Immunotherapy for metastatic breast cancer
Introduction
The immune system has developed to protect the host from continuous exposure to micro-organisms. This biological defense mechanism cannot work well unless it can distinguish between body cells (self) and micro-organisms or infected cells (non-self). Cancer cells are derived from body cells that gain malignant characteristics by a genetic alteration. The ‘foreignness’ of malignant cells, however, can often evade the immune system and develop into a clinically significant mass. In the past decades, the field of cancer immunology has oscillated between pessimism and optimism regarding the existence of biological defenses in the elimination of cancer cells. However, blocking monoclonal antibodies (mAbs) targeting cytotoxic T-lymphocyte associated antigen-4 (CTLA-4), programmed death-1 (PD-1) and programmed death ligand-1 (PD-L1) can restore pre-existing anti-cancer immunity and achieve a durable clinical response in various types of solid and hematological tumors that are refractory to standard therapies (1-8). The success of these immune checkpoint inhibitors demonstrates the existence of anti-cancer immunity in patients with malignant tumors. Breast cancer was traditionally thought to be poorly immunogenic compared to highly immunogenic (‘inflamed’) cancers, including malignant melanoma and non-small cell lung carcinoma. However, previous studies of tumor-infiltrating lymphocytes (TILs) and cancer genome sequences have demonstrated that estrogen receptor (ER)-negative, human epidermal growth factor receptor 2 (HER2)-positive subtype and triple-negative breast cancer (TNBC) are more immunogenic than ER-positive-HER2-negative subtypes (9,10). TILs represent pre-existing immunity and are potent biomarkers for predicting prognosis and possibly response to immunotherapy, especially for TNBC. In this review, we will discuss the basis of cancer immunity and current status of immune checkpoint therapy for metastatic breast cancer.
Adaptive immunity against breast cancer
Fifty years ago, in the history of cancer immunity, Burnet (11) proposed the immune surveillance theory that the body can eliminate transformed cells by immune cells, which are capable of recognizing and destroying the transformed cells before they become clinically significant tumors. Among immune cells, T lymphocytes are major players in adaptive immunity, and how to activate them has been a key issue in the development of cancer immunotherapy. However, the outcomes of conventional immunotherapies were not as satisfying as expected. In the past 15 years, previous studies elucidated that the function of immune surveillance was a part of anti-cancer immunity, and the new extended concept of immunoediting was proposed by Schreiber to understand the immunodynamics between tumors and the immune system (12,13). Immunoediting describes a series of interactions between tumor cells and immune cells which lead to cancer suppression and promotion in three phases: elimination, equilibrium, and escape (14,15). In the elimination phase, tumors have relatively high immunogenicity and innate immunity by natural killer (NK) cells, and the subsequent adaptive immunity by T cells can eliminate tumor cells before forming a clinically significant mass. In the equilibrium phase, some tumor cells have evolved with genetic and epigenetic changes to be resistant to attack by the immune system. Tumor cells become dormant as occult cancer, caught in the balance between anti-tumor and pro-tumor immunity in the tumor microenvironment. In the escape phase, tumor cells evolve to exhibit poor immunogenicity and proliferate to form clinically significant masses. For tumor cells, the absence of antigens, the loss of major histocompatibility complex (MHC), acquired anti-apoptosis and increased survival leads to immuno-evasion. In the tumor microenvironment, expression of immune checkpoints increased the frequency of immunosuppressive cells including regulatory T cells, myeloid-derived suppressor cells (MDSC), and immunomodulatory factors, including tumor growth factor (TGF) and vascular endothelial growth factor (VEGF), which also cause peripheral tolerance. Currently, how to release the brake on the immune system is a key issue in the reactivation of anti-tumor immunity (16).
