The emerging role of oncolytic virus therapy against cancer
Introduction
In recent years, there has been an explosion in the number of immuno-oncology drugs approved or in development for cancer treatment, and some of these therapies have demonstrated objective responses not seen with small molecules (1-3). Clinical trials testing immuno-oncology agents alone and in combinations are well underway and many of these combination drug trials have been shown to improve patient overall survival over monotherapies (4,5). In this review, we focus on a promising class of immuno-oncology drugs, oncolytic viruses (OVs) which are replicating viruses engineered to have tumor selective killing activity. We will first overview cancer treatment from a historical perspective, followed by a brief history of oncolytic virotherapy, design of OV, and focus on some of the viruses currently in clinical testing. We apologize to our colleagues whose primary work we could not cite due to space constraints and instead have referred to excellent reviews published.
Cancer treatment: a historical perspective
Seishu Hanaoka performed the first successful surgical partial mastectomy under general anesthesia for a patient with breast cancer in 1804 (6). To date, surgery remains one of the principle mainstay ways to treat localized cancer; complete tumor removal and potential cures are possible if the cancer is detected early and has not metastasized. Radiation therapy began to emerge as a new modality for cancer treatment with the discovery of X-ray by Wilhelm Roentgen in 1895, and the discovery of radioactive radium and polonium by Marie and Pierre Curie in 1898 (7). In 1903, S.W. Goldberg and Efim London successfully used radium to achieve complete responses (CR) in two patients with basal cell carcinoma of the skin (8). Since then, surgery and radiation therapy dominated the field of cancer treatment until the late 1940s where anti-metabolites (methotrexate) and alkylating agents (nitrogen mustard) were used as chemotherapy agents for cancer (9,10). By the 1950s, in spite of the powerful impact of combination chemotherapy in leukemia and lymphoma, physicians realized the limitations of chemotherapy to achieve the same success rates of complete remission of many advanced solid tumors (9). An earnest effort began thereafter with the research and development of preclinical tumor models to study the basic biology of carcinogenesis, develop novel drugs and drug combinations, and the use of adjuvant chemotherapy after surgery in 1970s to improve overall survival. In the following years, cancer treatment became more targeted, focusing on specific pathways, such as anti-angiogenesis, signaling pathways, or specific mutations (11).
The history of oncolytic virotherapy
Oncolytic virotherapy is the use of a replication-competent virus for the treatment of cancer (12). There are more than 3,000 species of viruses but not all are suitable as oncolytic agents. The OV must be non-pathogenic, have intrinsic cancer selective killing activity, or can be engineered to express attenuating genes or arming genes (13). Tumor selectivity could be at the level of receptor-mediated cell entry, intracellular antiviral responses and/or restriction factors that determine how susceptible the infected cell is to support viral gene expression and replication (14,15). Historically, there have been anecdotal reports of temporary tumor regression and cancer remission after the patient contracted natural viral infection, including responses of lymphoma after wild type measles virus infection (16,17). In the 1950s–1970s, live viruses were deliberately injected into cancer patients and showed promising activity, particularly notable were Egypt 101 West Nile virus (4/34 transient regressions), adenovirus lysates (26/40 showing localized tumor necrosis), and Urabe strain mumps virus [37/90 complete remission or partial responses (PR)] (16). However, toxicity was also noted in these early studies using viral isolates that were not engineered for tumor selectivity, especially in immune suppressed patients with leukemia or lymphoma whereby 5 of 8 patients experienced severe encephalitis after receiving Egypt 101 isolate of West Nile virus (16,18).
Present day: commercially available OVs
With genetic engineering, we can now design live replicating viruses to not only be highly tumor selective through cell entry and transcription targeting but also armed with reporter genes for noninvasive monitoring of the pharmacokinetics of virotherapy, and for enhancing cytotoxic activity or immunogenic cell death, or immune modulators. To date, three OVs are available commercially for the treatment of cancer. These include Rigvir, approved in Latvia, Georgia, and Armenia, Oncorine H101 approved in China, and talimogene laherparepvec (T-VEC) approved in the USA.
Rigvir
Rigvir (Riga virus) is an unmodified Enteric Cytopathogenic Human Orphan type 7 (ECHO-7) picornavirus approved for the treatment of melanoma in Latvia since 2004, Georgia as of 2015, and Armenia as of 2016 (19). Its 2004 approval made it the first oncolytic virus to gain regulatory approval anywhere in the world (19). However, while it was granted regulatory approval, limited data has been published to describe its efficacy. Three English language articles relating to Rigvir are publicly available, including one review article, one case study on 3 patients, and one retrospective analysis of early stage melanoma patients (19-21). The retrospective study found that early stage Latvian melanoma patients (IB, IIA, IIB, and IIC) who received surgical resection and Rigvir (n=52) survived significantly longer than patients who received surgical resection alone (n=27), though all patients were pronounced disease-free following surgery, and Rigvir was administered post-surgery only after the surgical wounds had healed. Rigvir administration was performed by locoregional intramuscular injection at a minimum TCID50 dose level of 106/mL in a volume of 2 mL, but dose level was not precisely quantified for any patient (19), and each patient received approximately 33 doses. The administration schedule involved decreasing frequency of Rigvir doses over 3 years: Rigvir was administered on 3 consecutive days every 4 weeks for 3 cycles, then a single dose every 4 weeks for the remainder of year 1, every 6 weeks for the following 6 months, every 8 weeks for the next 6 months, and every 12 weeks for the third year (19). Rigvir appeared to have an effect on tumor recurrence following surgical resection of low-grade melanomas, however, the potential of Rigvir to treat high-grade melanoma patients remains unclear, as English language reports include only case studies with no larger trials yet reported (20). Future studies on both the mechanism of action of Rigvir and its efficacy in treating non-resectable melanoma patients would improve our understanding of the virus and its potential for oncolytic therapy.
Oncorine (H101)
Oncorine became the first approved oncolytic virus for clinical use in China, and the world’s first recombinant oncolytic virus to gain regulatory approval (22). Oncorine was approved by the Chinese State Food and Drug Administration (SFDA) in 2005 for patients with head and neck cancer in combination with chemotherapy (22). Oncorine (formerly Onyx-015) is an attenuated serotype 5 adenoviral vector deleted for viral E1B-55k and with four deletions in viral E3 (23). It has been hypothesized that Oncorine selectively replicates in p53 deficient tumors, as E1B-55k is a strong p53 repressor. E1B-55k inhibits infection-induced apoptosis and allows viral replication in p53-normal cells (23), however, E1B-55k deleted adenoviruses have been shown to infect and replicate in p53 positive tumors indicating that an alternate mechanism of tumor selectivity may be involved (24-26). It has now been hypothesized that p14ARF plays a role in this circumvention, as well as YB-1 in an RNA export-dependent mechanism of tumor selectivity (27). Oncorine was patient-approved following a large, multi-center, open, randomized trial of Oncorine plus chemotherapy versus chemotherapy alone in patients with squamous cell carcinoma of the head and neck or esophageal cancer (28). Patients received combination cisplatin and 5-fluorouracil (5-FU) with or without Oncorine at 5e11 to 1.5e12 vp/day for 5 consecutive days in between 2- and 43-week cycles. Patients in the Oncorine plus chemotherapy arm had a 78.8% response rate compared with a 39.6% response rate for patients receiving chemotherapy alone (28). A new phase III study in patients with non-small cell lung cancer in combination with chemotherapy has been planned to open (NCT02579564), however, since the trial was initially listed on clinicaltrials.gov in 2015, patient accrual has yet to begin. High seroprevalence against several adenovirus serotypes (including the backbone of Oncorine, serotype 5) limits the ability to deliver Oncorine intravenously to treat highly metastatic disease (29,30). However, strategies to circumvent this employ adenoviral vectors with lower seroprevalence or modified knob proteins which are now in clinical trials to test their safety and efficacy following intravenous delivery (VCN-01 NCT02045602, Enadenotucirev NCT02028442). While oncolytic adenoviruses have been in development for over 20 years (31), Oncorine remains the only approved adenovirus for cancer therapy, and only when given in combination with chemotherapy.
