full table
Phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT)/mechanistic target of rapamycin (mTOR) pathway
Deregulation of the phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT)/mechanistic target of rapamycin (mTOR) pathway contributes to the development and progression of tumors, and the PI3K-AKT-mTOR axis is one of the most commonly affected pathways in breast cancer. For example, Chen and colleagues reported the incidence of somatic mutations in PIK3CA (44%), PIK3R1 (17%), AKT3 (15%), and PTEN (12%) in Chinese breast cancer patients (33). Similarly, we have detected mutations in PIK3CA (38%), TP53 (15%), and AKT1 (13%) in 39 early hormone receptor-positive Japanese breast cancer patients (unpublished data).
PI3K inhibitors
Several PI3K inhibitors are currently under investigation (Figure 1) (34). Among them, buparlisib is in the most advanced stage of development. A phase III study (BELLE-2) compared buparlisib with placebo in combination with fulvestrant in postmenopausal hormone receptor-positive and HER2-negative breast cancer progressed on or after aromatase inhibitor (35). In the total patient population (n=1,147), progression-free survival (PFS) was significantly longer with buparlisib [median PFS 6.9 vs. 5.0 months, hazard ratio (HR) =0.78, P=0.00021]. The benefit of buparlisib was larger in PI3K pathway-activated patients (n=372, median PFS 6.8 vs. 4.0 months, HR =0.76, P=0.014). In another phase III study (BELLE-3), buparlisib was also compared with placebo in combination with fulvestrant in postmenopausal hormone receptor-positive and HER2-negative breast cancer patients who had relapsed on or after endocrine therapy and mTOR inhibitors (36). Buparlisib significantly improved PFS (3.9 vs. 1.8 months, HR =0.67, P=0.00030). However, approval application has not been submitted yet. Currently, pictilisib, alpelisib and taselisib are being evaluated in phase III studies.
mTOR inhibitor
The mTOR inhibitor everolimus is approved worldwide and widely used in combination with aromatase inhibitor exemestane. A pivotal phase III study (BOLERO-2) evaluated the efficacy of everolimus in combination with exemestane in 724 patients with hormone-receptor-positive advanced breast cancer after a nonsteroidal aromatase inhibitor (37). PFS was significantly longer with everolimus (median PFS 6.9 vs. 2.8 months, HR =0.43, P<0.001). To explore the predictive factor for everolimus, next-generation sequencing was performed to analyze genetic alterations in cancer-related genes in 302 archival tumor samples from the BOLERO-2 study (38). The benefit of PFS with everolimus was consistent across gene alterations in FGFR1, CCND1, and also PIK3CA or the pathways of which they are components.
AKT inhibitors
Several AKT inhibitors have been investigated in patients with breast cancer. In a randomized phase II trial (LOTUS), ipatasertib was evaluated in combination with paclitaxel (39). In the study, 124 inoperable, locally advanced or metastatic chemotherapy-naïve triple-negative breast cancer (TNBC) patients were enrolled. The median PFS was 6.2 months with ipatasertib versus 4.9 months with placebo (HR =0.60, P=0.037), and in the 48 patients with PTEN-low tumors, the benefit of ipatasertib seemed better (median PFS was 6.2 vs. 3.7 months, HR =0.59, P=0.18). In a basket study of another AKT inhibitor, AZD5363, the median PFS was 5.5 months in patients with AKT1 E17K-mutant ER-positive breast cancer (40). MK-2206 was investigated in neoadjuvant setting in patients with PIK3CA-mutant ER-positive and HER2-negative breast cancer (41). MK-2206 is unlikely to add to the efficacy of anastrozole alone in PIK3CA-mutant ER-positive breast cancer.
RAS-RAF-MAPK pathway
RAF inhibitor
The RAS/RAF signaling pathway plays an important role of cell proliferation and angiogenesis. Sorafenib is a multitargeted kinase inhibitor and one of its targets is Raf-1, a member of the RAF/MEK/ERK signaling pathway. RESILIENCE is a randomized phase III trial that compared capecitabine with sorafenib or placebo in patients with locally advanced/metastatic HER2-negative breast cancer resistant to a taxane and anthracycline in the first- or second-line setting. Sorafenib failed to prolong PFS (median PFS 5.5 vs. 5.4 months, HR =0.973, P=0.811) or overall survival (OS) (median OS 18.9 vs. 20.3 months, HR =1.195, P=0.140) (42).
