Resection of primary lesion for patients with metastatic breast cancer: where are we now?
Background
Breast cancer is the most commonly diagnosed cancer in women worldwide (1). The incidence rate increased rapidly in the past decades [an annual rate of increase: 6.1% (2-4)]. The American Cancer Society estimated that 249,260 Americans would be diagnosed with invasive breast cancer and 40,890 would die of the disease in the United States in 2016 (5). In 2012, there were around 190 thousand new cases and more than 47 thousand deaths due to breast cancer in China (4). It is also estimated that, by 2021, there will be about 2.5 million patients with breast cancer in China (6).
Around 3% to 8% of patients with newly diagnosed breast cancer were found with distant metastasis (7), which is the driving determinant in the prognosis of metastatic breast cancer (8). According to the “seed and soil” theory, the metastatic breast cancer should be recognized as a systemic disease (9). Systemic treatment (including chemotherapy, endocrine therapy, and molecular targeted therapy), together with local treatment (surgery and/or radiotherapy), could significantly ameliorate symptoms and improve survival (10,11).
Conventionally, the surgical removal of the primary lesion is not considered for metastatic breast cancer unless in the purpose of the palliative care, for instance, when complicating with pain, bleeding, ulcers, and fungation (10,12). During the past decade, accumulating evidence from retrospective studies have suggested that surgical resection of the primary lesion may improve the survival of patients with metastatic breast cancer (2.3–4.6 vs.1.2–2.6 years) (13-15). However, it is possibly explained by indication bias, namely patients opted for surgery were generally in better conditions (16). Recently, two randomized controlled trials (RCTs) landed on the opposite findings, suggesting comparable overall survival after surgery (10,17).
Motivated by the conflicting evidence, it is of paramount importance to assess the up-to-date evidence on surgical resection of the primary lesion for patients with metastatic breast cancer at initial presentation, or stage IV. In this review, we discuss the comparison between surgery and no surgery, risk modifiers, the type and timing of surgical procedures, Chinese and international guidelines, as well as the progress of clinical trials.
Surgery vs. no surgery
Some experimental studies showed that surgical resection of primary lesion may promote distant metastasis (18), and the mechanisms involved in increased circulating tumor cell (CTC) adhesion (19), immune suppression (20), surgery-induced angiogenic switch, and inflammation (21). However, the others lend support to the opposite. It was reported that primary lesion resection may lead to a reduction in CTCs and subsequently better prognosis (median OS 26.9 vs. 15.3 months, P=0.0389) (22). Moreover, cancer stem cells exist in the primary lesion and are likely resistant to systemic therapy (23). The resection of primary lesion reduces the tumor burden including cancer stem cells, that may potentially delay the drug resistance induced by cancer stem cells (if any), as well as increase the efficacy of systemic treatment by reactivating immune regulation (24).
The conflicting results are also found in clinical studies. An RCT (MF07-01) revealed that the median survival was significantly superior among patients with metastatic breast cancer underwent surgery, as compared with no surgery (46 vs. 37 months, P=0.005) (25). In our previous work, compared with metastatic breast cancer without surgery, the median survival times were improved for patients received surgical resection of primary lesion (45.6 vs. 21.3 months, P<0.001) (26). Although in line with many retrospective studies (13-15,27-30), a handful retrospective studies showed that surgical resection of primary lesion was not associated with improved survival among patients with metastatic breast cancer (31-33) (Table 1).