T-VEC
T-VEC (Imlygic™) was approved by the US Food and Drug Administration (FDA) in 2015 for the treatment of non-resectable metastatic melanoma, and later in the EU for locally advanced or metastatic cutaneous melanoma, making it the most recent oncolytic virus to gain national regulatory approval, and the first to gain approval in the USA (https://www.fda.gov/downloads/BiologicsBloodVaccines/CellularGeneTherapyProducts/ApprovedProducts/UCM469670.pdf). T-VEC is a recombinant human herpes simplex virus type 1 (HSV1) deleted for both copies of the HSV1 gamma34.5 and viral ICP47, which accelerates the expression of US11, and encodes 2 copies of human granulocyte-macrophage colony stimulating factor (GM-CSF) under cytomegalovirus (CMV) promoters (32). Currently, T-VEC is approved for intratumoral injection into cutaneous high-grade melanoma lesions, and shows single-agent efficacy in this indication (33). Single-agent efficacy is also being evaluated in patients with liver, pancreatic, and advanced non-central nervous system solid tumors, and as of February 2018, ClinicalTrials.gov lists 23 ongoing clinical trials evaluating the safety and efficacy of T-VEC alone or in combination with checkpoint inhibitors, chemotherapy, or radiation therapy in melanoma and other indications. Promising results from a phase II trial in late-stage melanoma patients treated with combination T-VEC and PD-1 inhibitor pembrolizumab were published in 2017 (34). This combination resulted in enhanced efficacy with limited adverse events and showed particularly evident efficacy in uninjected visceral metastases which had a 7% response rate with T-VEC alone (33). Patients receiving combination anti-CTLA4 antibody ipilimumab with T-VEC versus ipilimumab alone showed improved progression-free survival (PFS) of visceral metastases from 0% PFS with ipilimumab to 23% with T-VEC plus ipilimumab (35).
OVs in clinical development
Measles virus
Measles virus is a negative strand RNA paramyxovirus which is highly fusogenic and induces extensive cytopathic effects of syncytial formation (36). Intercellular fusion (F) increases bystander killing of tumor cells, and induces immunogenic danger signals which can elicit host mediated cellular antitumor activity (37,38). Recombinant Edmonston strain measles virus encoding the sodium iodide symporter (MV-NIS) or soluble carcinoembryonic antigen (MV-CEA) are in Phase I/II clinical testing in patients with relapsed or recurrent cancers including multiple myeloma, ovarian cancer, glioma, breast cancer and mesothelioma (39,40). Intratumoral injections of Edmonston-Zagreb vaccine strain was also tested in 5 patients with cutaneous T cell lymphoma (41). Overall, no drug related dose limiting toxicities were observed in the trials even with high intravenous dosing, and in one study, MV-NIS induced complete remission of disseminated multiple myeloma after one systemic administration of 1011 infectious virus (42). Immunological analysis of peripheral blood T cells in ovarian cancer patients who received MV-NIS showed induction of tumor antigen specific cytotoxic T cells after measles virus therapy (43). A unique feature of MV-NIS is that it permits serial monitoring of the pharmacokinetics of viral replication in the infected tumors through noninvasive SPECT or PET imaging, enabling validation of virus delivery and infection of tumor metastases. Other MV engineering strategies include retargeting the H attachment glycoprotein (G) to obtain highly tumor selective viruses (44), encoding the wild type P accessory protein to enhance viral spread by antagonizing the host cellular antiviral immunity (45), potency enhancing cytotoxic genes to pair with a prodrug for chemovirotherapy (46), radiotracer enhancing transgene for imaging and radiosensitization (radiovirotherapy) (47), and immune modulatory transgenes such as anti-CTLA-4 and PDL-1 antibodies (48).
Newcastle disease virus (NDV)
NDV is an avian paramyxovirus and has been tested as an oncolytic or oncolysate cancer vaccine, for more than 50 years (49,50). NDV strains, MTH-68/H (veterinary vaccine strain), HUJ, a nonvirulent lentogenic strain, and PV701, have been tested clinically. In the United States, PV701 has been given intravenously to 113 patients with advanced cancers in 3 Phase 1 trials (51). In a trial of 79 patients, a CR was observed for 1 patient, and a PR in 1 patient (50). It was also shown that higher doses of PV701 can be better tolerated with less infusion reactions if the patients first received a 5-10-fold lower dose for desensitization (52). Clinical development of a mesogenic strain (intermediate virulence) of NDV as an oncolytic agent for cancer therapy has been hampered by its select agent status due to its pathogenicity in avian species (53). As such, a recombinant NDV based on the mesogenic NDV-73T strain with compromised infection of avian cells but not mammalian cells and encoding GM-CSF (Medimmune, MEDI5395) was generated and is in preclinical testing (54).
Rhabdoviruses
Rhabdoviruses are negative sense RNA viruses with rapid, ~12-hour lytic replication cycles in permissive cells (55,56). The best studied oncolytic rhabdovirus Vesicular Stomatitis Virus (VSV) uses the low-density lipoprotein (LDL) receptor for cell entry, allowing VSV to infect nearly all cell types and cause lytic infection in a wide variety of permissive cells (57). Oncolytic VSV cell entry has been made tumor selective through retargeting strategies, involving replacing the VSV G with a more selective entry G as the measles F and H (hemagglutinin) proteins (58), receptor targeted measles H and F proteins (59,60), the lymphocytic choriomeningitis virus WE54-strain glycoprotein (LCMV-GP) (61) and Lassa virus G (62).
VSV and other rhabdoviruses are exquisitely sensitive to type 1 interferon (63) which can be exploited in cancer cells that commonly lack a robust interferon response (56,64). To this end, two major modifications have been made to oncolytic rhabdoviruses to ensure their interferon sensitivity and therefore tumor selectivity. The first is an amino acid modification or deletion in the viral matrix (M) protein at the 51 position. The M protein methionine at position 51 is essential for the virus’s ability to inhibit a host interferon response (65), and its loss allows infected cells to produce and release interferon in response to infection. The two commonly exploited M51 mutations are M51R (methionine to arginine), and deltaM51 (deletion of methionine 51) (65-67). The alternative modification used to exploit VSV’s exquisite interferon sensitivity is the inclusion of an interferon beta transgene in the virus construct (68). Both the M51 mutation and interferon beta transgene inhibit viral spread by allowing an interferon-induced antiviral state in neighboring uninfected non-neoplastic cells but allows for VSV infection of cancer cells which lack an interferon response and thus remain permissive to virus replication (66,69).