Tumor mutation burden (TMB)
Innate immunity recognizes conserved structures in pathogens via Toll-like receptors (pattern recognition), whereas acquired immunity recognizes approximately 10 peptides derived from pathogens in context with MHC class I and class II (17-19). Naïve T cells are primed and activated by professional antigen presenting cells (dendritic cells). Primed T cells differentiate into antigen -specific T cells, and clonally expanded T cells can eliminate tumor cells in the tumor microenvironment. Cancer cells originate from normal cells with viral infections or gene mutations. Driver mutations are critical for malignant transformation and shared across any given type of cancer. Therefore, these mutated genes are ideal targets for drug treatments. Recent cancer genomic analyses have demonstrated that tumor cells accumulate gene mutations during development, and the altered proteins can be recognized by the immune system (20). These neoantigens are related to malignant transformations (driver mutations) or the products of increasing genetic instability (passenger mutations). Differences in TMB are observed among malignant tumors, and higher somatic mutations (10/Mb) are found in melanoma and non-small cell lung cancer (21). Breast cancer yields a rather low mutation rate of 1/Mb, which might cause relatively low immunogenicity. Some missense mutations yield mutational epitopes (neoepitope) in context with the patient’s MHC class I and class II.
The study using The Cancer Genome Atlas has demonstrated that TMB is associated with local anti-tumor immunity (22). Melanoma, non-small cell lung cancer and miss match repair deficiency colon cancer (Lynch syndrome) are thought to be immunogenic (‘inflamed’) tumor due to high genetic instability (23-26). For breast cancer, TNBC and HER2-enriched breast cancer yield relatively higher TMB and more T cell infiltration compared to the luminal subtype. As TMB is reversely correlated with prognosis in the luminal subtype (27), TMB may not reflect immunogenicity in this subtype.
Tumor-infiltrating lymphocyte (TIL)
TILs represent pre-existing immunity, and lymphocyte-predominant breast cancer (LPBC) has more than 50–60% lymphocyte infiltration in the stroma. Incidence of LPBC is 20% for TNBC, 16% for HER2 subtype and 6% for ER-positive luminal subtype (28). Since Aaltomaa (29) reported that TIL was an independent prognostic factor for highly proliferative breast cancer, accumulating results demonstrate that high frequencies of TIL were associated with a favorable prognosis and a better clinical response to chemotherapy in TNBC. In an adjuvant setting, the BIG 02-98 trial, Loi (9) reported that every 10% increase of stromal TIL was associated with a risk reduction of TNBC recurrence (HR =0.85, P=0.025), and a better clinical outcome was observed in LPBC compared to non LPBC (HR =0.30, P=0.018). When combined, the results of the ECOG2197 and ECOG119 trials demonstrated that every 10% increment of stromal TIL was associated with better disease-free survival (DFS) and overall survival (OS) in TNBC (30). For the neoadjuvant setting, TIL was considered to be a reliable biomarker for predicting pathological complete response (pCR) for breast cancer. According to a recent meta-analysis that included 3,771 patients, a high frequency of stromal TIL was a good biomarker for predicting pCR in all molecular subtypes (31). Better clinical outcomes were observed for LPBC in HER2-positive breast cancer for adjuvant and neoadjuvant settings (32-35). However, TIL was associated with a shorter overall survival for HER2-negative luminal subtype (31,36). These results indicated that the biology of TIL was different in the subtypes of breast cancer.
In the subset analyses of TILs, increased frequencies of CD8-positive effector T cells (37) and CD4-positive Th1 cells were associated with a better prognosis, while an increased frequency of CD4-positive Th2 cells was negatively associated (38,39). The prognostic impact of regulatory T cell (Treg) infiltration in breast cancer remains controversial. A recent meta-analysis reported that a high infiltration of Treg was associated with a poor prognosis in breast cancer (40). However, the clinical impact of Treg infiltration was varied in the subtypes of breast cancer. The association with a favorable prognosis was observed in ER-negative HER2-positive breast cancer but not ER-positive luminal breast cancer (41). The CD8/FoxP3 was significantly associated with OS (40) and residual tumor was a significant parameter to predict a better DFS in residual TNBC after neoadjuvant chemotherapy (42). Standardization of TIL assessment in residual cancer is now under way.
Analyses of the molecular profiles of tumors and tumor-associated cells have demonstrated that plasma cell signatures were associated with a favorable prognosis, while tumor-associated neutrophil signatures were associated with the opposite in breast cancer (43). Collectively, evaluation of both frequencies and composition of TIL is required to understand the immunogenicity of breast cancer and dynamic immune response in the tumor microenvironment.