MAPK inhibitor
p38 MAPK is a protein kinase activated by cytokine stimulation, ultraviolet irradiation, and various stresses. p38 MAPK plays an important role on regulation for cytokines and cell survival. Ralimetinib is a selective small-molecule inhibitor of p38 MAPK (43). Ralimetinib demonstrated acceptable safety, tolerability, and pharmacokinetics in patients with advanced cancer. A randomized phase II study of tamoxifen and ralimetinib in advanced or metastatic breast cancer after aromatase inhibitors (OLYMPE) is awaiting results (44).
Histone deacetylase (HDAC) inhibitors
Epigenetics refers to alterations in gene expression that are not accompanied by changes in the corresponding DNA sequence (45). Epigenetics has increasingly investigated to find new ways of prevention and to overcome the resistant to endocrine therapy in breast cancer. HDAC controls the level of acetylation of histones and consequently, gene expression. Several HDAC inhibitors are investigated in oncology. Entinostat is an oral isoform-selective HDAC inhibitor targeting resistance to endocrine therapies in ER-positive breast cancer. A randomized phase II trial (ENCORE301) evaluated entinostat in combination with exemestane in comparison with exemestane alone (46). Entinostat significantly improved PFS (median PFS 4.3 vs. 2.3 months, HR =0.73, P=0.055). A phase III study (E2112) is underway (47).
Cyclin dependent kinase (CDK) inhibitors
The cyclin D-CDK 4/6-inhibitor of CDK4 (INK4)-retinoblastoma (Rb) pathway plays a critical role in controlling cell cycle. CDK4/6 inhibitors induce cell cycle arrest in the G1 phase, which can eventually prevent the proliferation of cancer cells (48). The efficacies of three CDK4/6 inhibitors (palbociclib, ribociclib, and abemaciclib) in patients with ER-positive relapsed/metastatic breast cancer have been reported (Table 5) (49-53). However, the predictive factor of these CDK4/6 inhibitors has not yet been determined.

Full table
PARP inhibitors
BRCA1 and BRCA2 are critical proteins in the process of homologous recombination repair of double-strand DNA breaks. Germline BRCA mutations (gBRCA mutation) were found in 5–10% of breast cancers (54). In patients with gBRCA mutations, the function of BRCA might be deficient and homologous recombination repair of double-strand DNA breaks is impaired, therefore PARP inhibitors induce synthetic lethality. Iniparib was the first drug for which a phase III study was reported (55) (recently iniparib is suspected not to be a potential PARP inhibitor). Patients with stage IV/locally recurrent TNBC were randomly allocated to gemcitabine/carboplatin alone or in combination with iniparib. The benefit of iniparib was not statistically significant (OS HR =0.88, P=0.28; PFS HR =0.79, P=0.027). Olaparib, another PARP inhibitor, was investigated in a phase III trial (OlympiaD) (56). In the study, olaparib as a single agent was compared with standard chemotherapy (vinorelbine, eribulin, or capecitabine) in patients with a gBRCA-mutated HER2-negative metastatic breast cancer who had received no more than two lined of chemotherapy for metastatic disease. PFS was significantly improved in the olaparib group (median PFS 7.0 vs. 4.2 months, HR =0.58, P<0.001). The data for OS is preliminary, but the effect on OS seems not to be significant. Recently, a randomized phase III trial (EMBRACA) of talazoparib in patients with advanced breast cancer and a gBRCA mutation was reported (57). Talazoparib is a dual-mechanism PARP inhibitor showing to inhibit the PARP enzyme and also to trap PARP on DNA, thus preventing DNA damage repair, leading to death of BRCA-mutated cells. Talazoparib significantly improved PFS compared to placebo (median PFS 8.6 vs. 5.6 months, HR =0.542, P<0.0001). To test BRCA alterations, BRACAnalysis CDx was approved as a companion diagnostic by the FDA (58).
Miscellaneous
HER2 mutation
Somatic HER2 (encoded by ERBB2) mutations, apart from gene amplification, have been reported recurrently. Mutations in HER2 are clustered in the extracellular, transmembrane and kinase domains. Also, HER2 mutations are infrequent in a wide variety of cancers but targetable. In breast cancers, activating mutations were identified as follows: G309A, D769H/Y, V777L, P780ins, V842I, and R896C (59). L755S was associated with lapatinib resistance. All of these mutations were sensitive to the irreversible kinase inhibitor, neratinib. Recently, phase II SUMMIT trial, which is a HER2 mutant basket trial, showed mutation status can contribute to response to neratinib regardless of tumor type (60).