Due to the low seroprevalence of VSV-neutralizing antibodies in human patients, these viruses can be delivered systemically through intravenous injection (70). Intravenous delivery can lead to infection and lytic destruction of metastatic tumors, with the virus able to infect multiple tumor lesions simultaneously. While a primary mechanism of oncolysis for rhabdoviruses is lytic tumor destruction, some engineered viruses expressing immune modulators can also initiate adaptive immune response against tumor (neo)antigens, potentially enhancing antitumor responses (71-74).
Ongoing Phase 1 clinical trials are evaluating safety of intratumorally and intravenously delivered VSV-IFNβ, VSV-IFNβ-NIS, and Maraba MG1 virus encoding the tumor-associated antigen MAGEA3. MAGEA3 is found on non-small cell lung cancer cells (75), and infection of MAGEA3 positive tumors with an oncolytic virus expressing this antigen can increase the chances of developing adaptive, MAGEA3-specific T-cell responses (76). VSV-IFNβ and VSV-IFNβ-NIS express human IFNβ which acts both to attenuate virus infection of non-neoplastic cells and to enhance an innate immune response to virus infection which can lead to increased adaptive immune cell recruitment and activation against both the virus and tumor cells (77,78). VSV-IFNβ-NIS also encodes human NIS, which can be harnessed for non-invasive virus tracking and/or enhanced oncolysis through the use of iodine radioisotopes (77).
Adenovirus
Adenoviruses are non-enveloped icosahedral double-stranded DNA viruses with long fiber knobs protruding from each capsid vertex (79,80). At least 70 serotypes of human adenovirus exist, with serotype 5 being the most commonly used; 6 of 7 oncolytic adenoviruses in clinical testing use a serotype 5 backbone. Clinical data has been published for telomelysin in solid tumors (81), CG0070 in bladder cancer (82), DNX-2401 in malignant brain tumors (83). A list of these viruses is shown in Table 1.
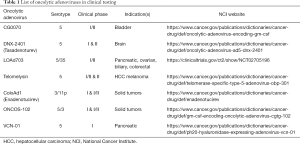
Full table
Adenovirus receptor binding occurs at both at RGD motifs of penton base proteins and at fiber knobs that extend from them (84). Receptor specificity is dependent on virus subgroup and serotype and to date at least 11 receptors have been shown to facilitate adenovirus binding (85). Many adenoviruses bind integrins via penton RGD motifs, facilitating entry and infection of permissive cells (86). Ad5, a subgroup C virus, also infects cells through coxsackie adenovirus receptor (CAR), heparan sulfate glycosaminoglycans (HS-GAG), and through other receptors such as MHC-I, VCAM-1, and DPPC, while Ad3, a subgroup B virus, binds CD46, CD80, and CD86 (85). Efforts to modify tissue tropism include modifications to the penton base RGD binding domain and serotype switching or modifications of fiber knob proteins (87). Examples include the RGD-4C motif used in DNX-2401 which binds cell adhesion molecules and allows entry through any fibronectin-binding integrin receptor (88), the chimeric ONCOS-102 and LOAd703 viruses which respectively incorporate CD46-tropic serotype 3 and 35 fiber knobs into serotype 5 backbones (89), and inclusion of an RGDK motif in the HS-GAG binding domain of the fiber shaft of VCN-01 (90) which detargets the virus from the liver and enhances tumor selectivity in vivo (91).
Attenuation strategies for oncolytic adenoviral vectors have revolved around 2 central mechanisms: targeting Rb-deficient tumors through E1A modifications and, in theory, targeting p53 deficient tumors through E1B modifications. Of these, E1A modifications are more common in the clinical landscape. CG0070 places E1A under the tumor-specific E2F-1 promoter (92), while DNX-2401 and ONCOS-102 incorporate a 24-bp deletion within E1A that deletes the Rb binding function from E1A (93). LOAd703 combines these strategies, driving an E1A deleted for its Rb binding region via an E2F promoter (89). Telomelysin uses a separate tumor-specific promoter, hTERT, to drive both E1A and E1B (94). ColoAd1, a serotype 3/11p chimeric virus, was uniquely designed through directed evolution and replicates and kills colorectal cancer cells more efficiently than normal human epithelial cells, but contains no obviously attenuating mutations (95).
Several oncolytic adenoviruses incorporate payloads to enhance antitumor activity. These payloads include GM-CSF, expressed by CG0070 and ONCOS-102, which activates antigen presenting cells (APCs) and may enhance the uptake and presentation of both viral and tumor-associated antigens following oncolysis (89). LOAd703 expresses CD40 and 4-1BB ligands, activating APCs and T-cells respectively through co-stimulation (96), VCN-01 expresses soluble hyaluronidase which degrades extracellular M hyaluronic acid and may enhance virus spread in solid tumors (97).
Vaccinia viruses
Vaccinia, closely related to cowpox virus, is a large, enveloped, double-stranded DNA virus with a linear genome approximately 190 kb in length and is the namesake virus for vaccination following its widespread use in the eradication of smallpox (98). To date, 3 oncolytic vaccinia viruses are being used clinically, derived from the Wyeth (Sillajen, JX-594, Pexastimogene devacirepvec/PexaVec), Western Reserve (Transgene, TG6002), and Lister (GeneLux, GL-ONC1/GLV-1h68) vaccinia strains (99-101).
Attenuation or tumor-specific targeting of these viruses has been accomplished using a variety of deletions and insertional mutations, with loss of thymidine kinase function being a common denominator among the clinical oncolytic vaccinia viruses. JX-594 is deleted for viral thymidine kinase (99), TG6002 is doubly deleted for thymidine kinase and viral ribonucleotide reductase (101), and GL-ONC1 has insertional mutations in its thymidine kinase (J2R), hemagglutinin HA (A56R), and F14.5L genes (100). The TK loss of function limits the virus’ ability to replicate in non-dividing cells, and the deletion of viral ribonucleotide reductase further limits this ability.
Strategies to enhance oncolytic efficacy of vaccinia vectors center around transgene incorporation. To this end, two clinical vectors include transgenes designed to improve tumor cell killing: JX-594, like T-VEC, includes GM-CSF (99), and TG6002 incorporates a nucleoside analog converting enzyme FCU1, which converts 5-fluorocytosine (5-FC) to 5-FU in infected cells (101). Combination treatment with 5-FC should result in lytic tumor destruction along with 5-FU conversion, from where 5-FU can be disseminated to uninfected tumor cells and inhibit DNA elongation during mitosis. The rationale for GM-CSF incorporation was solidified in 1993 (102), and provided evidence that the combination of dead or dying tumor cells along with high levels of locally secreted GM-CSF could enhance anti-tumor immunity in tumor-bearing mice.
PexaVec and GL-ONC1 have been proven safe and tolerable in humans across a multitude of indications through phase I and I/II clinical trials (103,104), and a phase I/II trial for brain cancer patients receiving TG6002 with 5-FC began enrolling in late September 2017 (NCT03294486). PexaVec is now enrolling in a phase III registration trial in combination with Sorafenib for patients with hepatocellular carcinoma (HCC) (NCT02562755). Results of the phase II trial for the same indication and treatment regimen revealed improved Choi tumor responses, disease control, and tolerable adverse events in patients receiving combination PexaVec and Sorafenib versus PexaVec (105).