Immunogenic cell death
In the cancer immunity cycle (44), the release of cancer cell antigens is the first step in initiating the anti-tumor response. Chemotherapy was traditionally thought to suppress immune response. However, there is a growing consensus that anti-cancer agents can induce immunogenic cell death (ICD) to modulate anti-tumor immunity (45). When tumor cells are exposed to chemotherapeutics, they release ‘danger signals’ including extracellular ATP, calreticulin (CRT), high mobility group box 1 (HMGB1) and heat shock proteins (HSPs). For example, expression of CRT, HSP70, and HSP90 on tumor cells can promote the up-take of dying cells by dendritic cells to present the tumor antigen to T cells (‘eat-me’ signal). The release of HMGB1 promotes the synthesis of pro-inflammatory factors including type I interferon (IFN), interleukin (IL)-1, IL-12 and tumor necrosis factor (TNF). The secretion of type I IFNs from dying cells also promotes the synthesis of chemokine CXCL10 which mediates chemotactic effects on T cells. These danger signals lead to the priming of the adaptive immune response against tumor cells (Table 1). Moreover, cyclophosphamide, docetaxel and gemcitabine are known to decrease the immunosuppressive immune cells including Treg and MDSC (46,47). These immune modulations are the rationale for the combination of chemotherapy with checkpoint inhibitors.

Full table
It is well known that radiation therapy achieves local tumor control, but sometimes leads to tumor shrinkage at a distant site out of the radiation field. This abscopal effect is thought to be associated with ICD-releasing danger signals from irradiated tumor cells. Recent studies have demonstrated that CTLA-4 blockade following radiation therapy broadens the T cell receptor (TCR) repertoire, and a diverse TCR repertoire is required to elicit tumor rejection (48,49). The combination of radiotherapy with immunotherapy can increase the abscopal effect, which leads to systemic acquired T cell responses.
Single agent immunotherapy
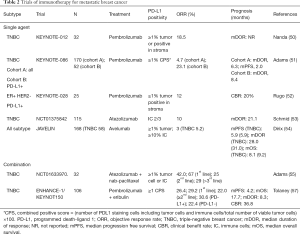
TNBC is known to be the most immunogenic subtype; therefore, most of the trials using immune checkpoint inhibitors have been focused on metastatic TNBC. In the KEYNOTE-012 phase Ib trial, monotherapy with the anti-PD-1 inhibitor, pembrolizumab, was explored in 32 patients with PD-L1-positive metastatic TNBC (50). PD-L1 positivity was defined as ≥1% on tumor cells or expression in the stroma by immunohistochemistry using 22C3 mAb. Pembrolizumab achieved a durable response in the patients with heavy prior treatment with anthracycline, taxanes and platinum. Among the 27 patients who were evaluated for tumor response, the objective response rate (ORR) reached 18.5%, the median time to response was 17.9 weeks (range, 7.3 to 32.4 weeks), and the median duration of response was not yet reached. The median treatment with pembrolizumab was five doses (range, 1 to 36 doses), and 15.6% of patients experienced at least one grade 3 or 4 adverse event. In the KEYNOTE-086 phase II trial, there were three cohorts categorized by PD-L1 expression and the history of metastatic treatment (51). TNBC patients were assigned to cohort A regardless of PD-L1 expression. Cohort B contained patients who were PD-L1-positive on tumor cells or in the stroma and who had no prior metastatic treatment. Cohort C involved patients with PD-L1 positivity after prior metastatic treatment. For 170 patients assigned to cohort A, 4.7% of ORR was observed (one CR and seven PRs). The median progression free survival (PFS) and duration of response (DOR) was 2 and 6.3 months, respectively. Fifty-two patients in cohort B experienced a higher ORR of 23.1% (two CRs, ten PRs) and the median DOR was 8.4 months. In the KEYNOTE-028 study, 25 heavily pretreated patients with ER-positive and HER2-negative disease, with PD-L1 expression in the stroma or in ≥1% tumor cells, were evaluated for tumor response (52). With a median duration of follow-up of 7.3 months, the ORR was 12%, and the clinical benefit rate was 20%. Progression of disease was observed in 5 (60%) patients. Overall, 16% of patients experienced at least one grade 3 or 4 adverse event.