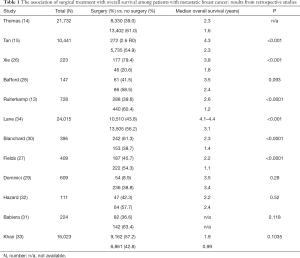
Full table
However, recently published RCTs failed to confirm the potential benefit of surgical resection for metastatic breast cancer. TATA, an open-label RCT from India, enrolled 350 patients with newly diagnosed advanced breast cancer from 2005 to 2013. One hundred seventy-three patients underwent surgical resection of the primary lesion, by means of modified radical mastectomy or breast-conserving surgery. They found that the surgical resection was not significantly associated with improved overall survival (19.5 vs. 20.5 months, P=0.79, HR 1.04, 95% CI: 0.81–1.34) (10). Later, a multicenter RCT from USA (TBCRC 013), also assessed the effect of surgical treatment among patients with stage IV breast cancer. Leveraging 112 patients with a median age of 51 years and a 3-year survival rate of 70%, TBCRC 013 found that surgical treatment for metastatic breast cancer was not associated with superior survival (35). No significant difference was noted for patients with different molecular types or responses to systemic therapies. In TBCRC 013, 39 patients received surgery among those who were well-responded to systematic treatment. Given the small number, it is not surprising that no significant survival benefit was detected as compared to their non-surgical counterpart (35). However, if patients with metastatic breast cancer patients were well-responded to systematic treatment and in a relatively well condition, the more active/aggressive treatment plan may be considered. However, the TBCRC 013 trial questioned the indications of surgical resection of the primary lesion. Was the null association attributable to the nature of selection bias nature in RCTs (36)? Or was the follow-up too short to capture the altered trajectory in the later course of the disease (37)? It, therefore, calls for large-scale RCTs with longer follow-up to further assess the potential survival benefits from resection of the primary lesion for patients with metastatic breast cancer.
It is also important to know, beyond the potential benefits on prognosis, whether the primary lesion resection entails side effects and impacts on the quality of life (QoL) in patients with metastatic breast cancer. To the best of our knowledge, there are however very few reports specific to metastatic breast cancer. Several studies suggest that containing primary tumor is associated with increased QoL among patients with metastatic breast cancer (32,38). Further studies are therefore warranted to evaluate the risks of side effects and benefits on QoL after the primary tumor resection among patients with metastatic breast cancer.
Risk modifiers
It is not clear that which subpopulation will benefit from resection of primary lesion in patients with metastatic breast cancer. In order to better carry out clinical work, we need to find the risk modifiers more accurately. In 2006, American Society of Clinical Oncology (ASCO) reported a population study based on the Geneva Cancer Registry, which included 300 patients with metastatic breast cancer. In this study, the surgical group could reduce the risk of death by 40% compared with the nonsurgical group, especially the patients with negative surgical margins. Further subgroup analysis found that the metastatic breast cancer patients with only bone metastases benefit more from the surgical resection of primary lesion (39). Multivariate analysis showed that surgery, positive estrogen receptor, and smaller primary lesions were predictors of good prognosis, whereas no difference was found in positive human epidermal growth factor receptor and triple negative receptor (40). However, there were also studies which demonstrated that oligo-metastasis (one-site metastasis), regardless of bone metastasis or visceral metastasis, was an independent predictor of better prognosis. However, estrogen receptor, progesterone receptor and human epidermal growth factor receptor status were not prognostic predictors. More interestingly, patients with positive axillary lymph nodes benefited significantly less from surgery and should not be recommended for surgical resection of the primary lesion (37). A Meta-analysis of 28,693 patients found that surgery, smaller primary lesions (T0–T2), fewer complications, and lower metastatic tumor burden (one site metastasis) were predictors of better prognosis, whereas not related with metastatic sites, World Health Organization (WHO) grade, and receptor status (41). Real-world studies conducted by our team found that median overall survival and progression-free survival of advanced breast cancer patients with oligo-metastasis were 139.6 and 24 months, respectively, which were significantly different from those advanced breast cancer patients with two or more metastatic sites 34.9–42.1 and 8.1 months (P<0.001) (unpublished data). Thomas et al. found that younger patients with metastatic breast cancer were more inclined to undergo surgical treatment and confirmed that age <45 years was an independent predictor of better survival (14). Thus, surgical resection of the primary lesion should be actively recommended in breast cancer patients younger than 45 years of age or with oligo-metastasis. To date, some patients are likely to benefit from surgical resection of the primary lesion, but there is no uniform standard to define this part of the patients. In clinical practice, multidisciplinary teams are needed to discuss together and formulate a treatment plan. It is also necessary to understand the patients’ willingness and balance the benefits and risks of surgical resection of the primary lesion. Therefore, we look forward to developing a prediction model combining molecular subtype and new biomarker to identify those subpopulation who may have a better survival after surgical resection of the primary tumors among patients with metastatic breast cancer.