Herpes viruses
HSV1 is a large double stranded DNA virus approximately 152 kb in length (106). Herpes was the first virus backbone to be genetically engineered to combat cancer with the demonstration in 1991 that HSV-dlspTK, a thymidine kinase-deleted HSV-1, enhanced overall survival in a murine model of glioblastoma (107). Further development led to the generation, preclinical and clinical testing of novel HSV gamma34.5-deficient viruses which lack both neurovirulence and the ability to inhibit the antiviral PKR response (108). Clinically evaluated gamma34.5-deficient viruses include the now FDA-approved T-VEC (109), HSV1716 (Seprehvir) (110), G207 (111), and RP1 which was announced in clinical trials as of November 2017. NV1020, which retains a single copy of gamma34.5 but also includes additional attenuating mutations to TK, UL24, UL55 and UL56 has also been tested in human patients (112). Separately, a naturally occurring HSV mutant HF-10 which retains both copies of gamma34.5 has been clinically evaluated in patients with breast, head and neck, and pancreatic cancers (113).
High seroprevalence of HSV-1 neutralizing antibodies in US population remains a barrier to systemic delivery of oncolytic HSV vectors (114). HSV-based OVs are therefore delivered locoregionally or intratumorally, avoiding intravenous administration. Attempts to boost the anti-cancer effects of HSV in metastatic diseases involve the inclusion of therapeutic transgenes used to simultaneously boost anti-cancer and antiviral immunity, with the goal of developing an adaptive anti-tumor response in treated patients. To this end, constitutively active GM-CSF is incorporated in T-VEC and RP1 viruses, and combinations with immune-stimulating therapies are ongoing (34).
Coxsackievirus
Coxsackievirus is a single stranded positive RNA picornavirus of approximately 7.4 kb, enclosed in an icosahedral capsid. Oncolytic CVA21 (Viralytics, CAVATAK) is derived from the Kuykendall strain and uses ICAM-1 as the primary receptor for cell entry (115). It has been tested in intratumoral or intravenous administration, a single agent or in combination with immune checkpoint blockade, in a number of Phase I/II clinical trials in patients with breast cancer, prostate cancer, bladder cancer, multiple myeloma, melanoma and non-small cell lung cancer (114). Phase I testing of intratumoral CVA21 virus injection in combination with pembrolizumab or ipilimumab is ongoing to enhance the overall efficacy of these drugs (116). Early analysis of the combination trial with pembrolizumab met its primary statistical futility endpoint of ≥2 confirmed objective responses (CR or PR) in the first 12 patients enrolled, and will be expanded to recruit up to 50 patients (116).
Reovirus
Reovirus is a double stranded RNA virus, non-enveloped and has an icosahedral capsid composed of an outer and inner protein shell. It naturally infects the gastrointestinal tract but does not cause serious disease and it is estimated that up to 100% of the healthy adult population has pre-existing antibodies to reovirus (117). Cells with an activated Ras pathway, such as cancer cells, are highly susceptible to reovirus infection (118). Reovirus as a monotherapy was investigated in several Phase I trials (Oncolytics Biotech, Reolysin™) as an intratumoral or intravenous administration but recently, the team has focused predominantly in combining reovirus with standard chemotherapy or radiation therapy. In a Phase I/II trial of reovirus with carboplatin and paclitaxel in patients with solid tumors, out of 26 patients, the best overall response was CR in 1 patient (3.8%), PR in 6 patients (23.1%) (119). A number of clinical trials testing reovirus with other chemotherapy or immune modulators are ongoing or planned (120).
Retrovirus
Retroviral replicating vector (Tocagen, Toca-511, vocimagene amiretrorepvec) encodes yeast cytosine deaminase (CD) that converts the prodrug 5-FC to the anticancer drug, 5-FU, thereby enhancing local concentration of 5-FU in tumor, and decreasing overall systemic toxicity of the drug (121,122). In contrast to the OVs discussed above, these vectors are nonlytic, but instead, selectively replicate in dividing cells with defective innate immunity and interferon responsiveness (122). A phase 1 trial of Toca-511 in patients with recurrent high-grade glioma resulted in overall survival of 13.6 months (95% confidence interval, 10.8 to 20.0) and was statistically improved relative to an external control (hazard ratio, 0.45; P=0.003) (123). In 2015, Toca-511 received orphan drug status from the US FDA and is in Phase II/III clinical testing for patients with glioblastoma (NCT02414165).
Future directions
The single-agent approval of T-VEC by the US FDA in 2015 was a watershed moment for the OV field, however, T-VEC delivered intralesionally to cutaneous melanoma failed to provide a significant benefit to patients with visceral metastases (33). To enhance overall response rates, OVs are increasingly being combined with anticancer drugs including standard of care chemotherapy, checkpoint inhibitors, and radiation therapy. Notable chemotherapy combinations include Phase III trials of PexaVec plus Sorafenib for hepatocellular carcinoma patients, Reolysin plus carboplatin and paclitaxel for head and neck cancer patients (120), and Toca-511 plus Toca-FC (a modified 5-FC) for high grade glioma patients.
The effect of oncolytic virotherapy on CD8+ T-cell recruitment is well documented and supports the rationale to enhance the efficacy of therapy using strategies to enhance adaptive immune cell activation (124,125). Promising results are emerging from clinical trials; T-VEC given intralesionally in combination with a standard regimen of pembrolizumab gave a response rate of 38% versus 16% with pembrolizumab or T-VEC alone (34), and this has led to the pursuit of combinations with checkpoint inhibitors for a wide array of viruses currently being explored in the clinic. Preclinical studies have shown that viruses encoding transgenes that activate host cell-mediated immune responses have greater efficacy in tumor models, including viruses encoding anti-PDL1 scFv fragments, bi-specific T-cell engager antibodies that recognize T-cells and a tumor-associated antigen, both activating T-cells and bringing them into close proximity with tumor targets (126,127).
Systemic delivery of oncolytic therapies can allow viruses to disseminate throughout a patient’s body and target metastatic cancers more effectively than other delivery methods. A major barrier to effective systemic therapy has been the presence of preexisting antiviral antibodies in vaccinated or seropositive patients (12). Strategies to evade neutralization are being actively explored, including the use of cell carriers for delivery of measles virus in patients with ovarian cancer (128). Intact innate responses of tumor cells or immune cells in the tumor microenvironment pose a barrier to viral replication. As such, incorporation of inhibitors of the innate immune response into the virus construct aim to prolong productive infection in the target tumor (129).