The clinical efficacy of targeted therapy against PD-L1 was also investigated for patients with metastatic TNBC. Atezolizumab, the human IgG1 PD-L1 targeting mAb, was tested in 115 patients with metastatic TNBC (53). For the 113 patients who were evaluated for response, the ORR reached 10% (three CRs, eight PRs) according to regular RECIST criteria, and 13% according to immuno-related RECIST criteria. The ORR of 26% was observed in patients who received first line therapy and 11% in those who received second or further line therapies. The median DOR reached 21.1 months. The 1-year overall survival rate was 41%, and the 2-year survival rate was 22% in all patients. However, 1- and 2-year survival rates for responders was 100% compared to 38% and 11% for non-responders. The results indicated that a small group of patients who responded to atezolizumab monotherapy experienced a longer clinical outcome.
In this study, PD-L1 expression was assessed by immunohistochemistry using clone SP142 mAb. The definition of PD-L1 positivity was ≥5% [immune cell (IC) 2/3] on immune cells in the stroma as opposed to <5% (IC 1/0), and the positive rate was 34%. The ORR for PD-L1 IC 2/3 and IC 1/0 patients were 17% and 8%, respectively. The one-year OS for patients with IC2/3 was 45% vs. 37% for those with IC0/1. There was a trend toward favorable response for patients with higher PD-L1 expression, but patients with lower PD-L1 expression also benefited from atezolizumab. Exploratory analysis revealed that higher response rates seemed to be associated with higher levels of TIL (≥10%) and CD8 T cell infiltration (≥1.35%).
In the JAVELIN trial, another anti-PD-L1 mAb, avelumab, was tested in 168 patients with all subtypes of metastatic breast cancer in (54). PD-L1 expression was determined by 22C3 mAb and the definition of positivity was ≥1% on tumor cells or ≥10% in stromal immune cells. The ORR was 3.0% (1 CR, 7 PRs) in all patients, and the ORR reached 5.2% in 58 patients with TNBC. There was a trend toward a higher ORR in patients with PD-L1 expression (16.7% vs. 1.6% for all patients, 22.2% vs. 2.6% for TNBC).
Immuno-oncology combinations
In breast cancer, combination therapy with chemotherapy was intensively investigated. Adams et al. (55) reported the clinical efficacy of atezolizumab combined with nab-paclitaxel in patients with metastatic TNBC. It is still unclear whether or not steroids during chemotherapy may reduce the effectiveness of immunotherapy; however, nab-paclitaxel does not require steroids as opposed to conventional taxane. Thirty-two patients were assigned, and 87% underwent prior treatment with taxane. Twenty-four out of 32 patients were evaluated for treatment efficacy, and the ORR reached 42% at a median follow-up of 5.2 months. The confirmed ORR was 67% in the first line, 25% in the second line, and 29% in the third or further lines. There was a trend towards a better response to combined treatment in patients whose tumors expressed PD-L1. The following phase III IMpassion 130 trial is investigating the combination of atezolizumab plus nab-paclitaxel in patients with previously untreated metastatic TNBC (56).
To evaluate eribulin combined with pembrolizumab, the ENHANCE-1/KEYNOTE-150 phase Ib/II study was considered for patients with metastatic TNBC (57). In evaluable 106 patients, the ORR with the combination increased to 26.4% (95% CI: 16.8–35.4). Patients with no prior metastatic treatment (n=65) had the ORR of 29.2% (95% CI: 18.6–41.8) whereas those with one or two prior treatments (n=41) had the ORR of 22.0% (95% CI: 10.6–37.6). This combination therapy was superior to monotherapy with eribulin in the historical control (ORR 10–20%). Clinical response was observed regardless of PD-L1 expression. The ORR with combination treatment was 30.6% for PD-L1-positive patients (n=49) and 22.4% for PD-L1-negative patients (n=49). The median PFS and the median OS was 4.2 months (95% CI: 4.1–5.6) and 17.7 months, (95% CI: 13.5– not estimable), respectively.