The type and timing of surgical procedures
Halsted radical surgery has pioneered the surgical treatment of early breast cancer since 1880 (42). For more than 100 years, surgery has undergone the evolution of extended radical mastectomy, modified radical mastectomy, breast-conserving surgery, and breast cancer sentinel lymph node biopsy (SLNB). Although surgical techniques have been very mature, are the surgical time and methods for patients with metastatic breast cancer same as the early breast cancer?
The timing of surgery
Surgery before or after the systemic treatment may affect the survival of metastatic breast cancer (34). It is generally believed that patients with metastatic lesions who benefit from systemic treatment should be recommended to consider surgical resection of the primary lesion following systemic treatment (43). So far, the timing of surgery in metastatic breast cancer has not been conclusive. Although many researchers want to clarify this issue, these studies come to a different conclusion (29,30,44,45). In the TBCRC 013 study, 85% patients (94 cases) benefited from first-line systemic therapy, among whom there were 43% (39 cases) then underwent surgery and 57% (51 cases) continued the systemic therapy. However, it concluded that the surgical resection of primary lesion didn’t improve the survival of primary stage IV breast cancer (35). In the TATA study, patients who benefited from systemic therapy were treated surgically or non-surgically at random, and surgical resection of primary lesion did not result in survival benefit either (10). In contrast, in the study of MF07-01, the trial group was treated with systemic treatment following surgery, and the control group was treated with systemic treatment throughout. The 5-year OS was 41.6% and 24.4%, respectively, in the trial group and the control group (P=0.005). Stratified analysis showed that the trial group had a survival advantage compared with the control group among the patients with estrogen receptor positive (ER+), human epidermal growth factor receptor 2 negative (HER2−), less than 55 years of age and single bone metastasis. However, there was no statistically significant difference between the two groups among patients with multiple lungs or liver metastases (25). A prospective, controlled study SUBMIT, comparing head-to-head first surgery follow-up systemic treatment with first systemic treatment follow-up surgery, was to answer the question of the timing of surgical resection of primary lesion in advanced breast cancer (46), but it was terminated due to low accrual rate.
The type of surgical procedures
Patients with early breast cancer are still mainly treated with modified radical mastectomy and breast-conserving surgery. The technology is very mature. Which surgical technique is optimal for the patients with metastatic breast cancer? Modified radical mastectomy, breast-conserving surgery, or back to the previously extended radical mastectomy? Some scholars believed that breast-conserving surgery should be used as much as possible in patients with primary stage IV breast cancer due to its fewer complications and rapid recovery. These advantages might allow patients to receive systemic treatment as soon as possible (31). In the TATA study, modified radical mastectomy and breast-conserving surgery combined with axillary lymph node dissection were performed in patients with metastatic breast cancer, and some patients received supraclavicular lymph node dissection (10). However, the authors did not describe any difference among surgical procedures. A retrospective study from the Surveillance, Epidemiology, and End Results program (SEER) found that 4,578 (47%) of the 9,734 patients with primary IV breast cancer who underwent surgical resection of the primary lesion. Among them, 1,844 (40.3%) underwent partial mastectomies, and 2,485 (54.3%) underwent mastectomies (47). Taken together, several surgical modalities are currently applied for patients with metastatic breast cancer. Partial modified radical mastectomy and breast-conserving surgery are the most common choices. However, surgeons should minimize the risk of the complications associated with surgery and ensure a negative surgical margin regardless of surgical modalities.