OVs are poised to play an important part in the future of cancer therapy, and while there is intense activity with combination trials of virus with standard therapies, we must not lose sight to also develop OV as monotherapies that could have a meaningful impact on overall survival of cancer patients. As the pace of OV development increases and we learn more from current clinical trials about the benefits and shortcomings of OVs, we hope to see a future generation of viruses able to combat cancer as single agents, delivered systemically and with minimal repeat dosing necessary to achieve efficacy.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Morrissey KM, Yuraszeck TM, Li CC, et al. Immunotherapy and Novel Combinations in Oncology: Current Landscape, Challenges, and Opportunities. Clin Transl Sci 2016;9:89-104. [Crossref] [PubMed]
- Brudno JN, Kochenderfer JN. Chimeric antigen receptor T-cell therapies for lymphoma. Nat Rev Clin Oncol 2018;15:31-46. [Crossref] [PubMed]
- Patel SA, Minn AJ. Combination Cancer Therapy with Immune Checkpoint Blockade: Mechanisms and Strategies. Immunity 2018;48:417-33. [Crossref] [PubMed]
- Antonia SJ, Larkin J, Ascierto PA. Immuno-oncology combinations: a review of clinical experience and future prospects. Clin Cancer Res 2014;20:6258-68. [Crossref] [PubMed]
- Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science 2018;359:1350-5. [Crossref] [PubMed]
- Izuo M. Medical history: Seishu Hanaoka and his success in breast cancer surgery under general anesthesia two hundred years ago. Breast Cancer 2004;11:319-24. [Crossref] [PubMed]
- Connell PP, Hellman S. Advances in radiotherapy and implications for the next century: a historical perspective. Cancer Res 2009;69:383-92. [Crossref] [PubMed]
- NCI. Milestones in Cancer Research and Discovery. National Cancer Institute. 2015. Available online: https://www.cancer.gov/research/progress/250-years-milestones
- DeVita VT Jr, Chu E. A history of cancer chemotherapy. Cancer Res 2008;68:8643-53. [Crossref] [PubMed]
- Lichtman MA. Battling the hematological malignancies: the 200 years' war. Oncologist 2008;13:126-38. [Crossref] [PubMed]
- Kalyn R. Overview of targeted therapies in oncology. J Oncol Pharm Pract 2007;13:199-205. [Crossref] [PubMed]
- Russell SJ, Peng KW, Bell JC. Oncolytic virotherapy. Nat Biotechnol 2012;30:658-70. [Crossref] [PubMed]
- Maroun J, Munoz-Alia M, Ammayappan A, et al. Designing and building oncolytic viruses. Future Virol 2017;12:193-213. [Crossref] [PubMed]
- Seymour LW, Fisher KD. Oncolytic viruses: finally delivering. Br J Cancer 2016;114:357-61. [Crossref] [PubMed]
- Kaufman HL, Kohlhapp FJ, Zloza A. Oncolytic viruses: a new class of immunotherapy drugs. Nat Rev Drug Discov 2015;14:642-62. [Crossref] [PubMed]
- Kelly E, Russell SJ. History of oncolytic viruses: genesis to genetic engineering. Mol Ther 2007;15:651-9. [Crossref] [PubMed]
- Lin E, Nemunaitis J. Oncolytic viral therapies. Cancer Gene Ther 2004;11:643-64. [Crossref] [PubMed]
- Southam CM, Moore AE. Clinical studies of viruses as antineoplastic agents with particular reference to Egypt 101 virus. Cancer 1952;5:1025-34. [Crossref] [PubMed]
- Donina S, Strele I, Proboka G, et al. Adapted ECHO-7 virus Rigvir immunotherapy (oncolytic virotherapy) prolongs survival in melanoma patients after surgical excision of the tumour in a retrospective study. Melanoma Res 2015;25:421-6. [Crossref] [PubMed]
- Babiker HM, Riaz IB, Husnain M, et al. Oncolytic virotherapy including Rigvir and standard therapies in malignant melanoma. Oncolytic Virother 2017;6:11-8. [Crossref] [PubMed]
- Alberts P, Olmane E, Brokane L, et al. Long-term treatment with the oncolytic ECHO-7 virus Rigvir of a melanoma stage IV M1c patient, a small cell lung cancer stage IIIA patient, and a histiocytic sarcoma stage IV patient-three case reports. APMIS 2016;124:896-904. [Crossref] [PubMed]
- Liang M. Oncorine, the World First Oncolytic Virus Medicine and its Update in China. Curr Cancer Drug Targets 2018;18:171-6. [Crossref] [PubMed]
- Ries S, Korn WM. ONYX-015: mechanisms of action and clinical potential of a replication-selective adenovirus. Br J Cancer 2002;86:5-11. [Crossref] [PubMed]
- Harada JN, Berk AJ. p53-Independent and -dependent requirements for E1B-55K in adenovirus type 5 replication. J Virol 1999;73:5333-44. [PubMed]
- Goodrum FD, Ornelles DA. The early region 1B 55-kilodalton oncoprotein of adenovirus relieves growth restrictions imposed on viral replication by the cell cycle. J Virol 1997;71:548-61. [PubMed]
- Goodrum FD, Ornelles DA. p53 status does not determine outcome of E1B 55-kilodalton mutant adenovirus lytic infection. J Virol 1998;72:9479-90. [PubMed]
- Larson C, Oronsky B, Scicinski J, et al. Going viral: a review of replication-selective oncolytic adenoviruses. Oncotarget 2015;6:19976-89. [Crossref] [PubMed]
- Xia ZJ, Chang JH, Zhang L, et al. Phase III randomized clinical trial of intratumoral injection of E1B gene-deleted adenovirus (H101) combined with cisplatin-based chemotherapy in treating squamous cell cancer of head and neck or esophagus. Ai Zheng 2004;23:1666-70. [PubMed]
- Zhang S, Huang W, Zhou X, et al. Seroprevalence of neutralizing antibodies to human adenoviruses type-5 and type-26 and chimpanzee adenovirus type-68 in healthy Chinese adults. J Med Virol 2013;85:1077-84. [Crossref] [PubMed]
- Nwanegbo E, Vardas E, Gao W, et al. Prevalence of neutralizing antibodies to adenoviral serotypes 5 and 35 in the adult populations of The Gambia, South Africa, and the United States. Clin Diagn Lab Immunol 2004;11:351-7. [PubMed]
- Heise C, Sampson-Johannes A, Williams A, et al. ONYX-015, an E1B gene-attenuated adenovirus, causes tumor-specific cytolysis and antitumoral efficacy that can be augmented by standard chemotherapeutic agents. Nat Med 1997;3:639-45. [Crossref] [PubMed]
- Liu BL, Robinson M, Han ZQ, et al. ICP34.5 deleted herpes simplex virus with enhanced oncolytic, immune stimulating, and anti-tumour properties. Gene Ther 2003;10:292-303. [Crossref] [PubMed]
- Andtbacka RH, Kaufman HL, Collichio F, et al. Talimogene Laherparepvec Improves Durable Response Rate in Patients With Advanced Melanoma. J Clin Oncol 2015;33:2780-8. [Crossref] [PubMed]
- Ribas A, Dummer R, Puzanov I, et al. Oncolytic Virotherapy Promotes Intratumoral T Cell Infiltration and Improves Anti-PD-1 Immunotherapy. Cell 2017;170:1109-19 e10.