Future direction of immunotherapy
Cancer immunotherapy achieved a durable clinical response in patients with advanced cancer that was refractory to the standard of care. Approximately 20% of patients experience a long-lasting life prolongation whereas more than half of patients are still non-responders to checkpoint blockade. Clarifying the reasons why checkpoint inhibitors still cannot restore anti-tumor immunity in these non-responders is a key issue for future immuno-oncology research.
Other immunosuppressive molecules are targeted to increase the activity of immune checkpoint inhibitors. Indoleamine 2,3 dioxygenase (IDO) induces immunosuppression through degradation of tryptophan, which is an important regulator of innate and adaptive immunity (58). Adenosine is a mediator of immunosuppression, and tumors can generate adenosine through CD73 in response to anti-PD-1/ani-PD-L1 (59). The adenosine-A2A receptor on NK and T cells is another target to release the brake on anti-tumor activity. The IDO-1 inhibitor (epacadostat) (60) and an oral antagonist of the adenosine-A2A receptor (CPI-444) (61) were investigated in combination with anti-PD-1 and anti-PD-L1 mAb for advanced solid tumors. The preliminary data demonstrated that these combination therapies were safe and their outcomes were promising. Arginase is a key immunosuppressive enzyme secreted from MDSC. The arginase inhibitors of CB-1158 can relive immune suppression and lead to high serum arginine. A tumor microenvironment with high arginine can activate effector cells, including NK and T cells. The phase I study showed that oral CB-1158 combined with anti-PD1 therapy was well tolerated, and peripheral NK cells and T cells were in the activation state (62).
Accumulating evidence has demonstrated that metabolic energetics of the tumor and the tumor microenvironment play an important role in the regulation of tumor immune response. Tumor cells can obtain energy from aerobic glycolysis (Warburg effect) whereas immune cells tend to starve in hypoxic microenvironments. Metformin, a type 2 diabetes drug, can inhibit oxygen consumption in tumor cells resulting in reduced intra-tumoral hypoxia. Combination therapy with metformin and PD-1 blockade has improved intra-tumoral T-cell function (63). Mitochondrial activity in cytotoxic T cells is associated with the anti-tumor activity of PD-1 blockade. In the mouse model, bezafibrate, an antilipemic agent, improves mitochondrial dysfunction and synergizes with the anti-tumor activity of anti-PD-1 therapy through expansion of CTLs in the tumor microenvironment (64).
Recent studies have reported that the intestinal microbiome is related to the response to checkpoint blockade (65-67). In each study, specific bacterial strains in stool were associated with response to immunotherapy. A relative abundance of Bifidobacterium, Akkermans and Ruminococcus was observed in responders, whereas the Bacteroides strain was dominant in non-responders. Cytotoxic T cells in the tumor microenvironment were observed in the submucosa of the colon. The effects of the microbiome can cross the mucosal barrier, but it is still unclear why specific strains of intestinal flora can induce systemic anti-tumor activity. These data raise important questions regarding limiting use of antibiotics and considering diet or pro-biotic intake to augment the anti-tumor activity of checkpoint blockade.
Taken together, triple-negative breast cancer is the most immunogenic subtype of breast cancer and some populations can be eliminated by re-activating anti-tumor immunity. The results of clinical trials descried in this review are summarized in Table 2. Accumulating results demonstrate that TMB and TIL represent antigenicity and pre-existing immunity. Recent studies have demonstrated that the efficacy of immunotherapy is also associated with cellular metabolism and host physiology. The development of these intrinsic and extrinsic biomarkers is required to maximize the clinical benefits of immunotherapy in patients with breast cancer.
Full table
Acknowledgements
None.
Footnote
Conflicts of Interest: The author received honoraria from Novartis, Astra Zeneca, Eisai and Chugai/Roche.