The Chinese and International Guidelines
The surgical procedures of the primary tumor recommended by the guidelines for metastatic breast cancer in different countries are not uniform, and even show obvious differences. The 2013 Austrian Arbeitsgemeinschaft für Gynäkologische Onkologie (AGO) guidelines suggested modified radical mastectomy for the primary tumor (48). In China, the correlation between survival and surgery of the primary tumor in patients with stage IV disease still remains not clear, according to the Chinese Guideline for Advanced Breast Cancer 2015 (49). The guideline only indicates that surgery could be considered if the patients had good responses to initial systemic therapy. According to The Third International Consensus Conference for Advanced Breast Cancer (ABC3), the resection of the primary tumor for de novo stage IV breast cancer patients usually might not improve an advance in survival unless for patients with only bone metastasis (50). Moreover, the 2017 National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology (NCCN Guidelines) for Breast Cancer suggest that systemic therapy should be the predominant treatment for de novo stage IV breast cancer. Surgery should be recommended only after systemic management to relieve symptoms and eliminate complications such as ulcers, bleeding, pain, and fungal infection (51).
The progress of ongoing clinical trials
The effect of surgical removal for primary breast cancer in metastatic breast cancer is currently being investigated further in prospective clinical studies. Four clinical trials are currently underway.
Local surgery for metastatic breast cancer (NCT00557986)
A phase III randomized controlled trial was carried out to compare the outcome of de novo stage IV breast cancer patients who receive locoregional treatment for intact primary tumor with those who do not receive such treatment. This study was done by Turkish Federation of the National Societies for Breast Diseases. The primary objective is to assess whether locoregional treatment of the primary tumor provides a better overall survival. And the secondary objectives include progression-free survival, quality-of-life, and morbidity related to locoregional treatment. The trial has now been completed and the results were published by ASCO in 2016. It showed that the 5-year OS was 41.6% and 24.4% (P=0.005) for the trial and control groups (26). It concluded that the surgical resection of primary lesion improved the survival of primary stage IV breast cancer.
Early surgery or standard palliative therapy in treating patients with stage IV breast cancer (NCT01242800)
This randomized phase III trial aims to see how well early surgery works compared to standard palliative therapy in treating patients with stage IV breast cancer. The principal investigators in the study are Seema A. Khan and Robert H from the Lurie Cancer Center. The experimental group included stage IV breast cancer patients with early surgical resection of the primary tumor, while the control group included those with standard palliative surgery or radiation therapy. The primary endpoint was overall survival. And the secondary objectives contained the time to uncontrolled chest wall disease, health-related quality of life (HRQL) and the CTCs burden. Now, the study is ongoing, and participants are receiving an intervention or being examined, but potential participants are not currently being recruited or enrolled.
Assessing impact of loco-regional treatment on survival in metastatic breast cancer at presentation (NCT00193778)
A one open-label and randomized controlled trial was promoted to compare the effect of locoregional treatment with no treatment on outcome in women with metastatic breast cancer at initial presentation. The primary endpoint was overall survival analyzed by intention to treat. And secondary objectives included the evaluation of locoregional treatment level and the change in expression of VEGF, bFGF, angiostatin, and endostatin. The trial was also called TATA, and the primary results were published in Lancet in 2015. Now, the trial is still ongoing and the expected completion time is December 2019.
The effect of primary surgery in patients with stage IV breast cancer with bone metastasis only (NCT02125630)
A prospective, randomized phase III study, is carried out by Istanbul scholars of Turkey University. The majority of researchers in this study are the same to MF 07-01. The test group is given surgical treatment, while the control group use the systemic treatment. Furthermore, the primary endpoint is the overall survival. And the secondary endpoint is progression-free survival.
A randomized controlled trial comparing primary tumor resection plus systemic therapy with systemic therapy alone in metastatic breast cancer (PRIM-BC): Japan Clinical Oncology Group Study JCOG1017
The JCOG1017 is an ongoing randomized controlled trial comparing primary tumor resection plus systemic therapy with systemic therapy alone in patients with stage IV breast cancer based on response to primary systemic therapy (52). It was commenced in May 2011 and planned to enroll 410 patients over a 5-year recruitment period. The primary endpoint is overall survival. We expect the results of JCOG 1017 to address primary tumor resection challenges in stage IV breast cancer.
In addition, there was also a randomized controlled study to determine benefits of surgery for the primary in patients with stage IV breast cancer from Thailand (NCT01906112). But it was terminate due to slow enrolment.