- Chesney J, Puzanov I, Collichio F, et al. Randomized, Open-Label Phase II Study Evaluating the Efficacy and Safety of Talimogene Laherparepvec in Combination With Ipilimumab Versus Ipilimumab Alone in Patients With Advanced, Unresectable Melanoma. J Clin Oncol 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Yanagi Y, Takeda M, Ohno S. Measles virus: cellular receptors, tropism and pathogenesis. J Gen Virol 2006;87:2767-79. [Crossref] [PubMed]
- Russell SJ, Peng KW. Measles virus for cancer therapy. Curr Top Microbiol Immunol 2009;330:213-41. [Crossref] [PubMed]
- Donnelly OG, Errington-Mais F, Steele L, et al. Measles virus causes immunogenic cell death in human melanoma. Gene Ther 2013;20:7-15. [Crossref] [PubMed]
- Msaouel P, Opyrchal M, Dispenzieri A, et al. Clinical Trials with Oncolytic Measles Virus: Current Status and Future Prospects. Curr Cancer Drug Targets 2018;18:177-87. [Crossref] [PubMed]
- Aref S, Bailey K, Fielding A. Measles to the Rescue: A Review of Oncolytic Measles Virus. Viruses 2016.8. [PubMed]
- Heinzerling L, Kunzi V, Oberholzer PA, et al. Oncolytic measles virus in cutaneous T-cell lymphomas mounts antitumor immune responses in vivo and targets interferon-resistant tumor cells. Blood 2005;106:2287-94. [Crossref] [PubMed]
- Russell SJ, Federspiel MJ, Peng KW, et al. Remission of disseminated cancer after systemic oncolytic virotherapy. Mayo Clin Proc 2014;89:926-33. [Crossref] [PubMed]
- Galanis E, Atherton PJ, Maurer MJ, et al. Oncolytic measles virus expressing the sodium iodide symporter to treat drug-resistant ovarian cancer. Cancer Res 2015;75:22-30. [Crossref] [PubMed]
- Nakamura T, Peng KW, Harvey M, et al. Rescue and propagation of fully retargeted oncolytic measles viruses. Nat Biotechnol 2005;23:209-14. [Crossref] [PubMed]
- Haralambieva I, Iankov I, Hasegawa K, et al. Engineering oncolytic measles virus to circumvent the intracellular innate immune response. Mol Ther 2007;15:588-97. [Crossref] [PubMed]
- Kaufmann JK, Bossow S, Grossardt C, et al. Chemovirotherapy of malignant melanoma with a targeted and armed oncolytic measles virus. J Invest Dermatol 2013;133:1034-42. [Crossref] [PubMed]
- Dingli D, Peng KW, Harvey ME, et al. Image-guided radiovirotherapy for multiple myeloma using a recombinant measles virus expressing the thyroidal sodium iodide symporter. Blood 2004;103:1641-6. [Crossref] [PubMed]
- Engeland CE, Grossardt C, Veinalde R, et al. CTLA-4 and PD-L1 checkpoint blockade enhances oncolytic measles virus therapy. Mol Ther 2014;22:1949-59. [Crossref] [PubMed]
- Schirrmacher V. Fifty Years of Clinical Application of Newcastle Disease Virus: Time to Celebrate! Biomedicines 2016.4. [PubMed]
- Zamarin D, Palese P. Oncolytic Newcastle disease virus for cancer therapy: old challenges and new directions. Future Microbiol 2012;7:347-67. [Crossref] [PubMed]
- Lorence RM, Pecora AL, Major PP, et al. Overview of phase I studies of intravenous administration of PV701, an oncolytic virus. Curr Opin Mol Ther 2003;5:618-24. [PubMed]
- Hotte SJ, Lorence RM, Hirte HW, et al. An optimized clinical regimen for the oncolytic virus PV701. Clin Cancer Res 2007;13:977-85. [Crossref] [PubMed]
- Cheng X, Wang W, Xu Q, et al. Genetic Modification of Oncolytic Newcastle Disease Virus for Cancer Therapy. J Virol 2016;90:5343-52. [Crossref] [PubMed]
- Carroll D, Harper J, Jon T, et al. Abstract 4556: MEDI5395: An armed oncolytic Newcastle disease virus. Cancer Research 2017;77:4556. [Crossref] [PubMed]
- Rose J, Whitt M. Rhabdoviridae: the viruses and their replication. In: Knipe DM, PM H. editors. Fields Virology. 4th ed. Philadelphia, PA: Lippincott Williams and Wilkins, 2001:1221-44.
- Hastie E, Grdzelishvili VZ. Vesicular stomatitis virus as a flexible platform for oncolytic virotherapy against cancer. J Gen Virol 2012;93:2529-45. [Crossref] [PubMed]
- Finkelshtein D, Werman A, Novick D, et al. LDL receptor and its family members serve as the cellular receptors for vesicular stomatitis virus. Proc Natl Acad Sci U S A 2013;110:7306-11. [Crossref] [PubMed]
- Ayala-Breton C, Russell LO, Russell SJ, et al. Faster replication and higher expression levels of viral glycoproteins give the vesicular stomatitis virus/measles virus hybrid VSV-FH a growth advantage over measles virus. J Virol 2014;88:8332-9. [Crossref] [PubMed]
- Ayala Breton C, Wikan N, Abbuhl A, et al. Oncolytic potency of HER-2 retargeted VSV-FH hybrid viruses: the role of receptor ligand affinity. Mol Ther Oncolytics 2015;2:15012. [Crossref] [PubMed]
- Hanauer JDS, Rengstl B, Kleinlutzum D, et al. CD30-targeted oncolytic viruses as novel therapeutic approach against classical Hodgkin lymphoma. Oncotarget 2018;9:12971-81. [Crossref] [PubMed]
- Muik A, Kneiske I, Werbizki M, et al. Pseudotyping vesicular stomatitis virus with lymphocytic choriomeningitis virus glycoproteins enhances infectivity for glioma cells and minimizes neurotropism. J Virol 2011;85:5679-84. [Crossref] [PubMed]
- Wollmann G, Drokhlyansky E, Davis JN, et al. Lassa-vesicular stomatitis chimeric virus safely destroys brain tumors. J Virol 2015;89:6711-24. [Crossref] [PubMed]
- Rieder M, Conzelmann KK. Rhabdovirus evasion of the interferon system. J Interferon Cytokine Res 2009;29:499-509. [Crossref] [PubMed]
- Masters PS, Samuel CE. Mechanism of interferon action: inhibition of vesicular stomatitis virus replication in human amnion U cells by cloned human leukocyte interferon. I. Effect on early and late stages of the viral multiplication cycle. J Biol Chem 1983;258:12019-25. [PubMed]
- Ahmed M, McKenzie MO, Puckett S, et al. Ability of the matrix protein of vesicular stomatitis virus to suppress beta interferon gene expression is genetically correlated with the inhibition of host RNA and protein synthesis. J Virol 2003;77:4646-57. [Crossref] [PubMed]
- Stojdl DF, Lichty BD, tenOever BR, et al. VSV strains with defects in their ability to shutdown innate immunity are potent systemic anti-cancer agents. Cancer Cell 2003;4:263-75. [Crossref] [PubMed]
- Balachandran S, Barber GN. Vesicular stomatitis virus (VSV) therapy of tumors. IUBMB Life 2000;50:135-8. [Crossref] [PubMed]
- Obuchi M, Fernandez M, Barber GN. Development of recombinant vesicular stomatitis viruses that exploit defects in host defense to augment specific oncolytic activity. J Virol 2003;77:8843-56. [Crossref] [PubMed]
- Barber GN. Vesicular stomatitis virus as an oncolytic vector. Viral Immunol 2004;17:516-27. [Crossref] [PubMed]
- Ferguson MS, Lemoine NR, Wang Y. Systemic delivery of oncolytic viruses: hopes and hurdles. Adv Virol 2012;2012:805629. [PubMed]
- Galivo F, Diaz RM, Thanarajasingam U, et al. Interference of CD40L-mediated tumor immunotherapy by oncolytic vesicular stomatitis virus. Hum Gene Ther 2010;21:439-50. [Crossref] [PubMed]
- Pol JG, Zhang L, Bridle BW, et al. Maraba virus as a potent oncolytic vaccine vector. Mol Ther 2014;22:420-9. [Crossref] [PubMed]
- Shin EJ, Wanna GB, Choi B, et al. Interleukin-12 expression enhances vesicular stomatitis virus oncolytic therapy in murine squamous cell carcinoma. Laryngoscope 2007;117:210-4. [Crossref] [PubMed]
- Fernandez M, Porosnicu M, Markovic D, et al. Genetically engineered vesicular stomatitis virus in gene therapy: application for treatment of malignant disease. J Virol 2002;76:895-904. [Crossref] [PubMed]
- Peled N, Oton AB, Hirsch FR, et al. MAGE A3 antigen-specific cancer immunotherapeutic. Immunotherapy 2009;1:19-25. [Crossref] [PubMed]
- Jonker DJ, Hotte SJ, Razak ARA, et al. Phase I study of oncolytic virus (OV) MG1 maraba/MAGE-A3 (MG1MA3), with and without transgenic MAGE-A3 adenovirus vaccine (AdMA3) in incurable advanced/metastatic MAGE-A3-expressing solid tumours: CCTG IND.214. J Clin Oncol 2017;35:e14637-e.