Glembatumumab vedotin
Glycoprotein non-metastatic gene B (gpNMB) is an internalizable glycoprotein that is expressed in more than 40% of breast cancers as well as in other tumor types (61). gpNMB plays a crucial role in the migration, invasion, and metastasis of breast cancer. Glembatumumab vedotin is a gpNMB-specific monoclonal antibody-drug conjugated with the potent cytotoxin monomethyl auristatin E. In a randomized phase II trial (EMERGE), patients with refractory breast cancer that expressed gpNMB in ≥5% of epithelial or stromal cells as indicated by central IHC were randomized to glembatumumab vedotin or chemotherapy (62). Unplanned analysis showed an overall objective response rate of 18% versus 0% in patients with TNBC, and 40% versus 0% in gpNMB-overexpressing TNBC. Currently, a pivotal phase IIb study METRIC completed enrollment and the results are awaited (63).
A need for expert panels
As described above, druggable or actionable gene alterations are more common across the cancer types. In breast cancer, several gene alterations such as gBRCA mutations, PIK3CA mutations are promising as a predictor for treatment choice. Therefore, it is necessary to examine multiple gene alterations at once. To introduce pan-cancer next generation sequencing (NGS) gene panels into clinical practice, analytical validity, clinical validity, and clinical utility should be established prospectively. Moreover, there is a potential for the recognition and reporting of secondary findings, unrelated to the indication for testing the sequencing, but of clinical importance. For interpreting sequencing results, assigning annotations, and discussing concomitant ethical/legal/social implications, a multidisciplinary tumor board (expert panel) is essential (64). An expert panel is usually referred to as a multidisciplinary tumor board consisting of experts in medical oncology, pathology, molecular medicine, genetics, and bioinformatics. The utility of organizing expert panels for discussing sequencing results has been suggested, because most physicians have lacked the confidence in the interpretation of genomic alterations (65).
Future directions
Recent advances in molecular targeted agents, such as PI3K/mTOR inhibitors, CDK4/6 inhibitors, and PARP inhibitors, have continually improved the prognosis of breast cancer patients; however, the predictive factor of these agents are poorly described, except for BRCA mutations. Serial investigations of mutational status, for example using cell-free DNA (cfDNA) (66), may overcome such issues.
Acknowledgements
Funding: This work was supported in part by a Grant-in-Aid for National Cancer Center Research and Development Fund 27-A-1.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Centers for Disease Control and Prevention 2006-2010 top ten cancers. National Program of Cancer Registries 2014. Available online: http://apps.nccd.cdc.gov/uscs/toptencancers.aspx
- Pruitt SL, Lee SJ, Tiro JA, et al. Residential racial segregation and mortality among black, white, and Hispanic urban breast cancer patients in Texas, 1995 to 2009. Cancer 2015;121:1845-55. [Crossref] [PubMed]
- Perou CM, Sørlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature 2000;406:747-52. [Crossref] [PubMed]
- Sørlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A 2001;98:10869-74. [Crossref] [PubMed]
- Nielsen TO, Hsu FD, Jensen K, et al. Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin Cancer Res 2004;10:5367-74. [Crossref] [PubMed]
- Curigliano G, Burstein HJ, P, Winer E, et al. De-escalating and escalating treatments for early-stage breast cancer: the St. Gallen International Expert Consensus Conference on the Primary Therapy of Early Breast Cancer 2017. Ann Oncol 2017;28:1700-12. [PubMed]
- Collins FS, Varmus H. A new initiative on precision medicine. N Engl J Med 2015;372:793-5. [Crossref] [PubMed]