References
- Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711-23. [Crossref] [PubMed]
- Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443-54. [Crossref] [PubMed]
- Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med 2013;369:122-33. [Crossref] [PubMed]
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]
- Rosenberg JE, Hoffman-Censits J, Powles T, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicenter, phase 2 trial. Lancet 2016;387:1909-20. [Crossref] [PubMed]
- Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-Positive non-small-cell lung cancer. N Engl J Med 2016;375:1823-33. [Crossref] [PubMed]
- Kaufman HL, Russell J, Hamid O, et al. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: a multicentre, single-group, open-label, phase 2 trial. Lancet Oncol 2016;17:1374-85. [Crossref] [PubMed]
- Jelinek T, Mihalyova J, Kascak M, et al. PD-1/PD-L1 inhibitors in hematological malignancies: update 2017. Immunology 2017;152:357-71. [Crossref] [PubMed]
- Loi S, Sirtaine N, Piette F, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J Clin Oncol 2013;31:860-7. [Crossref] [PubMed]
- Budczies J, Bockmayr M, Denkert C, et al. Classical pathology and mutational load of breast cancer–integration of two worlds. J Pathol Clin Res 2015;1:225-38. [Crossref] [PubMed]
- Burnet M. Cancer; a biological approach. I. the process of control. Br Med J 1957;1:779-86. [Crossref] [PubMed]
- Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science 2011;331:1565-70. [Crossref] [PubMed]
- Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity 2004;21:137-48. [Crossref] [PubMed]
- Vesely MD, Kershaw MH, Schreiber RD, et al. Natural innate and adaptive immunity to cancer. Annu Rev Immunol 2011;29:235-71. [Crossref] [PubMed]
- Mittal D, Gubin MM, Schreiber RD, et al. New insights into cancer immunoediting and its three component phases--elimination, equilibrium and escape. Curr Opin Immunol 2014;27:16-25. [Crossref] [PubMed]
- Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature 2011;480:480-9. [Crossref] [PubMed]
- Brubaker SW, Bonham KS, Zanoni I, et al. Innate immune pattern recognition: a cell biological perspective. Annu Rev Immunol 2015;33:257-90. [Crossref] [PubMed]
- Roche PA, Furuta K. The ins and outs of MHC class II-mediated antigen processing and presentation. Nat Rev Immunol 2015;15:203-16. [Crossref] [PubMed]
- Joffre OP, Segura E, Savina A, et al. Cross-presentation by dendritic cells. Nat Rev Immunol 2012;12:557-69. [Crossref] [PubMed]
- Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science 2015;348:69-74. [Crossref] [PubMed]
- Alexandrov LB, Nik-Zainal S, Wedge DC, et al. Signatures of mutational processes in human cancer. Nature 2013;500:415-21. [Crossref] [PubMed]
- Rooney MS, Shukla SA, Wu CJ, et al. Molecular and genetic properties of tumors associated with local immune cytolytic activity. Cell 2015;160:48-61. [Crossref] [PubMed]
- Kandoth C, McLellan MD, Vandin F, et al. Mutational landscape and significance across 12 major cancer types. Nature 2013;502:333-9. [Crossref] [PubMed]
- Snyder A, Makarov V, Merghoub T, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med 2014;371:2189-99. [Crossref] [PubMed]
- Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015;348:124-8. [Crossref] [PubMed]
- Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med 2015;372:2509-20. [Crossref] [PubMed]
- Haricharan S, Bainbridge MN, Scheet P, et al. Somatic mutation load of estrogen receptor-positive breast tumors predicts overall survival: an analysis of genome sequence data. Breast Cancer Res Treat 2014;146:211-20. [Crossref] [PubMed]
- Stanton SE, Adams S, Disis ML. Variation in the incidence and magnitude of tumor-infiltrating lymphocytes in breast cancer subtypes: a systematic review. JAMA Oncol 2016;2:1354-60. [Crossref] [PubMed]
- Aaltomaa S, Lipponen P, Eskelinen M, et al. Lymphocyte infiltrates as a prognostic variable in female breast cancer. Eur J Cancer 1992;28A:859-64. [Crossref] [PubMed]
- Adams S, Gray RJ, Demaria S, et al. Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin Oncol 2014;32:2959-66. [Crossref] [PubMed]