- Naik S, Nace R, Federspiel MJ, et al. Curative one-shot systemic virotherapy in murine myeloma. Leukemia 2012;26:1870-8. [Crossref] [PubMed]
- Prestwich RJ, Harrington KJ, Pandha HS, et al. Oncolytic viruses: a novel form of immunotherapy. Expert Rev Anticancer Ther 2008;8:1581-8. [Crossref] [PubMed]
- Vellinga J, Van der Heijdt S, Hoeben RC. The adenovirus capsid: major progress in minor proteins. J Gen Virol 2005;86:1581-8. [Crossref] [PubMed]
- Ahi YS, Mittal SK. Components of Adenovirus Genome Packaging. Front Microbiol 2016;7:1503. [Crossref] [PubMed]
- Nemunaitis J, Tong AW, Nemunaitis M, et al. A phase I study of telomerase-specific replication competent oncolytic adenovirus (telomelysin) for various solid tumors. Mol Ther 2010;18:429-34. [Crossref] [PubMed]
- Packiam VT, Lamm DL, Barocas DA, et al. An open label, single-arm, phase II multicenter study of the safety and efficacy of CG0070 oncolytic vector regimen in patients with BCG-unresponsive non-muscle-invasive bladder cancer: Interim results. Urol Oncol 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Lang FF, Conrad C, Gomez-Manzano C, et al. Phase I Study of DNX-2401 (Delta-24-RGD) Oncolytic Adenovirus: Replication and Immunotherapeutic Effects in Recurrent Malignant Glioma. J Clin Oncol 2018.JCO2017758219. [PubMed]
- Cao C, Dong X, Wu X, et al. Conserved fiber-penton base interaction revealed by nearly atomic resolution cryo-electron microscopy of the structure of adenovirus provides insight into receptor interaction. J Virol 2012;86:12322-9. [Crossref] [PubMed]
- Zhang Y, Bergelson JM. Adenovirus receptors. J Virol 2005;79:12125-31. [Crossref] [PubMed]
- Mathias P, Wickham T, Moore M, et al. Multiple adenovirus serotypes use alpha v integrins for infection. J Virol 1994;68:6811-4. [PubMed]
- Arnberg N. Adenovirus receptors: implications for targeting of viral vectors. Trends Pharmacol Sci 2012;33:442-8. [Crossref] [PubMed]
- Fueyo J, Alemany R, Gomez-Manzano C, et al. Preclinical characterization of the antiglioma activity of a tropism-enhanced adenovirus targeted to the retinoblastoma pathway. J Natl Cancer Inst 2003;95:652-60. [Crossref] [PubMed]
- Kuryk L, Haavisto E, Garofalo M, et al. Synergistic anti-tumor efficacy of immunogenic adenovirus ONCOS-102 (Ad5/3-D24-GM-CSF) and standard of care chemotherapy in preclinical mesothelioma model. Int J Cancer 2016;139:1883-93. [Crossref] [PubMed]
- Martinez-Velez N, Xipell E, Vera B, et al. The Oncolytic Adenovirus VCN-01 as Therapeutic Approach Against Pediatric Osteosarcoma. Clin Cancer Res 2016;22:2217-25. [Crossref] [PubMed]
- Bayo-Puxan N, Gimenez-Alejandre M, Lavilla-Alonso S, et al. Replacement of adenovirus type 5 fiber shaft heparan sulfate proteoglycan-binding domain with RGD for improved tumor infectivity and targeting. Hum Gene Ther 2009;20:1214-21. [Crossref] [PubMed]
- Jakubczak JL, Ryan P, Gorziglia M, et al. An oncolytic adenovirus selective for retinoblastoma tumor suppressor protein pathway-defective tumors: dependence on E1A, the E2F-1 promoter, and viral replication for selectivity and efficacy. Cancer Res 2003;63:1490-9. [PubMed]
- Fueyo J, Gomez-Manzano C, Alemany R, et al. A mutant oncolytic adenovirus targeting the Rb pathway produces anti-glioma effect in vivo. Oncogene 2000;19:2-12. [Crossref] [PubMed]
- Fujiwara T, Urata Y, Tanaka N. Telomerase-specific oncolytic virotherapy for human cancer with the hTERT promoter. Curr Cancer Drug Targets 2007;7:191-201. [Crossref] [PubMed]
- Kuhn I, Harden P, Bauzon M, et al. Directed evolution generates a novel oncolytic virus for the treatment of colon cancer. PLoS One 2008;3:e2409. [Crossref] [PubMed]
- Eriksson E, Milenova I, Wenthe J, et al. Shaping the Tumor Stroma and Sparking Immune Activation by CD40 and 4-1BB Signaling Induced by an Armed Oncolytic Virus. Clin Cancer Res 2017;23:5846-57. [Crossref] [PubMed]
- Guedan S, Rojas JJ, Gros A, et al. Hyaluronidase expression by an oncolytic adenovirus enhances its intratumoral spread and suppresses tumor growth. Mol Ther 2010;18:1275-83. [Crossref] [PubMed]
- Verardi PH, Titong A, Hagen CJ. A vaccinia virus renaissance: new vaccine and immunotherapeutic uses after smallpox eradication. Hum Vaccin Immunother 2012;8:961-70. [Crossref] [PubMed]
- Kim JH, Oh JY, Park BH, et al. Systemic armed oncolytic and immunologic therapy for cancer with JX-594, a targeted poxvirus expressing GM-CSF. Mol Ther 2006;14:361-70. [Crossref] [PubMed]
- Zhang Q, Liang C, Yu YA, et al. The highly attenuated oncolytic recombinant vaccinia virus GLV-1h68: comparative genomic features and the contribution of F14.5L inactivation. Mol Genet Genomics 2009;282:417-35. [Crossref] [PubMed]
- Fend L, Remy-Ziller C, Foloppe J, et al. Oncolytic virotherapy with an armed vaccinia virus in an orthotopic model of renal carcinoma is associated with modification of the tumor microenvironment. Oncoimmunology 2015;5:e1080414. [Crossref] [PubMed]
- Dranoff G, Jaffee E, Lazenby A, et al. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci U S A 1993;90:3539-43. [Crossref] [PubMed]
- Breitbach CJ, Bell JC, Hwang TH, et al. The emerging therapeutic potential of the oncolytic immunotherapeutic Pexa-Vec (JX-594). Oncolytic Virother 2015;4:25-31. [Crossref] [PubMed]
- Mell LK, Brumund KT, Daniels GA, et al. Phase I Trial of Intravenous Oncolytic Vaccinia Virus (GL-ONC1) with Cisplatin and Radiotherapy in Patients with Locoregionally Advanced Head and Neck Carcinoma. Clin Cancer Res 2017;23:5696-702. [Crossref] [PubMed]
- Heo J, Breitbach C, Cho M, et al. Phase II trial of Pexa-Vec (pexastimogene devacirepvec; JX-594), an oncolytic and immunotherapeutic vaccinia virus, followed by sorafenib in patients with advanced hepatocellular carcinoma (HCC). J Clin Oncol 2013;31:4122.