- van 't Veer LJ, Dai H, van de Vijver MJ, et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature 2002;415:530-6. [Crossref] [PubMed]
- van de Vijver MJ, He YD, van't Veer LJ, et al. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med 2002;347:1999-2009. [Crossref] [PubMed]
- Drukker CA, Bueno-de-Mesquita JM, Retèl VP, et al. A prospective evaluation of a breast cancer prognosis signature in the observational RASTER study. Int J Cancer 2013;133:929-36. [Crossref] [PubMed]
- Cardoso F, van't Veer LJ, Bogaerts J, et al. 70-Gene Signature as an Aid to Treatment Decisions in Early-Stage Breast Cancer. N Engl J Med 2016;375:717-29. [Crossref] [PubMed]
- Clinical Trial Gov. Accessed on 28 April. Available online: https://clinicaltrials.gov/ct2/show/NCT00433589
- Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med 2004;351:2817-26. [Crossref] [PubMed]
- Dowsett M, Cuzick J, Wale C, et al. Prediction of risk of distant recurrence using the 21-gene recurrence score in node-negative and node-positive postmenopausal patients with breast cancer treated with anastrozole or tamoxifen: a TransATAC study. J Clin Oncol 2010;28:1829-34. [Crossref] [PubMed]
- Paik S, Tang G, Shak S, et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol 2006;24:3726-34. [Crossref] [PubMed]
- Albain KS, Barlow WE, Shak S, et al. Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. Lancet Oncol 2010;11:55-65. [Crossref] [PubMed]
- Sparano JA, Gray RJ, Makower DF, et al. Prospective Validation of a 21-Gene Expression Assay in Breast Cancer. N Engl J Med 2015;373:2005-14. [Crossref] [PubMed]
- Clinical Trial Gov. Hormone Therapy With or Without Combination Chemotherapy in Treating Women Who Have Undergone Surgery for Node-Negative Breast Cancer (The TAILORx Trial) (TAILORx). Accessed on 28 April, 2018. Available online: https://clinicaltrials.gov/ct2/show/NCT00310180
- Clinical Trial Gov. Tamoxifen Citrate, Letrozole, Anastrozole, or Exemestane With or Without Chemotherapy in Treating Patients With Invasive RxPONDER Breast Cancer. Accessed on 28 April, 2018. Available online: https://clinicaltrials.gov/ct2/show/NCT01272037
- Parker JS, Mullins M, Cheang MC, et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. J Clin Oncol 2009;27:1160-7. [Crossref] [PubMed]
- Dowsett M, Sestak I, Lopez-Knowles E, et al. Comparison of PAM50 risk of recurrence score with oncotype DX and IHC4 for predicting risk of distant recurrence after endocrine therapy. J Clin Oncol 2013;31:2783-90. [Crossref] [PubMed]
- Gnant M, Filipits M, Greil R, et al. Predicting distant recurrence in receptor-positive breast cancer patients with limited clinicopathological risk: using the PAM50 Risk of Recurrence score in 1478 postmenopausal patients of the ABCSG-8 trial treated with adjuvant endocrine therapy alone. Ann Oncol 2014;25:339-45. [Crossref] [PubMed]
- Sgroi DC, Sestak I, Cuzick J, et al. Prediction of late distant recurrence in patients with oestrogen-receptor-positive breast cancer: a prospective comparison of the breast-cancer index (BCI) assay, 21-gene recurrence score, and IHC4 in the TransATAC study population. Lancet Oncol 2013;14:1067-76. [Crossref] [PubMed]
- Kronenwett R, Bohmann K, Prinzler J, et al. Decentral gene expression analysis: analytical validation of the Endopredict genomic multianalyte breast cancer prognosis test. BMC Cancer 2012;12:456. [Crossref] [PubMed]
- Naoi Y, Kishi K, Tanei T, et al. Development of 95-gene classifier as a powerful predictor of recurrences in node-negative and ER-positive breast cancer patients. Breast Cancer Res Treat 2011;128:633-41. [Crossref] [PubMed]
- Naoi Y, Kishi K, Tsunashima R, et al. Comparison of efficacy of 95-gene and 21-gene classifier (Oncotype DX) for prediction of recurrence in ER-positive and node-negative breast cancer patients. Breast Cancer Res Treat 2013;140:299-306. [Crossref] [PubMed]
- Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 2004;350:2129-39. [Crossref] [PubMed]