Summary
The relationship between primary lesion resection and survival remains a subject of debate for patients with metastatic breast cancer at initial presentation. Partial modified radical mastectomy and breast-conserving surgery are by far the most common choices for those patients. Survival benefits from primary lesion resection are suggested for those patients younger than 45 years of age or with oligo-metastasis. The clinical practice guidelines for timing and procedures of surgery for metastatic breast cancer are different across countries. Forthcoming evidence from the ongoing clinical trials might help close the knowledge gaps in surgical treatment for patients with metastatic breast cancer and aid the decision-making in clinical practice.
Acknowledgements
Funding: This work was supported by grants from the National Natural Science Foundation of China (X Zhong, Grant No. 81202099).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Althuis MD, Dozier JM, Anderson WF, et al. Global trends in breast cancer incidence and mortality 1973-1997. Int J Epidemiol 2005;34:405-12. [Crossref] [PubMed]
- Park EH, Min SY, Kim Z, et al. Korean Breast Cancer, Basic Facts of Breast Cancer in Korea in 2014: The 10-Year Overall Survival Progress J Breast Cancer 2017;20:1-11. [Crossref] [PubMed]
- Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin 2008;58:71-96. [Crossref] [PubMed]
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359-86. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016 CA Cancer J Clin 2016;66:7-30. [Crossref] [PubMed]
- Ziegler RG, Anderson WF, Gail MH. Increasing breast cancer incidence in China: the numbers add up. J Natl Cancer Inst 2008;100:1339-41. [Crossref] [PubMed]
- Sant M, Allemani C, Berrino F, et al. Breast carcinoma survival in Europe and the United States. Cancer 2004;100:715-22. [Crossref] [PubMed]
- Management of advanced breast cancer. Available online: www.nhmrc.gov.au/_files_nhmrc/publications/attachments/cp76_management_advanced_breast_cancer_131223.pdf
- Pegat S. The distribution of secondary growths in cancer of the breast. Lancet 1889;133:571-3. [Crossref]
- Badwe R, Hawaldar R, Nair N, et al. Locoregional treatment versus no treatment of the primary tumour in metastatic breast cancer: an open-label randomised controlled trial. Lancet Oncol 2015;16:1380-8. [Crossref] [PubMed]
- Khan SA. De novo Stage IV breast cancer: breast conserving resection of the primary tumor? J Surg Oncol 2014;110:51-7. [Crossref] [PubMed]
- Singletary SE, Walsh G, Vauthey JN, et al. A role for curative surgery in the treatment of selected patients with metastatic breast cancer. Oncologist 2003;8:241-51. [Crossref] [PubMed]
- Ruiterkamp J, Ernst MF, van de Poll-Franse LV, et al. Surgical resection of the primary tumour is associated with improved survival in patients with distant metastatic breast cancer at diagnosis. Eur J Surg Oncol 2009;35:1146-51. [Crossref] [PubMed]
- Thomas A, Khan SA, Chrischilles EA, et al. Initial Surgery and Survival in Stage IV Breast Cancer in the United States, 1988-2011. JAMA Surg 2016;151:424-31. [Crossref] [PubMed]
- Tan Y, Li X, Chen H, et al. Hormone receptor status may impact the survival benefit of surgery in stage IV breast cancer: a population-based study. Oncotarget 2016;7:70991-1000. [Crossref] [PubMed]
- Criscitiello C, Giuliano M, Curigliano G, et al. Surgery of the primary tumor in de novo metastatic breast cancer: To do or not to do? Eur J Surg Oncol 2015;41:1288-92. [Crossref] [PubMed]
- Soran A, Ozbas S, Kelsey SF, et al. Randomized trial comparing locoregional resection of primary tumor with no surgery in stage IV breast cancer at the presentation (Protocol MF07-01): a study of Turkish Federation of the National Societies for Breast Diseases. Breast J 2009;15:399-403. [Crossref] [PubMed]