- Macdonald SJ, Mostafa HH, Morrison LA, et al. Genome sequence of herpes simplex virus 1 strain KOS. J Virol 2012;86:6371-2. [Crossref] [PubMed]
- Martuza RL, Malick A, Markert JM, et al. Experimental therapy of human glioma by means of a genetically engineered virus mutant. Science 1991;252:854-6. [Crossref] [PubMed]
- Mohr I, Gluzman Y. A herpesvirus genetic element which affects translation in the absence of the viral GADD34 function. EMBO J 1996;15:4759-66. [PubMed]
- Rehman H, Silk AW, Kane MP, et al. Into the clinic: Talimogene laherparepvec (T-VEC), a first-in-class intratumoral oncolytic viral therapy. J Immunother Cancer 2016;4:53. [Crossref] [PubMed]
- Streby KA, Geller JI, Currier MA, et al. Intratumoral Injection of HSV1716, an Oncolytic Herpes Virus, Is Safe and Shows Evidence of Immune Response and Viral Replication in Young Cancer Patients. Clin Cancer Res 2017;23:3566-74. [Crossref] [PubMed]
- Markert JM, Razdan SN, Kuo HC, et al. A phase 1 trial of oncolytic HSV-1, G207, given in combination with radiation for recurrent GBM demonstrates safety and radiographic responses. Mol Ther 2014;22:1048-55. [Crossref] [PubMed]
- Geevarghese SK, Geller DA, de Haan HA, et al. Phase I/II study of oncolytic herpes simplex virus NV1020 in patients with extensively pretreated refractory colorectal cancer metastatic to the liver. Hum Gene Ther 2010;21:1119-28. [Crossref] [PubMed]
- Eissa IR, Naoe Y, Bustos-Villalobos I, et al. Genomic Signature of the Natural Oncolytic Herpes Simplex Virus HF10 and Its Therapeutic Role in Preclinical and Clinical Trials. Front Oncol 2017;7:149. [Crossref] [PubMed]
- Bradley H, Markowitz LE, Gibson T, et al. Seroprevalence of herpes simplex virus types 1 and 2--United States, 1999-2010. J Infect Dis 2014;209:325-33. [Crossref] [PubMed]
- Shafren DR, Au GG, Nguyen T, et al. Systemic therapy of malignant human melanoma tumors by a common cold-producing enterovirus, coxsackievirus a21. Clin Cancer Res 2004;10:53-60. [Crossref] [PubMed]
- Silk AW, Kaufman H, Gabrail N, et al. Abstract CT026: Phase 1b study of intratumoral Coxsackievirus A21 (CVA21) and systemic pembrolizumab in advanced melanoma patients: Interim results of the CAPRA clinical trial. Cancer Res 2017;77:CT026. [Crossref]
- Harrington KJ, Vile RG, Melcher A, et al. Clinical trials with oncolytic reovirus: moving beyond phase I into combinations with standard therapeutics. Cytokine Growth Factor Rev 2010;21:91-8. [Crossref] [PubMed]
- Gong J, Mita MM. Activated ras signaling pathways and reovirus oncolysis: an update on the mechanism of preferential reovirus replication in cancer cells. Front Oncol 2014;4:167. [Crossref] [PubMed]
- Gong J, Sachdev E, Mita AC, et al. Clinical development of reovirus for cancer therapy: An oncolytic virus with immune-mediated antitumor activity. World J Methodol 2016;6:25-42. [Crossref] [PubMed]
- Chakrabarty R, Tran H, Selvaggi G, et al. The oncolytic virus, pelareorep, as a novel anticancer agent: a review. Invest New Drugs 2015;33:761-74. [Crossref] [PubMed]
- Yagiz K, Rodriguez-Aguirre ME, Lopez Espinoza F, et al. A Retroviral Replicating Vector Encoding Cytosine Deaminase and 5-FC Induces Immune Memory in Metastatic Colorectal Cancer Models. Mol Ther Oncolytics 2017;8:14-26. [Crossref] [PubMed]
- Perez OD, Logg CR, Hiraoka K, et al. Design and selection of Toca 511 for clinical use: modified retroviral replicating vector with improved stability and gene expression. Mol Ther 2012;20:1689-98. [Crossref] [PubMed]
- Cloughesy TF, Landolfi J, Hogan DJ, et al. Phase 1 trial of vocimagene amiretrorepvec and 5-fluorocytosine for recurrent high-grade glioma. Sci Transl Med 2016;8:341ra75. [Crossref] [PubMed]
- Shen W, Patnaik MM, Ruiz A, et al. Immunovirotherapy with vesicular stomatitis virus and PD-L1 blockade enhances therapeutic outcome in murine acute myeloid leukemia. Blood 2016;127:1449-58. [Crossref] [PubMed]
- Chen CY, Wang PY, Hutzen B, et al. Cooperation of Oncolytic Herpes Virotherapy and PD-1 Blockade in Murine Rhabdomyosarcoma Models. Sci Rep 2017;7:2396. [Crossref] [PubMed]
- Freedman JD, Hagel J, Scott EM, et al. Oncolytic adenovirus expressing bispecific antibody targets T-cell cytotoxicity in cancer biopsies. EMBO Mol Med 2017;9:1067-87. [Crossref] [PubMed]
- Yu F, Wang X, Guo ZS, et al. T-cell engager-armed oncolytic vaccinia virus significantly enhances antitumor therapy. Mol Ther 2014;22:102-11. [Crossref] [PubMed]
- Mader EK, Butler G, Dowdy SC, et al. Optimizing patient derived mesenchymal stem cells as virus carriers for a phase I clinical trial in ovarian cancer. J Transl Med 2013;11:20. [Crossref] [PubMed]
- Yin J, Markert JM, Leavenworth JW. Modulation of the Intratumoral Immune Landscape by Oncolytic Herpes Simplex Virus Virotherapy. Front Oncol 2017;7:136. [Crossref] [PubMed]