- Paez JG, Jänne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 2004;304:1497-500. [Crossref] [PubMed]
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57. [Crossref] [PubMed]
- Van Cutsem E, Köhne CH, Láng I, et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol 2011;29:2011-9. [Crossref] [PubMed]
- Douillard JY, Siena S, Cassidy J, et al. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol 2010;28:4697-705. [Crossref] [PubMed]
- cBioPortal. Accessed on 28 April 2018. Available online: http://www.cbioportal.org/
- Chen L, Yang L, Yao L, et al. Characterization of PIK3CA and PIK3R1 somatic mutations in Chinese breast cancer patients. Nat Commun 2018;9:1357. [Crossref] [PubMed]
- Clinical Trial Gov. Accessed on 28 April 2018. Available online: https://clinicaltrials.gov/
- Baselga J, Im SA, Iwata H, et al. Buparlisib plus fulvestrant versus placebo plus fulvestrant in postmenopausal, hormone receptor-positive, HER2-negative, advanced breast cancer (BELLE-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2017;18:904-16. [Crossref] [PubMed]
- Di Leo A, Johnston S, Lee KS, et al. Buparlisib plus fulvestrant in postmenopausal women with hormone-receptor-positive, HER2-negative, advanced breast cancer progressing on or after mTOR inhibition (BELLE-3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2018;19:87-100. [Crossref] [PubMed]
- Baselga J, Campone M, Piccart M, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med 2012;366:520-9. [Crossref] [PubMed]
- Hortobagyi GN, Chen D, Piccart M, et al. Correlative Analysis of Genetic Alterations and Everolimus Benefit in Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Advanced Breast Cancer: Results From BOLERO-2. J Clin Oncol 2016;34:419-26. [Crossref] [PubMed]
- Kim SB, Dent R, Im SA, et al. Ipatasertib plus paclitaxel versus placebo plus paclitaxel as first-line therapy for metastatic triple-negative breast cancer (LOTUS): a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol 2017;18:1360-72. [Crossref] [PubMed]
- Hyman DM, Smyth LM, Donoghue MTA, et al. AKT Inhibition in Solid Tumors With AKT1 Mutations. J Clin Oncol 2017;35:2251-9. [Crossref] [PubMed]
- Ma CX, Suman V, Goetz MP, et al. A Phase II Trial of Neoadjuvant MK-2206, an AKT Inhibitor, with Anastrozole in Clinical Stage II or III PIK3CA-Mutant ER-Positive and HER2-Negative Breast Cancer. Clin Cancer Res 2017;23:6823-32. [Crossref] [PubMed]
- Baselga J, Zamagni C, Gómez P, et al. RESILIENCE: Phase III Randomized, Double-Blind Trial Comparing Sorafenib With Capecitabine Versus Placebo With Capecitabine in Locally Advanced or Metastatic HER2-Negative Breast Cancer. Clin Breast Cancer 2017;17:585-94.e4. [Crossref] [PubMed]
- Patnaik A, Haluska P, Tolcher AW, et al. A First-in-Human Phase I Study of the Oral p38 MAPK Inhibitor, Ralimetinib (LY2228820 Dimesylate), in Patients with Advanced Cancer. Clin Cancer Res 2016;22:1095-102. [Crossref] [PubMed]
- Clinical Trial.gov. A Multicenter Trial Assessing the Efficacy and Safety of tamOxifen Plus LY2228820 in Advanced or Metastatic Breast Cancer Progressing on aromatasE Inhibitors (OLYMPE). Accessed on 28 April 2018. Available online: https://clinicaltrials.gov/ct2/show/NCT02322853
- Lakshmaiah KC, Jacob LA, Aparna S, et al. Epigenetic therapy of cancer with histone deacetylase inhibitors. J Cancer Res Ther 2014;10:469-78. [PubMed]
- Yardley DA, Ismail-Khan RR, Melichar B, et al. Randomized phase II, double-blind, placebo-controlled study of exemestane with or without entinostat in postmenopausal women with locally recurrent or metastatic estrogen receptor-positive breast cancer progressing on treatment with a nonsteroidal aromatase inhibitor. J Clin Oncol 2013;31:2128-35. [Crossref] [PubMed]