- Fisher B, Gunduz N, Coyle J, et al. Presence of a growth-stimulating factor in serum following primary tumor removal in mice. Cancer Res 1989;49:1996-2001. [PubMed]
- ten Kate M, Hofland LJ, van Grevenstein WM, et al. Influence of proinflammatory cytokines on the adhesion of human colon carcinoma cells to lung microvascular endothelium. Int J Cancer 2004;112:943-50. [Crossref] [PubMed]
- Benish M, Bartal I, Goldfarb Y, et al. Perioperative use of beta-blockers and COX-2 inhibitors may improve immune competence and reduce the risk of tumor metastasis. Ann Surg Oncol 2008;15:2042-52. [Crossref] [PubMed]
- Shien T, Iwata H. Significance of primary lesion resection in Stage IV breast cancer. Jpn J Clin Oncol 2017;47:381-4. [Crossref] [PubMed]
- Budd GT, Cristofanilli M, Ellis MJ, et al. Circulating tumor cells versus imaging--predicting overall survival in metastatic breast cancer. Clin Cancer Res 2006;12:6403-9. [Crossref] [PubMed]
- Kakarala M, Wicha MS. Implications of the cancer stem-cell hypothesis for breast cancer prevention and therapy. J Clin Oncol 2008;26:2813-20. [Crossref] [PubMed]
- Danna EA, Sinha P, Gilbert M, et al. Surgical removal of primary tumor reverses tumor-induced immunosuppression despite the presence of metastatic disease. Cancer Res 2004;64:2205-11. [Crossref] [PubMed]
- Soran A, Ozmen V, Ozbas S. A randomized controlled trial evaluating resection of the primary breast tumor in women presenting with de novo stage IV breast cancer: Turkish Study (Protocol MF07-01). J Clin Oncol 2016;34:abstr 1005.
- Xie Y, Lv X, Luo C, et al. Surgery of the primary tumor improves survival in women with stage IV breast cancer in Southwest China: A retrospective analysis. Medicine (Baltimore) 2017;96. [Crossref] [PubMed]
- Fields RC, Jeffe DB, Trinkaus K, et al. Surgical resection of the primary tumor is associated with increased long-term survival in patients with stage IV breast cancer after controlling for site of metastasis. Ann Surg Oncol 2007;14:3345-51. [Crossref] [PubMed]
- Bafford AC, Burstein HJ, Barkley CR, et al. Breast surgery in stage IV breast cancer: impact of staging and patient selection on overall survival. Breast Cancer Res Treat 2009;115:7-12. [Crossref] [PubMed]
- Dominici L, Najita J, Hughes M, et al. Surgery of the primary tumor does not improve survival in stage IV breast cancer. Breast Cancer Res Treat 2011;129:459-65. [Crossref] [PubMed]
- Blanchard DK, Shetty PB, Hilsenbeck SG, et al. Association of surgery with improved survival in stage IV breast cancer patients. Ann Surg 2008;247:732-8. [Crossref] [PubMed]
- Babiera GV, Rao R, Feng L, et al. Effect of primary tumor extirpation in breast cancer patients who present with stage IV disease and an intact primary tumor. Ann Surg Oncol 2006;13:776-82. [Crossref] [PubMed]
- Hazard HW, Gorla SR, Scholtens D, et al. Surgical resection of the primary tumor, chest wall control, and survival in women with metastatic breast cancer. Cancer 2008;113:2011-9. [Crossref] [PubMed]
- Khan SA, Stewart AK, Morrow M. Does aggressive local therapy improve survival in metastatic breast cancer? Surgery 2002;132:620-6; discussion 626-7. [Crossref] [PubMed]
- Lane WO, Thomas SM, Blitzblau RC, et al. Surgical Resection of the Primary Tumor in Women With De Novo Stage IV Breast Cancer: Contemporary Practice Patterns and Survival Analysis. Ann Surg 2017. [Epub ahead of print]. [Crossref] [PubMed]
- King TA, Lyman J, Gonen M. A prospective analysis of surgery and survival in stage IV breast cancer (TBCRC 013). J Clin Oncol 2016;34:abstr 1006.