- Denkert C, von Minckwitz G, Darb-Esfahani S, et al. Tumor-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol 2018;19:40-50. [Crossref] [PubMed]
- Loi S, Michiels S, Salgado R, et al. Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: results from the FinHER trial. Ann Oncol 2014;25:1544-50. [Crossref] [PubMed]
- Salgado R, Denkert C, Campbell C, et al. Tumor-infiltrating lymphocytes and associations with pathological complete response and event-free survival in HER2-positive early-stage breast cancer treated with lapatinib and trastuzumab: A secondary analysis of the NeoALTTO trial. JAMA Oncol 2015;1:448-54. [Crossref] [PubMed]
- Perez EA, Ballman KV, Tenner KS, et al. Association of stromal tumor-infiltrating lymphocytes with recurrence-free survival in the N9831 adjuvant trial in patients with early-stage HER2-positive breast cancer. JAMA Oncol 2016;2:56-64. [Crossref] [PubMed]
- Luen SJ, Salgado R, Fox S, et al. Tumour-infiltrating lymphocytes in advanced HER2-positive breast cancer treated with pertuzumab or placebo in addition to trastuzumab and docetaxel: a retrospective analysis of the CLEOPATRA study. Lancet Oncol 2017;18:52-62. [Crossref] [PubMed]
- Chung YR, Kim HJ, Jang MH, et al. Prognostic value of tumor infiltrating lymphocyte subsets in breast cancer depends on hormone receptor status. Breast Cancer Res Treat 2017;161:409-20. [Crossref] [PubMed]
- Mahmoud SM, Paish EC, Powe DG, et al. Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J Clin Oncol 2011;29:1949-55. [Crossref] [PubMed]
- Gu-Trantien C, Loi S, Garaud S, et al. CD4+ follicular helper T cell infiltration predicts breast cancer survival. J Clin Invest 2013;123:2873-92. [Crossref] [PubMed]
- Pedroza-Gonzalez A, Xu K, Wu TC, et al. Thymic stromal lymphopoietin fosters human breast tumor growth by promoting type 2 inflammation. J Exp Med 2011;208:479-90. [Crossref] [PubMed]
- Shang B, Liu Y, Jiang SJ, et al. Prognostic value of tumor-infiltrating FoxP3+ regulatory T cells in cancers: a systematic review and meta-analysis. Sci Rep 2015;5:15179. [Crossref] [PubMed]
- Liu S, Foulkes WD, Leung S, et al. Prognostic significance of FOXP3+ tumor-infiltrating lymphocytes in breast cancer depends on estrogen receptor and human epidermal growth factor receptor-2 expression status and concurrent cytotoxic T-cell infiltration. Breast Cancer Res 2014;16:432. [Crossref] [PubMed]
- Miyashita M, Sasano H, Tamaki K, et al. Prognostic significance of tumor-infiltrating CD8+ and FOXP3+ lymphocytes in residual tumors and alterations in these parameters after neoadjuvant chemotherapy in triple-negative breast cancer: a retrospective multicenter study. Breast Cancer Res 2015;17:124. [Crossref] [PubMed]
- Gentles AJ, Newman AM, Liu CL, et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat Med 2015;21:938-45. [Crossref] [PubMed]
- Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity 2013;39:1-10. [Crossref] [PubMed]
- Galluzzi L, Buqué A, Kepp O, et al. Immunogenic cell death in cancer and infectious disease. Nat Rev Immunol 2017;17:97-111. [Crossref] [PubMed]
- Kroemer G, Galluzzi L, Kepp O, et al. Immunogenic cell death in cancer therapy. Annu Rev Immunol 2013;31:51-72. [Crossref] [PubMed]
- Lake RA, Robinson B. Immunotherapy and chemotherapy — a practical partnership. Nature Reviews Cancer 2005;5:397-405. [Crossref] [PubMed]
- Twyman-Saint Victor C, Rech AJ, Maity A, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature 2015;520:373-7. [Crossref] [PubMed]
- Rudqvist NP, Pilones KA, Lhuillier C, et al. Radiotherapy and CTLA-4 blockade shape the TCR repertoire of tumor-infiltrating T cells. Cancer Immunol Res 2018;6:139-50. [Crossref] [PubMed]
- Nanda R, Chow LQ, Dees EC, et al. Pembrolizumab in Patients with Advanced Triple-Negative Breast Cancer: Phase Ib KEYNOTE-012 Study. J Clin Oncol 2016;34:2460-7. [Crossref] [PubMed]
- Adams S, Loi S, Toppmeyer D, et al. Phase 2 study of pembrolizumab as first-line therapy for PD-L1-positive metastatic triple-negative breast cancer (mTNBC): Preliminary data from KEYNOTE-086 cohort B. J Clin Oncol 2017;35:1088.