- Clinical Trial.gov. A Randomized Phase III Trial of Endocrine Therapy Plus Entinostat/Placebo in Patients with Hormone Receptor-Positive Advanced Breast Cancer. Accessed on 28 April 2018. Available online: https://clinicaltrials.gov/ct2/show/NCT02115282
- Kwapisz D. Cyclin-dependent kinase 4/6 inhibitors in hormone receptor-positive early breast cancer: preliminary results and ongoing studies. Breast Cancer 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Finn RS, Martin M, Rugo HS, et al. Palbociclib and Letrozole in Advanced Breast Cancer. N Engl J Med 2016;375:1925-36. [Crossref] [PubMed]
- Turner NC, Ro J, André F, et al. PALOMA3 Study Group. Palbociclib in Hormone-Receptor-Positive Advanced Breast Cancer. N Engl J Med 2015;373:209-19. [Crossref] [PubMed]
- Hortobagyi GN, Stemmer SM, Burris HA, et al. Ribociclib as First-Line Therapy for HR-Positive, Advanced Breast Cancer. N Engl J Med 2016;375:1738-48. [Crossref] [PubMed]
- Goetz MP, Toi M, Campone M, et al. MONARCH 3: Abemaciclib As Initial Therapy for Advanced Breast Cancer. J Clin Oncol 2017;35:3638-46. [Crossref] [PubMed]
- Sledge GW Jr, Toi M, Neven P, et al. MONARCH 2: Abemaciclib in Combination With Fulvestrant in Women With HR+/HER2- Advanced Breast Cancer Who Had Progressed While Receiving Endocrine Therapy. J Clin Oncol 2017;35:2875-84. [Crossref] [PubMed]
- Walsh CS. Two decades beyond BRCA1/2: Homologous recombination, hereditary cancer risk and a target for ovarian cancer therapy. Gynecol Oncol 2015;137:343-50. [Crossref] [PubMed]
- O'Shaughnessy J, Schwartzberg L, Danso MA, et al. Phase III study of iniparib plus gemcitabine and carboplatin versus gemcitabine and carboplatin in patients with metastatic triple-negative breast cancer. J Clin Oncol 2014;32:3840-7. [Crossref] [PubMed]
- Robson M, Im SA, Senkus E, et al. Olaparib for Metastatic Breast Cancer in Patients with a Germline BRCA Mutation. N Engl J Med 2017;377:523-33. [Crossref] [PubMed]
- Litton J, Rugo HS, Ettl J, et al. EMBRACA: A phase 3 trial comparing talazoparib, an oral PARP inhibitor, to physician's choice of therapy in patients with advanced breast cancer and a germline BRCA mutation [abstract]. In: Proceedings of the 2017 San Antonio Breast Cancer Symposium; 2017 Dec 5-9; San Antonio, TX. Philadelphia (PA): AACR; Cancer Res 2018;78:abstr nr GS6-07.
- BRACAnalysis CDx. Accessed on 28 April 2018. Available online: https://myriad.com/products-services/companion-diagnostics/bracanalysis-cdx/
- Bose R, Kavuri SM, Searleman AC, et al. Activating HER2 mutations in HER2 gene amplification negative breast cancer. Cancer Discov 2013;3:224-37. [Crossref] [PubMed]
- Hyman DM, Piha-Paul SA, Won H, et al. HER kinase inhibition in patients with HER2- and HER3-mutant cancers. Nature 2018;554:189-94. [Crossref] [PubMed]
- Rose AA, Grosset AA, Dong Z, et al. Glycoprotein nonmetastatic B is an independent prognostic indicator of recurrence and a novel therapeutic target in breast cancer. Clin Cancer Res 2010;16:2147-56. [Crossref] [PubMed]
- Yardley DA, Weaver R, Melisko ME, et al. EMERGE: A Randomized Phase II Study of the Antibody-Drug Conjugate Glembatumumab Vedotin in Advanced Glycoprotein NMB-Expressing Breast Cancer. J Clin Oncol 2015;33:1609-19. [Crossref] [PubMed]
- Clinical Trial. Gov. Study of Glembatumumab Vedotin (CDX-011) in Patients with Metastatic, gpNMB Over-Expressing, Triple Negative Breast Cancer (METRIC). Accessed on 28 April 2018. Available online: https://clinicaltrials.gov/ct2/show/NCT01997333
- Naito Y, Takahashi H, Shitara K, et al. Feasibility study of cancer genome alterations identified by next generation sequencing: ABC study. Jpn J Clin Oncol 2018;48:559-64. [Crossref] [PubMed]
- Gray SW, Hicks-Courant K, Cronin A, et al. Physicians' attitudes about multiplex tumor genomic testing. J Clin Oncol 2014;32:1317-23. [Crossref] [PubMed]
- Rohanizadegan M. Analysis of circulating tumor DNA in breast cancer as a diagnostic and prognostic biomarker. Cancer Genet 2018. [Epub ahead of print]. [Crossref] [PubMed]