- Olson JA Jr, Marcom PK. Benefit or bias? The role of surgery to remove the primary tumor in patients with metastatic breast cancer. Ann Surg 2008;247:739-40. [Crossref] [PubMed]
- Quinn EM, Kealy R, O'Meara S, et al. Is there a role for locoregional surgery in stage IV breast cancer? Breast 2015;24:32-7. [Crossref] [PubMed]
- Dalberg K, Liedberg A, Johansson U, et al. Uncontrolled local disease after salvage treatment for ipsilateral breast tumour recurrence. Eur J Surg Oncol 2003;29:143-54. [Crossref] [PubMed]
- Rapiti E, Verkooijen HM, Vlastos G, et al. Complete excision of primary breast tumor improves survival of patients with metastatic breast cancer at diagnosis. J Clin Oncol 2006;24:2743-9. [Crossref] [PubMed]
- AlJohani B, AlMalik O, Anwar E, et al. Impact of Surgery on Survival in Stage IV Breast Cancer. Breast J 2016;22:678-82. [Crossref] [PubMed]
- Harris E, Barry M, Kell MR. Meta-analysis to determine if surgical resection of the primary tumour in the setting of stage IV breast cancer impacts on survival. Ann Surg Oncol 2013;20:2828-34. [Crossref] [PubMed]
- Halsted WS. I. The Results of Operations for the Cure of Cancer of the Breast Performed at the Johns Hopkins Hospital from June, 1889, to January, 1894. Ann Surg 1894;20:497-555. [Crossref] [PubMed]
- Harris E, Barry M, Kell MR. Supporting trials for primary tumor resection in stage IV breast cancer is paramount. Ann Surg Oncol 2013;20:3151-2. [Crossref] [PubMed]
- Rao R, Feng L, Kuerer HM, et al. Timing of surgical intervention for the intact primary in stage IV breast cancer patients. Ann Surg Oncol 2008;15:1696-702. [Crossref] [PubMed]
- Ruiterkamp J, Voogd AC, Bosscha K, et al. Presence of symptoms and timing of surgery do not affect the prognosis of patients with primary metastatic breast cancer. Eur J Surg Oncol 2011;37:883-9. [Crossref] [PubMed]
- Ruiterkamp J, Voogd AC, Tjan-Heijnen VC, et al. SUBMIT: Systemic therapy with or without up front surgery of the primary tumor in breast cancer patients with distant metastases at initial presentation. BMC Surg 2012;12:5. [Crossref] [PubMed]
- Gnerlich J, Jeffe DB, Deshpande AD, et al. Surgical removal of the primary tumor increases overall survival in patients with metastatic breast cancer: analysis of the 1988-2003 SEER data. Ann Surg Oncol 2007;14:2187-94. [Crossref] [PubMed]
- Harbeck N, Scharl A, Thomssen C, et al. AGO Recommendations for Diagnosis and Treatment of Patients with Advanced and Metastatic Breast Cancer: Update 2013. Breast Care (Basel) 2013;8:181-5. [Crossref] [PubMed]
- China Medical Women's Association of Clinical Oncology, Chinese Anti-Cancer Association of Breast Cancer. Chinese Guideline for Advanced Breast Cancer 2015(CABC 2015). Oncol Prog 2016;3:223-45.
- Untch M, Augustin D, Ettl J, et al. ABC3 Consensus Commented from the Perspective of the German Guidelines: Third International Consensus Conference for Advanced Breast Cancer (ABC3), Lisbon, 07. 11. 2015. Geburtshilfe Frauenheilkd 2016;76:156-63. [Crossref] [PubMed]
- Gradishar WJ, Anderson BO, Balassanian R, et al. NCCN Guidelines Insights: Breast Cancer, Version 1.2017. Available online: www.nccn.org
- Shien T, Nakamura K, Shibata T, et al. A randomized controlled trial comparing primary tumour resection plus systemic therapy with systemic therapy alone in metastatic breast cancer (PRIM-BC): Japan Clinical Oncology Group Study JCOG1017. Jpn J Clin Oncol 2012;42:970-3. [Crossref] [PubMed]