- Rugo HS, Delord JP, Im SA, et al. Preliminary efficacy and safety of pembrolizumab (MK-3475) in patients with PD-L1–positive, estrogen receptor-positive (ER+)/HER2-negative advanced breast cancer enrolled in KEYNOTE-028. Cancer Res 2016;76:Abstract S5-07.
- Schmid P, Cruz C, Braiteh FS. Atezolizumb in metastatic TNBC (mTNBC): Long-term clinical outcomes and biomarker analyses. Cancer Res 2017;77:Abstract nr 2986.
- Dirix LY, Takacs I, Jerusalem G, et al. Avelumab, an anti-PD-L1 antibody, in patients with locally advanced or metastatic breast cancer: a phase 1b JAVELIN Solid Tumor study. Breast Cancer Res Treat 2018;167:671-86. [Crossref] [PubMed]
- Adams S, Diamond RJ, Hamilton EP, et al. Phae Ib trial of atezolizumab in combination with nab-paclitaxel in patients with metastatic triple-negative breast cancer (mTNBC). J Clin Oncol 2016;34:1009.
- Emens LA, Adams S, Loi S, et al. IMpassion130: A Phase III randomized trial of atezolizumab with nab-paclitaxel for first-line treatment of patients with metastatic triple-negative breast cancer (mTNBC). J Clin Oncol 2016.34.
- Tolaney SM, Kalinsky K, Kaklamani V, et al. Phae 1b/2 study to evaluate eribulin mesylate in combination with pembrolizuamb in patients with metastatic triple-negative breast cancer. Cancer Res 2018;78:Abstract nr PD6-13.
- Moon YW, Hajjar J, Hwu P, et al. Targeting the indoleamine 2,3-dioxygenase pathway in cancer. J Immunother Cancer 2015;3:51. [Crossref] [PubMed]
- Beavis PA, Slaney CY, Milenkovski N, et al. CD73: A potential biomarker for anti-PD-1 therapy. Oncoimmunology 2015;4. [Crossref] [PubMed]
- Hamid O, Bauer TM, Spira AI, et al. Safety of epacadostat 100mg bid plus pembrolizumab 200 mg Q3W in advanced solid tumors: Phase 2 data from ECHO-202/ KEYNOTE-037. J Clin Oncol 2017;35:3012.
- Emens L, Powderly J, Fong L, et al. CPI-444, an oral adenosine A2a receptor (A2aR) antagonist, demonstrates clinical activity in patients with advanced solid tumors. Cancer Res 2017;77:Abstr nr CT119.
- Papadopoulos KP, Tsai F, Bauer T, et al. CX-1158-101: A first-in human phase 1 study of CB-1158, a small molecule inhibitor of arginase, as monotherapy and in combination with an anti-PD-1 checkpoint inhibitor in patients with solid tumor. J Clin Oncol 2017;35:3005.
- Scharping NE, Menk AV, Whetstone RD, et al. Efficacy of PD-1 blockade is potentiated by metformin-induced reduction of tumor hypoxia. Cancer Immunol Res 2017;5:9-16. [Crossref] [PubMed]
- Chamoto K, Chowdhury PS, Kumar A, et al. Mitochondrial activation chemicals synergize with surface receptor PD-1 blockade for T cell-dependent antitumor activity. Proc Natl Acad Sci U S A 2017;114:E761-70. [Crossref] [PubMed]
- Routy B, Le Chatelier E, Derosa L, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 2018;359:91-7. [Crossref] [PubMed]
- Gopalakrishnan V, Spencer CN, Nezi L, et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 2018;359:97-103. [Crossref] [PubMed]
- Matson V, Fessler J, Bao R, et al. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science 2018;359:104-8. [Crossref] [PubMed]


